Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antioxidant activity of cerium dioxide nanoparticles and nanorods in scavenging hydroxyl radicals†
Alexander Filippi‡
a,
Fobang Liu‡ab,
Jake Wilsona,
Steven Lelieveld a,
Karsten Korscheltc,
Ting Wangad,
Yueshe Wangd,
Tobias Reiche,
Ulrich Pöschl
a,
Karsten Korscheltc,
Ting Wangad,
Yueshe Wangd,
Tobias Reiche,
Ulrich Pöschl a,
Wolfgang Tremel
a,
Wolfgang Tremel c and
Haijie Tong
c and
Haijie Tong *a
*a
aMultiphase Chemistry Department, Max Planck Institute for Chemistry, Mainz, 55128, Germany. E-mail: h.tong@mpic.de
bSchool of Chemical and Biomolecular Engineering, Georgia Institute of Technology, Atlanta, Georgia 30332, USA
cInstitute for Inorganic Chemistry and Analytical Chemistry, Johannes Gutenberg University Mainz, Mainz, 55128, Germany
dState Key Laboratory of Multiphase Flow in Power Engineering, Xi'an Jiaotong University, Xi'an 710049, China
eInstitute of Nuclear Chemistry, Johannes Gutenberg University Mainz, Mainz, 55099, Germany
First published on 9th April 2019
Abstract
Cerium oxide nanoparticles (CeNPs) have been shown to exhibit antioxidant capabilities, but their efficiency in scavenging reactive oxygen species (ROS) and the underlying mechanisms are not yet well understood. In this study, cerium dioxide nanoparticles (CeNPs) and nanorods (CeNRs) were found to exhibit much stronger scavenging activity than ·OH generation in phosphate buffered saline (PBS) and surrogate lung fluid (SLF). The larger surface area and higher defect density of CeNRs may lead to higher ·OH scavenging activity than for CeNPs. These insights are important to understand the redox activity of cerium nanomaterials and provide clues to the role of CeNPs in biological and environmental processes.
Reactive oxygen species (ROS) generally describe reduction products of oxygen molecules, including H2O2 and hydroxyl radicals (·OH).1 ROS play a central role in biological processes exerting both beneficial and adverse health effects.2 Several studies have looked into the redox balance between ROS and antioxidants3 as well as the underlying mechanisms.4 Among all ROS, ·OH is considered as one of the most reactive species; it can attack biomolecules and cause irreversible damage.5 Thus, experimental quantification and abiotic regulation of ·OH under physiologically relevant conditions is an important yet challenging task.
In the last decade, cerium dioxide nanoparticles (CeNPs) have drawn much attention due to their redox properties6 and potential therapeutic applications (such as treating cardiac ischemia).7–9 Efforts have been made to explore the potential use of CeNPs as medicine.7,10,11 The ability of CeNPs in switching the oxidation state of Ce3+ and Ce4+ makes it a good candidate to mediate ROS.6,12 Direct scavenging of ·OH (process ① in Scheme 1), NO·, and OONO− by CeNPs have been investigated.13–16 Moreover, previous studies indicated that CeNPs have catalase- and superoxide dismutase (SOD)-like effects (processes ③ and ⑤ in Scheme 1).17,18 Both effects are closely correlated with the Ce3+ and Ce4+ surface concentrations, pH, H2O2 and chelating ligand concentrations.19–23
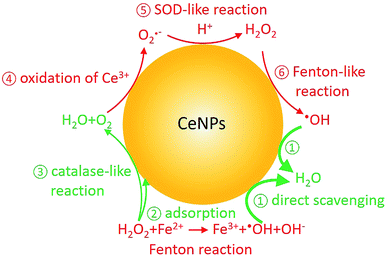 | ||
| Scheme 1 Fenton reaction and reactive oxygen chemistry of CeNPs. Red and green colors indicate ROS formation and scavenging processes, respectively. | ||
In contrast to research about the antioxidant activity of CeNPs, inhalable CeNPs have been detected in ambient air and concerns have been raised about their potential adverse health effect.24,25 Besides this, additional studies suggested that CeNPs can induce oxidative stress, inflammatory signaling response, and cell death upon generating ROS (processes ④–⑥ in Scheme 1) or ROS-messengers.26–30 Given the controversies about the beneficial and toxic effects of CeNPs, it is necessary to distinguish the anti- and prooxidant activities of CeNPs under physiologically relevant conditions.31 In this study, we compared the ·OH formation and scavenging ability of commercial CeNPs (∅ 25 and 50 nm) and homemade cerium nanorods (CeNRs) with different physicochemical properties in phosphate buffered saline (PBS) buffer, antioxidant solutions, and a surrogate lung fluid (SLF). The SLF was used to mimic the key interface between human respiratory tract and inhaled air.
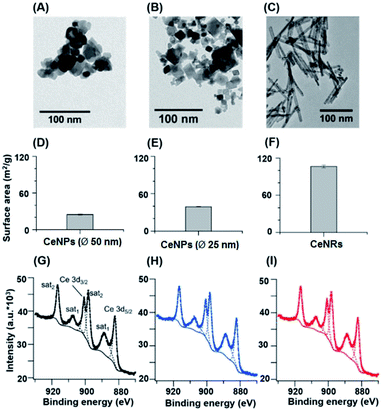
Fig. 1 shows the size, morphology, surface composition, and mass normalized surface area of CeNPs and cerium dioxide nanorods (CeNRs). More information about the applied techniques, sample handling and instrument settings is compiled in Sections S1–S5.† Fig. 1A and B indicate that CeNPs (∅ 50 nm) and CeNPs (∅ 25 nm) have a heterogeneous size distribution with average diameters of <50 nm and <25 nm respectively. Moreover, samples of these commercial CeNPs contain predominantly cubic NPs. In contrast, the morphology of CeNRs (Fig. 1C) is more uniform with a length of ∼100 nm. Details about the CeNRs can be found from our previous study.32 In addition to the detection of size and morphology, the specific surface areas of the cerium nanoparticles were determined to be 24.8 ± 0.4 m2 g−1 (∅ 50 nm CeNPs) (Fig. 1D), 39.2 ± 0.7 m2 g−1 (∅ 25 nm CeNPs) (Fig. 1E), and 106.5 ± 2.4 m2 g−1 (CeNRs) (Fig. 1F). Moreover, the similar Ce 3d XPS spectra of CeNPs (∅ 50 nm) (Fig. 1G), CeNPs (∅ 25 nm) (Fig. 1H), and CeNRs (Fig. 1I) indicate that the distribution of the cerium surface oxidation states (Ce3+ and Ce4+) on these NPs are quite similar. The six most prominent peaks of these spectra are attributable to Ce4+ ions.33 This indicates that Ce4+ was the dominant cerium species in all three samples. The peak fittings (dashed lines) in panels G, H and I are based on the method by Maslakov et al..33 The fitting based deconvolution of Ce 3d XPS spectra indicates that the concentration of surface Ce3+ in all these samples is <3%. Such a low abundance of surface Ce3+ is also supported by the absence of a shoulder peak of Ce 4f electrons at ∼1.1 eV in the XPS valence band spectrum of the CeNRs samples (Fig. S3†). Furthermore, the deconvolution of the XPS spectrum of the O 1s region of the NPs (Fig. S2 and Table S3†) indicates that the CeNRs surface contains a much higher concentration of hydroxide than CeNPs. This may correlate with the synthesis method of CeNRs using NaOH as reagent34 and may play a role in the higher ·OH scavenging activity of CeNRs. These differences in chemical composition, morphology, and surface area between CeNPs and CeNRs may result in variations of their redox activity.
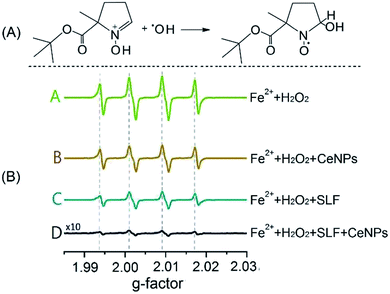
Fig. 2 shows the trapping mechanism of 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO, panel A) and EPR spectra of aqueous mixtures of Fe2+, H2O2, SLF, and CeNPs (panel B). Fig. 2A shows that BMPO can react with OH radicals and form a BMPO–OH radical adduct. In this way, short lifetime radicals can be probed and characterized by electron paramagnetic resonance (EPR) spectroscopy (EMXplus10/12, Bruker, Germany, see details in Section S5 and Table S4†). The grey dashed lines in panel B indicate the characteristic hyperfine splitting of BMPO–OH, in agreement with previous assignment.35 The peak intensities of the spectra in Fig. 2B decrease in the order A (Fe2+ + H2O2) > B (Fe2+ + H2O2 + CeNPs) > C (Fe2+ + H2O2 + SLF) > D (Fe2+ + H2O2 + CeNPs + SLF). This implies that the amount of ·OH decreases accordingly. Based on the spin-counting method,32 we quantified the concentration of BMPO–OH in these solutions. The results are shown in Fig. 3 and Tables S5–S7.†
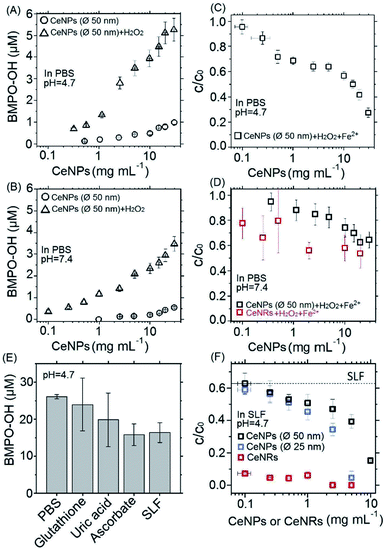
Fig. 3A and B show the positive correlation of ·OH yields of CeNPs (∅ 50 nm) without (black circles) and with the addition of H2O2 (black triangles) under different CeNPs (∅ 50 nm) loading conditions. In the absence of H2O2, 0.1–30 mg mL−1 CeNPs (∅ 50 nm) can generate 0–0.8 and 0–0.5 μM ·OH in pH = 4.7 (Fig. 3A) and 7.4 (Fig. 3B) PBS, respectively. The generation of ·OH by pure CeNPs (∅ 50 nm) in acidic PBS is consistent with previous hypothesis that acid can catalyze the ·OH formation by CeNPs.36 In contrast to pure CeNPs (∅ 50 nm), mixtures of 0.1–30 mg mL−1 CeNPs (∅ 50 nm) with 10 mM of H2O2 can generate 0–5 (pH = 4.7) and 0–3 μM (pH = 7.4) ·OH, which also shows a positive correlation with the loading of CeNPs (∅ 50 nm) as shown in Fig. 3A and B. These hydroxyl radicals may be formed through Fenton-like reactions initiated by CeNPs:37 H2O2 + Ce3+ → Ce4+ + ·OH + OH−.
To evaluate the ·OH scavenging activity of CeNPs in aqueous solution, we measured the ·OH yield by mixtures of CeNPs (∅ 50 nm) or CeNRs, Fe2+, and H2O2 in acidic and neutral PBS. Fig. 3C and D show that the ·OH concentration decreased with increasing CeNPs or CeNRs loading, characterized by the decreasing remaining OH radical concentration. In the absence of CeNPs, Fenton reactions of 1 mM Fe2+ and 10 mM H2O2 generated ∼53 and ∼17 μM ·OH in pH = 4.7 (Fig. 3C) and 7.4 (Fig. 3D) PBS. At 30 mg mL−1 CeNPs (∅ 50 nm), concentration of ·OH decreased to 15 μM (pH = 4.7) and 11 μM (pH = 7.4), respectively (Tables S5 and S6†). In contrast to CeNPs, CeNRs exhibited higher ·OH scavenging efficiency, with 20–50% of ·OH to be scavenged by 0.1–20 mg mL−1 CeNRs. This implies that the scavenging activity of CeNPs (∅ 50 nm) is more pronounced under acidic conditions. The decrease of ·OH concentration may be induced by the following processes: first, CeNPs (∅ 50 nm) or CeNRs could scavenge ·OH directly (process ① in Scheme 1).13 Second, the adsorption of H2O2 on CeNPs (∅ 50 nm) or CeNRs surfaces (like process ② in Scheme 1) may decrease the available H2O2 concentration.38 In this case, due to the lower availability of the H2O2 precursor, the amount of ·OH formed by Fenton reactions will decrease. Third, the surface-bound H2O2 can be decomposed via catalase-like reactions (process ③ in Scheme 1).21 This process will form H2O and O2 rather than ·OH. Beyond these two pathways, iron ion-initiated redox processes may also influence the measured ·OH concentrations. For instance, it has been suggested that upon interaction with the surface of CeNPs, Fe2+ can enhance the dissolution of Ce3+ and cause the formation of 6-line ferrihydrite, which can increase the colloidal stability of the CeNPs.39 Such a reaction may alter the redox activity of CeNPs (∅ 50 nm) or CeNRs.
Recently Baldim et al.38 measured the H2O2 surface adsorption potential of CeNPs with different sizes. They found that 5–28 nm diameter CeNPs could adsorb 2–20 H2O2 molecules nm−2, depending on the surface composition of the nanomaterial. We used the adsorption potential from Baldim et al. and estimated that only <1% of H2O2 (∼8 μM) can be adsorbed on the surface of the CeNPs. Therefore, the surface adsorption of H2O2 by CeNPs cannot fully explain the reduction of ·OH concentration in Fig. 3. Furthermore, Pirmohamed et al.21 observed a H2O2 decomposition rate of ∼2.7 nmol min−1 through catalase-like reactions. Based on this value, we estimate that a concentration of 0.1 mg mL−1 of CeNPs would result in a H2O2 loss of <2% in our studies. Therefore, we suggest the direct scavenging process (① in Scheme 1), rather than the surface adsorption (② in Scheme 1) and catalase-like (③in Scheme 1) processes to be the dominant reduction pathways of ·OH.
Fig. 3E shows the ·OH scavenging activity of typical epithelial lung fluid antioxidants and a surrogate lung fluid (SLF). Here, 0.1 mM of glutathione, 0.1 mM of uric acid, and 0.2 mM of ascorbate solutions could scavenge ∼8%, ∼14%, and ∼39% of hydroxyl radicals originating from Fenton reactions of 1 mM Fe2+ and 10 mM H2O2 in PBS. The SLF showed a similar activity as 0.2 mM ascorbate, i.e. the ·OH scavenging activities of individual antioxidants are not additive and decrease in the order ascorbate > uric acid > glutathione. This trend is consistent with previous findings.38
To assess the antioxidant activity of CeNPs under quasi-physiological conditions, we explored the ·OH scavenging activity of CeNPs and CeNRs in SLF. Fig. 3F shows the hydroxyl radical yield by Fenton reactions in SLF as a function of the CeNPs (∅ 25 and 50 nm) and CeNRs loading. As the loading of CeNPs (∅ 50 nm) increased from 0.1 to 10 mg mL−1, the concentration of ·OH in SLF decreased by 38–85%. Within the same loading range, the CeNPs (∅ 25 nm) exhibited a similar efficiency. Whereas at higher loadings (1–5 mg mL−1), the ·OH scavenging potential of CeNPs (∅ 25 nm) was 9–55% higher than that of their 50 nm counterparts. In contrast to CeNPs, the CeNRs showed a much higher ·OH scavenging efficiency. Even with a loading as low as 0.1 mg mL−1, the CeNRs could reduce 88% of the ·OH. For CeNRs loadings that exceeded 1 mg mL−1, no ·OH could be observed. The trend of the ·OH scavenging efficiency according to CeNRs > CeNPs (∅ 25 nm) >CeNPs (∅ 50 nm) is in the same order as the surface area of these NPs (Fig. 1D–F). Given the low abundance of Ce3+ on fresh CeNPs and CeNRs surface (Fig. 1G–H), we suggest that substantial amount of Ce3+ may be formed upon interactions of NPs with water.13 The larger surface area of CeNRs may increase the density of Ce3+ per unit particle mass and subsequently their ·OH scavenging activity. Previous works showed that CeNRs are prone to expose their (110) facets to reactive species.34 These facets were described as reactive “hybrid structures” between the (111) and (100) surfaces of CeNPs. Furthermore, the distinct crystallographic surface structure of CeNRs may act as binding site for reactive species (·OH and H2O2) exerting peroxidase-like effects. Additionally, Fe2+-dependent reactive oxygen chemistry may contribute to the observed ·OH scavenging processes.39 Finally, it has been suggested that glutathione could interact with CeNPs and influence the redox couple of Ce3+/Ce4+.40
It is worthy to note that a real physiologic environment is more complicated than SLF. A large number of redox chemistry processes may alter the agglomeration and distribution of CeNPs and relevant materials,41 which may eventually influence its properties including ·OH scavenging efficiency and SOD-like characteristics.42 Thus, characterizing CeNPs or their functionalized derivatives in more realistic environments will be beneficial and promising in follow-up studies.
Conclusions
In this study, we compared the ability of CeNPs and CeNRs in scavenging hydroxyl radicals (·OH) under physiologically relevant conditions. We found that CeNPs and CeNRs exert high ·OH scavenging activity in both PBS and SLF. In SLF, the ·OH scavenging potential of CeNPs increased 4-fold as the loading increases from 0.1 to 10 mg mL−1. In the same loading range, CeNRs showed 5–50-fold higher ·OH scavenging potential than CeNPs, which may be attributable to the higher surface area and defect density of CeNRs. Furthermore, we found the scavenging activity of CeNPs is pH-dependent, exhibiting higher scavenging efficiency under lower pH condition. The observed ·OH scavenging efficiency of CeNPs and CeNRs in SLF took into account the effect of antioxidants at concentrations close to the epithelial lung fluid, reflecting the redox activity of CeNPs and CeNRs under more realistic in vitro conditions than previous studies. These findings are of critical importance for a better understanding of the relative ROS scavenging efficiency of CeNPs comparing to conventional antioxidants. Moreover, these results are also important for making accurate dose–response curves predicting the toxicity or antioxidant characteristics of CeNPs or their functionalized derivatives in biological and environmental processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Max Planck Society, the Deutsche Forschungsgemeinschaft (DFG), and the National Natural Science Foundation of China (No. 51576160). We thank Jakob Drebert for his support of the XPS measurements. Open Access funding provided by the Max Planck Society.Notes and references
- A. A. Alfadda and R. M. Sallam, J. Biomed. Biotechnol., 2012, 2012, 936486 CrossRef PubMed.
- C. C. Winterbourn, Nat. Chem. Biol., 2008, 4, 278–286 CrossRef CAS PubMed.
- B. Poljsak, D. Suput and I. Milisav, Oxid. Med. Cell. Longevity, 2013, 2013, 956792 Search PubMed.
- S. B. Nimse and D. Pal, RSC Adv., 2015, 5, 27986–28006 RSC.
- A. Phaniendra, D. B. Jestadi and L. Periyasamy, Indian J. Clin. Biochem., 2015, 30, 11–26 CrossRef CAS PubMed.
- A. Karakoti, S. Singh, J. M. Dowding, S. Seal and W. T. Self, Chem. Soc. Rev., 2010, 39, 4422–4432 RSC.
- J. M. Perez, A. Asati, S. Nath and C. Kaittanis, Small, 2008, 4, 552–556 CrossRef CAS PubMed.
- S. Rajeshkumar and P. Naik, Biotechnol. Rep., 2018, 17, 1–5 CrossRef CAS PubMed.
- M. Culcasi, L. Benameur, A. Mercier, C. Lucchesi, H. Rahmouni, A. Asteian, G. Casano, A. Botta, H. Kovacic and S. Pietri, Chem.-Biol. Interact., 2012, 199, 161–176 CrossRef CAS PubMed.
- C. Walkey, S. Das, S. Seal, J. Erlichman, K. Heckman, L. Ghibelli, E. Traversa, J. F. McGinnis and W. T. Self, Environ. Sci.: Nano, 2015, 2, 33–53 RSC.
- C. K. Kim, T. Kim, I. Y. Choi, M. Soh, D. Kim, Y. J. Kim, H. Jang, H. S. Yang, J. Y. Kim and H. K. Park, Angew. Chem., Int. Ed., 2012, 51, 11039–11043 CrossRef CAS PubMed.
- K. M. Dunnick, R. Pillai, K. L. Pisane, A. B. Stefaniak, E. M. Sabolsky and S. S. Leonard, Biol. Trace Elem. Res., 2015, 166, 96–107 CrossRef CAS PubMed.
- Y. Xue, Q. Luan, D. Yang, X. Yao and K. Zhou, J. Phys. Chem. C, 2011, 115, 4433–4438 CrossRef CAS.
- J. M. Dowding, T. Dosani, A. Kumar, S. Seal and W. T. Self, Chem. Commun., 2012, 48, 4896–4898 RSC.
- J. M. Dowding, S. Seal and W. T. Self, Drug Delivery Transl. Res., 2013, 3, 375–379 CrossRef CAS PubMed.
- W. He, Y. Liu, W. G. Wamer and J. J. Yin, J. Food Drug Anal., 2014, 22, 49–63 CrossRef CAS.
- C. Korsvik, S. Patil, S. Seal and W. T. Self, Chem. Commun., 2007, 10, 1056–1058 RSC.
- Y. Yang, Z. Mao, W. Huang, L. Liu, J. Li, J. Li and Q. Wu, Sci. Rep., 2016, 6, 35344 CrossRef CAS PubMed.
- B. C. Nelson, M. E. Johnson, M. L. Walker, K. R. Riley and C. M. Sims, Antioxidants, 2016, 5, 15–36 CrossRef PubMed.
- E. Grulke, K. Reed, M. Beck, X. Huang, A. Cormack and S. Seal, Environ. Sci.: Nano, 2014, 1, 429–444 RSC.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- V. K. Ivanov, A. B. Shcherbakov and A. V. Usatenko, Russ. Chem. Rev., 2009, 78, 855–871 CrossRef CAS.
- V. Patel, M. Singh, E. L. H. Mayes, A. Martinez, V. Shutthanandan, V. Bansal, S. Singh and A. S. Karakoti, Chem. Commun., 2018, 54, 13973–13976 RSC.
- C. Guo, S. Robertson, R. J. M. Weber, A. Buckley, J. Warren, A. Hodgson, J. Z. Rappoport, K. Ignatyev, K. Meldrum, I. Römer, S. Macchiarulo, J. K. Chipman, T. Marczylo, M. O. Leonard, T. W. Gant, M. R. Viant and R. Smith, Nanotoxicology, 2019, 1–18 CrossRef CAS PubMed.
- S. Dekkers, L. Ma-Hock, I. Lynch, M. Russ, M. R. Miller, R. P. Schins, J. Keller, I. Römer, K. Küttler and V. Strauss, Inhalation Toxicol., 2018, 30, 273–286 CrossRef CAS PubMed.
- A. Srinivas, P. J. Rao, G. Selvam, P. B. Murthy and P. N. Reddy, Toxicol. Lett., 2011, 205, 105–115 CrossRef CAS PubMed.
- E. J. Park, J. Choi, Y. K. Park and K. Park, Toxicology, 2008, 245, 90–100 CrossRef CAS PubMed.
- H. J. Eom and J. Choi, Toxicol. Lett., 2009, 187, 77–83 CrossRef CAS PubMed.
- E.-J. Park, W.-S. Cho, J. Jeong, J.-h. Yi, K. Choi, Y. Kim and K. Park, J. Health Sci., 2010, 56, 387–396 Search PubMed.
- M. S. Wason, J. Colon, S. Das, S. Seal, J. Turkson, J. Zhao and C. H. Baker, Nanomedicine, 2013, 9, 558–569 CrossRef CAS PubMed.
- H. Zhang, X. He, Z. Zhang, P. Zhang, Y. Li, Y. Ma, Y. Kuang, Y. Zhao and Z. Chai, Environ. Sci. Technol., 2011, 45, 3725–3730 CrossRef CAS PubMed.
- K. Korschelt, R. Schwidetzky, F. Pfitzner, J. Strugatchi, C. Schilling, M. von der Au, K. Kirchhoff, M. Panthofer, I. Lieberwirth, M. N. Tahir, C. Hess, B. Meermann and W. Tremel, Nanoscale, 2018, 10, 13074–13082 RSC.
- K. I. Maslakov, Y. A. Teterin, A. J. Popel, A. Y. Teterin, K. E. Ivanov, S. N. Kalmykov, V. G. Petrov, P. K. Petrov and I. Farnan, Appl. Surf. Sci., 2018, 448, 154–162 CrossRef CAS.
- K. Herget, P. Hubach, S. Pusch, P. Deglmann, H. Gotz, T. E. Gorelik, I. A. Gural'skiy, F. Pfitzner, T. Link, S. Schenk, M. Panthofer, V. Ksenofontov, U. Kolb, T. Opatz, R. Andre and W. Tremel, Adv. Mater., 2017, 29, 1603823 CrossRef PubMed.
- H. Tong, P. S. J. Lakey, A. M. Arangio, J. Socorro, F. Shen, K. Lucas, W. H. Brune, U. Poschl and M. Shiraiwa, Environ. Sci. Technol., 2018, 52, 11642–11651 CrossRef CAS PubMed.
- W. Lin, Y. W. Huang, X. D. Zhou and Y. Ma, Int. J. Toxicol., 2006, 25, 451–457 CrossRef CAS PubMed.
- A. D. Bokare and W. Choi, J. Hazard. Mater., 2014, 275, 121–135 CrossRef CAS PubMed.
- V. Baldim, F. Bedioui, N. Mignet, I. Margaill and J. F. Berret, Nanoscale, 2018, 10, 6971–6980 RSC.
- X. Liu, J. R. Ray, C. W. Neil, Q. Li and Y. S. Jun, Environ. Sci. Technol., 2015, 49, 5476–5483 CrossRef CAS PubMed.
- F. Rollin-Genetet, C. Seidel, E. Artells, M. Auffan, A. Thiery and C. Vidaud, Chem. Res. Toxicol., 2015, 28, 2304–2312 Search PubMed.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00642g |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |