Open Access Article
Open Access ArticleDesign and synthesis of acetaminophen probe APAP-P1 for identification of the toxicity targets thioredoxin reductase-1 in HepaRG cells†
Shan Wang‡
 abcde,
Yu Tian‡abcde,
Shan Luabcde,
Ruiying Wangabcde,
Hai Shangabcde,
Xuelian Zhangabcde,
Chenyang Zhangabcde,
Guibo Sun*abcde,
Xudong Xu*abcde and
Xiaobo Sun*abcde
abcde,
Yu Tian‡abcde,
Shan Luabcde,
Ruiying Wangabcde,
Hai Shangabcde,
Xuelian Zhangabcde,
Chenyang Zhangabcde,
Guibo Sun*abcde,
Xudong Xu*abcde and
Xiaobo Sun*abcde
aBeijing Key Laboratory of Innovative Drug Discovery of Traditional Chinese Medicine (Natural Medicine) and Translational Medicine, Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100193, P. R. China. E-mail: sun_xiaobo163@163.com
bKey Laboratory of Bioactive Substances and Resources Utilization of Chinese Herbal Medicine, Ministry of Education, Beijing 100193, P. R. China
cKey Laboratory of Efficacy Evaluation of Chinese Medicine against Glycolipid Metabolic Disorders, State Administration of Traditional Chinese Medicine, Beijing 100193, P. R. China
dZhong guan cun Open Laboratory of the Research and Development of Natural Medicine and Health Products, Beijing 100193, P. R. China
eKey Laboratory of New Drug Discovery Based on Classic Chinese Medicine Prescription, Beijing 100193, P. R. China
First published on 15th May 2019
Abstract
Drug-induced liver injury is one of the main causes of drug non-approval and drug withdrawal by the Food and Drug Administration (FDA). Acetaminophen (APAP) is a widely used non-steroidal anti-inflammatory drug for treating fever and headache. APAP is considered safe at therapeutic doses; however, there have been reports of acute liver injury following the administration of APAP. To explore APAP hepatotoxicity and its mechanisms, we designed and synthesized a new click chemistry probe, APAP-P1, in our current study. We introduced the PEG-azide probe linker into the acetyl group of acetaminophen. First, we evaluated the probe toxicity in HepaRG cells and found that it still retained hepatotoxicity. We also found that this probe APAP-P1 can be metabolized by HepaRG cells. This demonstrated that the APAP-P1 probe still kept its metabolism characteristics. Using this probe, we pulled down its potential targets in vivo and in vitro. APAP can directly target TrxR1; thus, we tested for this interaction by Western blotting of pull-down proteins. The results showed that APAP-P1 can pull down TrxR1 in vivo and in vitro.
Introduction
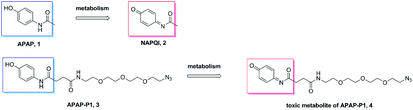
Drug-induced liver injury (DILI) is an unpredictable adverse drug reaction that affects only a small subset of treated patients and remains an increasingly recognized cause of hepatotoxicity and liver failure worldwide.1–3 Acetaminophen (APAP) is a widely used agent for the treatment of pain and fever around the world and is considered safe at therapeutic doses; however, overdose can cause severe hepatotoxicity leading to acute liver failure, which is the most common cause of drug-induced liver damage in the world.4,5 APAP causes potentially fatal hepatocellular necrosis when taken in nontherapeutic doses.6 APAP can undergo metabolism to form the reactive electrophile N-acetyl-p-benzoquinone imine (NAPQI), which causes extensive and rapid glutathione depletion, mitochondrial dysfunction, oxidant stress, peroxynitrite formation and nuclear DNA fragmentation in hepatocytes.1,7–9 Its molecular toxicology was proposed to be initiated by reactive oxygen species (ROS) formation in the mitochondria, leading to the subsequent activation of apoptosis signalling-regulating kinase 1 (ASK1) and the c-Jun N-terminal kinase (JNK) pathway.10,11 Thioredoxin reductase-1 (TrxR1), encoded by the Txnrd1 gene, is a NADPH-dependent enzyme and is known to be related to oxidative stress and the regulation of cell growth and development. Some studies have found that the GSH- or TrxR1-dependent redox pathway can independently support hepatocyte proliferation during liver growth.12 Previous research has found that NAPQI directly inactivates TrxR1, which may be associated with enhanced Nrf2 activity.13,14 TrxR1 is important for attenuating the activation of apoptosis signaling-regulating kinase 1 (ASK1) and the c-JunN-terminal kinase (JNK) pathway, which is caused by high doses of APAP.15 The Trx system impacts bioenergetics and drug metabolism, and some studies have indicated it to be a potential marker of DILI, as in the case of anti-tuberculosis drugs.16,17HepaRG cells express most of the enzymes and transcription factors involved in xenobiotic biotransformation and transport and have been shown to be a valuable model for studying the mechanism of hepatotoxicity induced by drugs and toxins.18 Research has demonstrated that HepaRG cells can be used to explore the mechanisms of APAP hepatotoxicity.19 APAP is converted to the reactive intermediate NAPQI in HepaRG cells, which has been thought to be the toxin for hepatotoxicity.20 In our research, we chose the HepaRG cell line as our in vitro model to evaluate the liver damage caused by APAP.
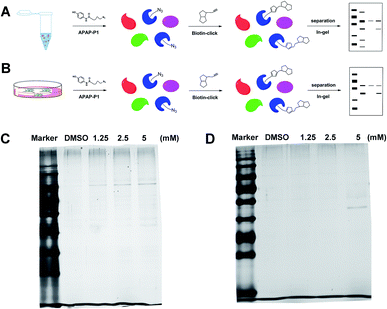
Chemical proteomics is a growing area of chemical biology that seeks to design small molecule probes to understand protein function.21 Activity-based protein profiling (ABPP) has emerged as a powerful functional chemical proteomic strategy that enables global profiling of proteome reactivity toward bioactive small molecules in complex biological and/or pathological processes.22 Activity-based probes (ABPs) usually consist of three fundamental building blocks with distinct functions: (1) an active warhead (WH) that contains reactive groups that target the conserved mechanistic or structural feature of a set of proteins; (2) a reporter tag for identification and purification of modified proteins; and (3) a linker region that can modulate the reactivity and specificity of the reactive group and binding group while providing enough space between the reporter and the reactive or binding group.23,24 In this study, we designed and synthesized a new ABPP probe, APAP-P1, for the identification of APAP toxicity targets in HepaRG cells.
Results and discussion
Chemistry
As illustrated in Scheme 1, commercially available p-aminophenol (1) was treated with tert-butyldimethylsilyl chloride (TBSCl) and imidazole in dry tetrahydrofuran (THF) to obtain a good yield of intermediate 2. Intermediate 2 reacted with succinic anhydride (SAA) in organic alkali conditions with dimethylaminopyridine (DMAP) to provide compound 3, which was subjected to an acylation reaction to obtain compound 4 in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) and DMAP. Then, APAP-P1 was obtained via the deprotection reaction of intermediate 4 with tetrabutylammonium fluoride in THF solution.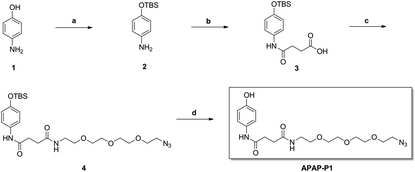 | ||
| Scheme 1 The synthesis of toxic probe APAP-P1. Reagents and conditions: (a) TBSCl, imidazole, THF; (b) succinic anhydride (SAA), DMAP, DCM; (c) EDCI, DMAP, DCM; (d) TBAF, THF. | ||
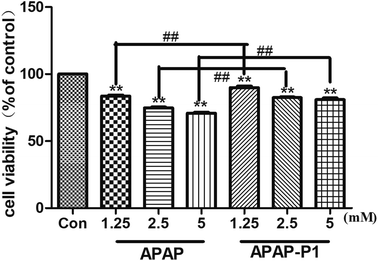
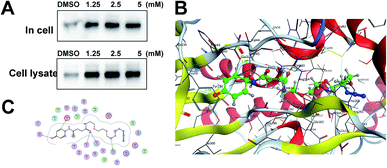
From our results, we found that APAP-P1 has a similar protein profiling in HepaRG cell lysates and in HepaRG cells. APAP-P1 can pull down 42–55 kDa bands; however, there are obvious differences. For the cell lysate, the probe mainly pulled down the 100 kDa protein bands, but 42–55 kDa bands were more obvious in cells. These results demonstrated that after APAP-P1 metabolism, its interaction protein profile was changed. This suggests that we should focus more on the APAP metabolism for hepatotoxicity. With this useful probe, we will perform additional in vivo research in the future.
Experimental
Chemistry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PE-EtOAc, 1
PE-EtOAc, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to offer pure white solid compound 2, 93% yield; 1H-NMR (600 MHz, CDCl3) δ: 6.69–6.68 (m, 2H, Ph-H), 6.58–6.56 (m, 2H, Ph-H), 3.45 (s, 2H, NH2), 1.01 (s, 9H, C-(CH3)3), 0.19 (m, 6H, Si-(CH3)2); 13C-NMR (150 MHz, CDCl3) δ: 148.0, 140.4, 120.6, 116.3, 25.8, 18.2, −4.48; HRMS (ESI): calcd for [M + H]+ C12H22NOSi: 224.1471, found 224.1484.
1) to offer pure white solid compound 2, 93% yield; 1H-NMR (600 MHz, CDCl3) δ: 6.69–6.68 (m, 2H, Ph-H), 6.58–6.56 (m, 2H, Ph-H), 3.45 (s, 2H, NH2), 1.01 (s, 9H, C-(CH3)3), 0.19 (m, 6H, Si-(CH3)2); 13C-NMR (150 MHz, CDCl3) δ: 148.0, 140.4, 120.6, 116.3, 25.8, 18.2, −4.48; HRMS (ESI): calcd for [M + H]+ C12H22NOSi: 224.1471, found 224.1484.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc) to offer pure white solid compound 3, 81% yield; 1H-NMR (600 MHz, CDCl3) δ: 8.29 (s, 1H, NH), 7.32–7.31 (m, 2H, Ph-H), 6.72–6.70 (m, 2H, Ph-H), 2.63 (m, 2H, CH2COOH), 2.53 (m, 2H, CH2CONH), 0.95 (s, 9H, C-(CH3)3), 0.14 (m, 6H, Si-(CH3)2); 13C-NMR (150 MHz, CDCl3) δ: 177.9, 170.9, 152.5, 131.5, 122.0, 120.4, 31.8, 30.1, 25.8, 18.3, −4.35; HRMS (ESI): calcd for [M + Na]+ C16H25NNaO4Si: 346.1451, found 346.1476.
EtOAc) to offer pure white solid compound 3, 81% yield; 1H-NMR (600 MHz, CDCl3) δ: 8.29 (s, 1H, NH), 7.32–7.31 (m, 2H, Ph-H), 6.72–6.70 (m, 2H, Ph-H), 2.63 (m, 2H, CH2COOH), 2.53 (m, 2H, CH2CONH), 0.95 (s, 9H, C-(CH3)3), 0.14 (m, 6H, Si-(CH3)2); 13C-NMR (150 MHz, CDCl3) δ: 177.9, 170.9, 152.5, 131.5, 122.0, 120.4, 31.8, 30.1, 25.8, 18.3, −4.35; HRMS (ESI): calcd for [M + Na]+ C16H25NNaO4Si: 346.1451, found 346.1476.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM-MeOH, 20
DCM-MeOH, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to offer pure white solid compound 4, 65% yield; 1H-NMR (600 MHz, CDCl3) δ: 8.66 (s, 1H, CONHPh), 7.37–7.35 (m, 2H, Ph-H), 6.75–6.73 (m, 2H, Ph-H), 6.67 (t, J = 5.7 Hz, 1H, CH2CONH), 3.66–3.57 (m, 10H, CH2(OCH2CH2)2), 3.53–3.51 (m, 2H, CH2O), 3.44–3.42 (m, 2H, CH2N3), 3.38–3.37 (m, 2H, CONHCH2), 2.67–2.65 (m, 2H, CONHCH2), 2.61–2.59 (m, 2H, CH2CONH), 0.95 (s, 9H, C-(CH3)3), 0.15 (m, 6H, Si-(CH3)2); 13C-NMR (150 MHz, CDCl3) δ: 172.7, 170.5, 152.0, 132.2, 121.3, 120.3, 70.7, 70.7, 70.6, 70.3, 70.1, 69.8, 50.7, 39.5, 32.9, 31.7, 25.8, 18.3, −4.38; HRMS (ESI): calcd for [M + Na]+ C24H41N5NaO6Si: 546.2724, found 546.2753.
1) to offer pure white solid compound 4, 65% yield; 1H-NMR (600 MHz, CDCl3) δ: 8.66 (s, 1H, CONHPh), 7.37–7.35 (m, 2H, Ph-H), 6.75–6.73 (m, 2H, Ph-H), 6.67 (t, J = 5.7 Hz, 1H, CH2CONH), 3.66–3.57 (m, 10H, CH2(OCH2CH2)2), 3.53–3.51 (m, 2H, CH2O), 3.44–3.42 (m, 2H, CH2N3), 3.38–3.37 (m, 2H, CONHCH2), 2.67–2.65 (m, 2H, CONHCH2), 2.61–2.59 (m, 2H, CH2CONH), 0.95 (s, 9H, C-(CH3)3), 0.15 (m, 6H, Si-(CH3)2); 13C-NMR (150 MHz, CDCl3) δ: 172.7, 170.5, 152.0, 132.2, 121.3, 120.3, 70.7, 70.7, 70.6, 70.3, 70.1, 69.8, 50.7, 39.5, 32.9, 31.7, 25.8, 18.3, −4.38; HRMS (ESI): calcd for [M + Na]+ C24H41N5NaO6Si: 546.2724, found 546.2753.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc), to offer pure white solid APAP-P1, 90% yield; 1H-NMR (600 MHz, CDCl3) δ: 8.68 (s, 1H, Ph-OH), 7.92 (s, 1H, CONHPh), 7.20–7.19 (m, 2H, Ph-H), 6.74 (t, J = 5.3 Hz, 1H, CH2CONH), 6.69–6.68 (m, 2H, Ph-H), 3.63–3.55 (m, 10H, CH2(OCH2CH2)2), 3.48–3.47 (m, 2H, CH2O), 3.38–3.33 (m, 4H, CH2N3, CONHCH2), 2.64–2.62 (m, 2H, CONHCH2), 2.56–2.54 (m, 2H, CH2CONH); 13C-NMR (150 MHz, CDCl3) δ: 172.9, 170.9, 153.4, 130.5, 122.5, 115.7, 70.7, 70.7, 70.6, 70.3, 70.1, 69.7, 50.8, 39.6, 32.5, 31.5; HRMS (ESI): calcd for [M + Na]+ C18H27N5NaO6: 432.1859, found 432.1875.
EtOAc), to offer pure white solid APAP-P1, 90% yield; 1H-NMR (600 MHz, CDCl3) δ: 8.68 (s, 1H, Ph-OH), 7.92 (s, 1H, CONHPh), 7.20–7.19 (m, 2H, Ph-H), 6.74 (t, J = 5.3 Hz, 1H, CH2CONH), 6.69–6.68 (m, 2H, Ph-H), 3.63–3.55 (m, 10H, CH2(OCH2CH2)2), 3.48–3.47 (m, 2H, CH2O), 3.38–3.33 (m, 4H, CH2N3, CONHCH2), 2.64–2.62 (m, 2H, CONHCH2), 2.56–2.54 (m, 2H, CH2CONH); 13C-NMR (150 MHz, CDCl3) δ: 172.9, 170.9, 153.4, 130.5, 122.5, 115.7, 70.7, 70.7, 70.6, 70.3, 70.1, 69.7, 50.8, 39.6, 32.5, 31.5; HRMS (ESI): calcd for [M + Na]+ C18H27N5NaO6: 432.1859, found 432.1875.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 5 min. The supernatant was transferred into a glass tube, evaporated to dryness under a stream of nitrogen.26 Then the dried metabolites extract was dissolved in 300 μL of methanol, and an aliquot of 2 μL was injected for UPLC/MS analysis. The lysate and cell samples were analysed on a Waters Acquity™ Ultra Performance LC system (Waters Corporation, Milford, MA, USA) equipped with a BEH C18 column (100 mm × 2.1 mm, 1.7 μm). The autosampler temperature was kept at 4 °C and the column compartment was set at 40 °C. The mobile phase was composed of solvents A (2 mM ammonium acetate in 95% H2O/5% acetonitrile + 0.1% acetic acid) and B (2 mM ammonium acetate in 95% acetonitrile/5% H2O + 0.1% acetic acid). A Waters SYNAPT G2 HDMS (Waters Corp., Manchester, UK) was used to carry out the mass spectrometry with an electrospray ionization source (ESI) operating in positive ion modes. The UPLC-Q-TOF/MS raw data were processed by MarkerLynx Applications Manager version 4.1 (Waters, Manchester, UK). The procedure included integration, normalization, and alignment of peak intensities, and a list of m/z and retention times with corresponding peaks was provided for all metabolites in every sample in the data set.
000g for 5 min. The supernatant was transferred into a glass tube, evaporated to dryness under a stream of nitrogen.26 Then the dried metabolites extract was dissolved in 300 μL of methanol, and an aliquot of 2 μL was injected for UPLC/MS analysis. The lysate and cell samples were analysed on a Waters Acquity™ Ultra Performance LC system (Waters Corporation, Milford, MA, USA) equipped with a BEH C18 column (100 mm × 2.1 mm, 1.7 μm). The autosampler temperature was kept at 4 °C and the column compartment was set at 40 °C. The mobile phase was composed of solvents A (2 mM ammonium acetate in 95% H2O/5% acetonitrile + 0.1% acetic acid) and B (2 mM ammonium acetate in 95% acetonitrile/5% H2O + 0.1% acetic acid). A Waters SYNAPT G2 HDMS (Waters Corp., Manchester, UK) was used to carry out the mass spectrometry with an electrospray ionization source (ESI) operating in positive ion modes. The UPLC-Q-TOF/MS raw data were processed by MarkerLynx Applications Manager version 4.1 (Waters, Manchester, UK). The procedure included integration, normalization, and alignment of peak intensities, and a list of m/z and retention times with corresponding peaks was provided for all metabolites in every sample in the data set.Biological studies
Conclusions
In conclusion, we designed and synthesized one new powerful probe APAP-P1, introducing the PEG-azide probe linker into the acetyl group of acetaminophen. This probe maintained hepatotoxicity in HepaRG cells by MTT assay, and demonstrated that the PEG-azide probe linker does not change APAP hepatotoxicity. Metabolite of APAP-P1 by HepaRG cells showed this probe still can be metabolised to hepatotoxic intermediate. Results above proved APAP-P1 worked well in HepaRG cells, so we can use this probe for target fishing and identification. By CC-ABPP pull-down assays in both cell lysate and cells, we identified their protein profiles. There was some difference under the two conditions. This change is due to the metabolism of APAP-P1. This is similar to the process of APAP in vivo. TrxR1 has been proved NAPQI hepatotoxicity target in previous research, finally, we also identified that APAP-P1 can target TrxR1 by pull-down protein Western blot assay and thus APAP-P1 can be used for further research in vivo.Conflicts of interest
The authors confirm that there are no conflicts of interest.Acknowledgements
This work was supported by the National Science and Technology Major Project (Grant No. 2015ZX09501004-001-003), the Special Research Project for TCM (Grant No. 201507004), and the CAMS Innovation Fund for Medical Science (CIFMS) (Grant No. 2016-I2M-1-012), Beijing Natural Science Foundation (Grant No. 7192129).Notes and references
- R. Chan and L. Z. Benet, Chem. Res. Toxicol., 2016, 30, 1017–1029 Search PubMed.
- G. A. Kullak-Ublick, R. J. Andrade, M. Merz, P. End, A. Benesic, A. L. Gerbes and G. P. Aithal, Gut, 2017, 66, 1154–1164 CrossRef CAS PubMed.
- D. Bissell, Hepatology, 2001, 33, 1009–1013 CrossRef CAS PubMed.
- W. M. Lee, J. Hepatol., 2017, 67, 1324–1331 CrossRef CAS.
- D. Bartlett, J. Emerg. Nurs., 2004, 3, 281–283 CrossRef.
- M. R. McGill and H. Jaeschke, Pharm. Res., 2013, 30, 2174–2187 CrossRef CAS PubMed.
- M. L. Bajt, Toxicol. Sci., 2004, 80, 343–349 CrossRef CAS PubMed.
- J. A. Hinson, A. B. Reid, S. S. McCullough and L. P. James, Drug Metab. Rev., 2004, 36, 805–822 CrossRef CAS.
- H. Jaeschke, C. D. Williams, A. Ramachandran and M. L. Bajt, Liver Int., 2012, 32, 8–20 CrossRef CAS.
- K. Du, Y. Xie, M. R. McGill and H. Jaeschke, Expert Opin. Drug Metab. Toxicol., 2015, 11, 1769–1779 CrossRef CAS PubMed.
- J. A. Hinson, D. W. Robert and L. P. James, Handb. Exp. Pharmacol., 2010, 196, 369–405 CAS.
- J. R. Prigge, S. Eriksson, S. V. Iverson, T. A. Meade, M. R. Capecchi, E. S. J. Arnér and E. E. Schmidt, Free Radical Biol. Med., 2012, 52, 803–810 CrossRef CAS PubMed.
- S. V. Iverson, S. Eriksson, J. Xu, J. R. Prigge, E. A. Talago, T. A. Meade, E. S. Meade, M. R. Capecchi, E. S. J. Arnér and E. E. Schmidt, Free Radical Biol. Med., 2013, 63, 369–380 CrossRef CAS PubMed.
- Y. Jan, D. E. Heck, A. Dragomir, C. R. Gardner, D. L. Laskin and J. D. Laskin, Chem. Res. Toxicol., 2014, 27, 882–894 Search PubMed.
- M. Saitoh, H. Nishitoh, M. Fujii, K. Takeda, K. Tobiume, Y. Sawada, M. Kawabata, K. Miyazono and H. Ichijo, EMBO J., 1998, 17, 2596–2606 CrossRef CAS PubMed.
- J. Kwon, E. Shin, J. Lee, S. Kim, S. Kim, Y. Jee, Y. Kim, H. Park, K. Min and H. Park, Allergy, Asthma Immunol. Res., 2012, 4, 132 CrossRef CAS PubMed.
- B. Fu, W. Meng, X. Zeng, H. Zhao, W. Liu and T. Zhang, BioMed Res. Int., 2017, 2017, 1–8 Search PubMed.
- A. Michaut, D. Le Guillou, C. Moreau, S. Bucher, M. R. McGill, S. Martinais, T. Gicquel, I. Morel, M. Robin, H. Jaeschke and B. Fromenty, Toxicol. Appl. Pharmacol., 2016, 292, 40–55 CrossRef CAS.
- M. R. McGill, H. Yan, A. Ramachandran, G. J. Murray, D. E. Rollins and H. Jaeschke, Hepatology, 2011, 53, 974–982 CrossRef CAS PubMed.
- Y. Xie, M. R. McGill, S. F. Cook, M. R. Sharpe, R. D. Winefield, D. G. Wilkins, D. E. Rollins and H. Jaeschke, Xenobiotica, 2015, 45, 921–929 CrossRef CAS PubMed.
- M. H. Wright and S. A. Sieber, Nat. Prod. Rep., 2016, 5, 681–708 RSC.
- J. Krysiak and R. Breinbauer, Top. Curr. Chem., 2012, 43–84 CAS.
- A. E. Speers and B. F. Cravatt, Curr. Protoc. Chem. Biol., 2009, 1, 29–41 Search PubMed.
- A. B. Berger, P. M. Vitorino and M. Bogyo, Am. J. PharmacoGenomics, 2004, 4, 371–381 CrossRef CAS PubMed.
- A. D. Patterson, B. A. Carlson, F. Li, J. A. Bonzo, M. Yoo, K. W. Krausz, M. Conrad, C. Chen, F. J. Gonzalez and D. L. Hatfield, Chem. Res. Toxicol., 2013, 26, 1088–1096 Search PubMed.
- L. Li, X. Chen, D. Li and D. Zhong, Drug Metab. Dispos., 2011, 39, 472–483 CrossRef CAS.
- S. Wang, M. Wang, M. Wang, Y. Tian, X. Sun, G. Sun and X. Sun, Toxins, 2018, 10, 154 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra00483a |
| ‡ These two authors contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2019 |