 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
VUV spectroscopy of an electron irradiated benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) carbon dioxide interstellar ice analogue
carbon dioxide interstellar ice analogue
Rachel L. James *a,
Nykola C. Jones
*a,
Nykola C. Jones b,
Søren V. Hoffmann
b,
Søren V. Hoffmann b and
Anita Dawes
b and
Anita Dawes a
a
aSchool of Physical Sciences, The Open University, Walton Hall, Milton Keynes, UK. E-mail: Rachel.James1@open.ac.uk; Fax: +44(0) 1908 654192; Tel: +44(0) 1908 332012
bISA, Centre for Storage Ring Facilities, Department of Physics and Astronomy, Aarhus University, Ny Munkegade 120, DK-8000 Aarhus C, Denmark
First published on 13th February 2019
Abstract
We present the first vacuum ultraviolet spectroscopic study of an interstellar ice analogue of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 benzene (C6H6)
100 benzene (C6H6)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) carbon dioxide (CO2) mixture which has been energetically processed with 1 keV electrons. We have exploited the fact that benzene has a relatively high photoabsorption cross section in the vacuum ultraviolet region to study this dilute mixture of benzene. Before irradiation with 1 keV electrons, we observed that the benzene electronic transition bands in the C6H6
carbon dioxide (CO2) mixture which has been energetically processed with 1 keV electrons. We have exploited the fact that benzene has a relatively high photoabsorption cross section in the vacuum ultraviolet region to study this dilute mixture of benzene. Before irradiation with 1 keV electrons, we observed that the benzene electronic transition bands in the C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture exhibits a blueshift in band position towards energies observed in the gas-phase compared with that of pure, amorphous benzene and we have attributed this to a matrix isolation effect. After irradiation, a lowering in intensity of both the carbon dioxide and benzene electronic transition bands was observed, as well as the formation of the small irradiation product, carbon monoxide. A residue was obtained at 200 K which showed characteristic features of the benzene electronic transition of 1E1u ← 1A1g, but with additional structure suggesting the formation of a benzene derivative.
CO2 mixture exhibits a blueshift in band position towards energies observed in the gas-phase compared with that of pure, amorphous benzene and we have attributed this to a matrix isolation effect. After irradiation, a lowering in intensity of both the carbon dioxide and benzene electronic transition bands was observed, as well as the formation of the small irradiation product, carbon monoxide. A residue was obtained at 200 K which showed characteristic features of the benzene electronic transition of 1E1u ← 1A1g, but with additional structure suggesting the formation of a benzene derivative.
1 Introduction
Polycyclic aromatic hydrocarbons (PAHs) have attracted considerable attention in the astrochemistry community due to their ubiquitous presence in the interstellar medium (ISM). It is thought that up to 10% of the interstellar carbon budget is in the form of PAHs1 and absorption features around young stellar objects contain characteristic aromatic C–C and C–H stretches suggesting the presence of condensed phase PAHs in icy mantles.2 However, despite the interest surrounding PAHs there still has not been a single PAH identified in the ISM. The structure of most PAHs makes them difficult to observe using rotational spectroscopy and observations with infrared spectroscopy generally lack the resolution to distinguish between different PAHs.Nevertheless, benzene, a prototypical aromatic molecule and building block of PAHs, has been detected in the gas-phase in the protoplanetary nebula CRL 618![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and the Large Magellanic Cloud object SMP LMC 11.4 Recently a derivative of benzene, benzonitrile, was detected in the gas-phase in the molecular cloud TMC-1.5 Formation routes for PAHs in the diffuse ISM include the top-down processes of SiC dust grain destruction from the intense UV radiation field and sputtering from shockwaves.6,7 Alternative bottom-up processes include the formation of PAHs from benzene derivatives or radicals in the gas-8 or solid-phase which are more likely in low temperature, molecular cloud environments. Within our own Solar System, PAHs and benzene derivatives have been detected on some Saturnian moons,9 interplanetary dust grains10 and meteorites. In fact, some of the PAHs and benzene derivatives detected in meteoritic materials show deuterium enrichment indicating that the formation origin of these molecules may be from molecular cloud environments.11
3 and the Large Magellanic Cloud object SMP LMC 11.4 Recently a derivative of benzene, benzonitrile, was detected in the gas-phase in the molecular cloud TMC-1.5 Formation routes for PAHs in the diffuse ISM include the top-down processes of SiC dust grain destruction from the intense UV radiation field and sputtering from shockwaves.6,7 Alternative bottom-up processes include the formation of PAHs from benzene derivatives or radicals in the gas-8 or solid-phase which are more likely in low temperature, molecular cloud environments. Within our own Solar System, PAHs and benzene derivatives have been detected on some Saturnian moons,9 interplanetary dust grains10 and meteorites. In fact, some of the PAHs and benzene derivatives detected in meteoritic materials show deuterium enrichment indicating that the formation origin of these molecules may be from molecular cloud environments.11
Although PAHs, benzene and its derivatives have not been directly identified in the solid-phase, which is most likely due to their weak infrared absorption features overlapping with intense ice absorption bands, they are believed to be incorporated into icy mantles around interstellar dust grains or operate as nucleation sites for the condensation of other species.12 Within molecular clouds, the icy mantles of interstellar dust grains may be subjected to energetic processing (protons, secondary UV photons or secondary electrons) which could induce molecular synthesis within the ice. Several experimental studies have looked at the formation of PAHs or benzene derivatives within interstellar ice analogues.13–15 In particular, aromatic molecules with the carboxylic acid moiety (–COOH) have been studied because of their role as precursors to amino acids with non-aromatic carboxylic acids detected in the ISM in the form of formic acid (HCOOH)16 and acetic acid (CH3COOH).17 Carboxylic acids with varying degrees of complexity have also been detected in the Tagish Lake,18–20 Murchison21,22 and Orgueil22,23 carbonaceous meteorites.
In this paper we investigate the energetic processing of a dilute mixture of benzene in CO2, as an interstellar ice analogue, with 1 keV electrons using vacuum ultraviolet (VUV) spectroscopy. While mid-IR spectroscopy is the more commonly used in situ laboratory technique for investigating the structure and composition of interstellar ices, and any changes upon energetic processing, this is the first time that VUV spectroscopy has been used to study the benzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 system. With VUV spectroscopy we can take advantage of the fact that benzene has appreciatively high photoabsorption cross sections due to strong electronic π → π* transitions compared to that of other interstellar ice constituents such as H2O and CO2. For example, cross sections of benzene at wavelengths <200 nm reach 50 Mb
CO2 system. With VUV spectroscopy we can take advantage of the fact that benzene has appreciatively high photoabsorption cross sections due to strong electronic π → π* transitions compared to that of other interstellar ice constituents such as H2O and CO2. For example, cross sections of benzene at wavelengths <200 nm reach 50 Mb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 which is almost 40 times more intense compared to that of the CO2 peak cross section of around 1.3 Mb.25 In contrast, the mid-IR band strength (A-value) of the strongest vibrational transition of solid benzene, the C–C stretch (v19), is 1.4 × 10−18 cm per molecule26 which is approximately 90 times less intense compared to the C–O stretch (v3) of CO2 with a band strength of 1.3 × 10−16 cm per molecule.27 This makes VUV spectroscopy a potentially more favourable region of the electromagnetic spectrum to study very dilute mixtures of benzene in ice matrices (e.g. benzene and water). In this paper we present the results of the first experimental study using VUV spectroscopy to investigate an electron irradiated, 1
24 which is almost 40 times more intense compared to that of the CO2 peak cross section of around 1.3 Mb.25 In contrast, the mid-IR band strength (A-value) of the strongest vibrational transition of solid benzene, the C–C stretch (v19), is 1.4 × 10−18 cm per molecule26 which is approximately 90 times less intense compared to the C–O stretch (v3) of CO2 with a band strength of 1.3 × 10−16 cm per molecule.27 This makes VUV spectroscopy a potentially more favourable region of the electromagnetic spectrum to study very dilute mixtures of benzene in ice matrices (e.g. benzene and water). In this paper we present the results of the first experimental study using VUV spectroscopy to investigate an electron irradiated, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture as an interstellar ice analogue.
CO2 mixture as an interstellar ice analogue.
2 Experimental
Experiments were carried out using The Open University's Portable Astrochemistry Chamber (PAC) attached to the AU-UV beamline at the ASTRID2 Synchrotron Facility in Aarhus, Denmark.28 This set-up has been used before for the investigation of condensed phase molecules and is described in detail elsewhere by Dawes et al.24 and only a brief description is given here.The experiments were carried out at a base pressure of low 10−8 mbar. Benzene (anhydrous; Sigma-Aldrich, 99.8% purity) was degassed via three freeze–pump–thaw cycles prior to use and pre-mixed with CO2 (Sigma-Aldrich, 99.995% purity) in the gas line. The mixture was then vapour deposited onto a cold (20 K) MgF2 (Crystran) substrate held in a copper holder attached to a closed cycle helium cryostat (Sumitomo). VUV spectra were acquired in the range 120–340 nm with 0.05 to 1 nm wavelength step size depending on the width of the spectral features to be resolved. Absorbance spectra were calculated from the recorded incident and transmitted intensities as a function of wavelength (nm).
Film thickness was determined from in situ laser interferometry measurements from the use of a HeNe laser beam reflected off the substrate during deposition. Deposition rates were between 0.12 and 0.55 nm s−1 depending on the thickness of the ice being grown. The final thickness of the films were 349 nm for the unirradiated mixture and a 1.28 μm for the irradiated mixture. For the unirradiated mixture a thinner ice was prepared to avoid saturation of the electronic transition bands, to allow for accurate band assignments and observe any changes in the band profiles upon annealing. A thicker ice was prepared for irradiation to ensure a sufficient quantity of products were formed for observation/monitoring and to ensure that the ice thickness was greater than the penetration depth of electrons so that there was no substrate effect. This thicker ice was grown in stages to confirm that no thickness-dependent effects were present until saturation of the peaks was observed.
The mixture was irradiated with 1 keV electrons (Kimball Physics FRA-2X1-2/EGPS-1011A) with a current of 10 μA and the bombarded area was about 3.5 cm−2. The electron flux at the sample was approximately 1.77 × 1013 electrons per cm2 per s. After 180 min of irradiation this corresponded to a fluence of 1.91 × 1017 electrons per cm2. A penetration depth of 40 nm for 1 keV electrons was estimated using Monte Carlo simulations from the CASINO code.29
Samples were annealed gradually at a rate of 1 K min−1 by heating the sample using a Kapton heater and regulated with a temperature controller (Oxford Instruments). The samples were held at the required temperature for 2 min and then returned to base temperature (20 K) prior to measuring the spectra.
3 Results and discussion
From previous studies24,30 we know that when solid-phase benzene is diluted with amorphous solid water a blueshift in the electronic bands compared to that of pure benzene is observed. We therefore used an unirradiated control sample to characterise the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture and any effects annealing might produce, the results of which are discussed in Section 3.1. In Section 3.2 we discuss the results of irradiating the 1
CO2 mixture and any effects annealing might produce, the results of which are discussed in Section 3.1. In Section 3.2 we discuss the results of irradiating the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture with 1 keV electrons for 180 min and in Section 3.2.1 we discuss the results on the residue left at 200 K.
CO2 mixture with 1 keV electrons for 180 min and in Section 3.2.1 we discuss the results on the residue left at 200 K.
3.1 Characterisation of an unirradiated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) CO2 mixture
CO2 mixture
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) CO2 mixture at 20 K. Fig. 1 shows the VUV photoabsorption spectrum of a 1
CO2 mixture at 20 K. Fig. 1 shows the VUV photoabsorption spectrum of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture deposited at 20 K compared with spectra of a 1
CO2 mixture deposited at 20 K compared with spectra of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture deposited at 24 K,30 pure benzene deposited at 24 K and gas-phase benzene.24 Two broad bands centred around 127 and 142 nm are due to CO2 and correspond to the
H2O mixture deposited at 24 K,30 pure benzene deposited at 24 K and gas-phase benzene.24 Two broad bands centred around 127 and 142 nm are due to CO2 and correspond to the 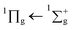 and
and 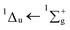 transitions respectively. The higher energy
transitions respectively. The higher energy  transition exhibits vibronic structure the positions of which are in good agreement (to within 10 meV) with a study conducted by Mason et al.25 where pure CO2 was deposited at 20 K.
transition exhibits vibronic structure the positions of which are in good agreement (to within 10 meV) with a study conducted by Mason et al.25 where pure CO2 was deposited at 20 K.
 | ||
Fig. 1 VUV photoabsorption spectra of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6 100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture deposited at 20 K (red) compared with pure, amorphous benzene deposited at 24 K (black), 1 CO2 mixture deposited at 20 K (red) compared with pure, amorphous benzene deposited at 24 K (black), 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6 100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture deposited at 24 K (green) and gas-phase benzene (blue) from ref. 24 and 30. The band assignments and positions are summarised in Table 1. The 1B2u ← 1A1g vibronic bands between 230–270 nm have been scaled x 20 and offset for clarity. The spectra are normalised to the integrated area of the overlapping 1E1u ← 1A1g and 1B1u ← 1A1g transitions of the 1 H2O mixture deposited at 24 K (green) and gas-phase benzene (blue) from ref. 24 and 30. The band assignments and positions are summarised in Table 1. The 1B2u ← 1A1g vibronic bands between 230–270 nm have been scaled x 20 and offset for clarity. The spectra are normalised to the integrated area of the overlapping 1E1u ← 1A1g and 1B1u ← 1A1g transitions of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6 100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture deposited at 20 K. CO2 mixture deposited at 20 K. | ||
Three benzene bands in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture are observed in Fig. 1 and correspond to the 1E1u ← 1A1g, 1B1u ← 1A1g and 1B2u ← 1A1g transitions. The 1E1u ← 1A1g transition is the most intense benzene band and it is blueshifted by 0.30 eV from 191.8 nm (6.47 eV) in pure benzene to 183.2 nm (6.77 eV) in the 1
CO2 mixture are observed in Fig. 1 and correspond to the 1E1u ← 1A1g, 1B1u ← 1A1g and 1B2u ← 1A1g transitions. The 1E1u ← 1A1g transition is the most intense benzene band and it is blueshifted by 0.30 eV from 191.8 nm (6.47 eV) in pure benzene to 183.2 nm (6.77 eV) in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture. The 1B1u ← 1A1g transition appears as a structured shoulder on the long wavelength side of the 1E1u ← 1A1g transition (assignments are given in Table 1) and is again blueshifted compared to that of pure benzene. Interestingly the benzene band assignments for the 1
CO2 mixture. The 1B1u ← 1A1g transition appears as a structured shoulder on the long wavelength side of the 1E1u ← 1A1g transition (assignments are given in Table 1) and is again blueshifted compared to that of pure benzene. Interestingly the benzene band assignments for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture are in good agreement with a 1
CO2 mixture are in good agreement with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture. In the previous study by Dawes et al.30 several ratios (1
H2O mixture. In the previous study by Dawes et al.30 several ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) of C6H6
100) of C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O were studied and as the benzene was diluted, successive blueshifts from pure benzene were observed and attributed to a matrix isolation effect. Also, the 1
H2O were studied and as the benzene was diluted, successive blueshifts from pure benzene were observed and attributed to a matrix isolation effect. Also, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture showed structure within in the 1E1u ← 1A1g transition band which resembled the band profile of gas-phase benzene and was not observed in the less diluted benzene mixtures. This gas-phase like band profile is also observed in the 1
H2O mixture showed structure within in the 1E1u ← 1A1g transition band which resembled the band profile of gas-phase benzene and was not observed in the less diluted benzene mixtures. This gas-phase like band profile is also observed in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture of the 1E1u ← 1A1g transition band and further supports the theory of matrix isolation as a cause of the shifts observed in the electronic band rather than a solvent effect. While the vibronic peaks of the 1B2u ← 1A1g electronic transition are not completely resolved, the data is sufficient to suggest a blue shift in position towards energies observed in the gas-phase in a similar fashion to that of the 1
CO2 mixture of the 1E1u ← 1A1g transition band and further supports the theory of matrix isolation as a cause of the shifts observed in the electronic band rather than a solvent effect. While the vibronic peaks of the 1B2u ← 1A1g electronic transition are not completely resolved, the data is sufficient to suggest a blue shift in position towards energies observed in the gas-phase in a similar fashion to that of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O mixture.
H2O mixture.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture deposited at 20 K, annealed to 80 K and then 90 K compared with pure benzene at 24 K and gas-phase benzene from ref. 24 and 30. The gas to solid energy shifts (in eV) are shown under headings ‘g–s’
CO2 mixture deposited at 20 K, annealed to 80 K and then 90 K compared with pure benzene at 24 K and gas-phase benzene from ref. 24 and 30. The gas to solid energy shifts (in eV) are shown under headings ‘g–s’
| Band assignment | C6H6 (solid, 20 K)24 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6 100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 CO2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6 100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O30 H2O30 |
C6H6 (gas)24 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| nm | eV | g–s/eV | nm | eV | g–s/eV | nm | eV | g–s/eV | nm | eV | |
| 1E1u ← 1A1g | |||||||||||
| 191.8 | 6.47 | 0.50 | 183.2 | 6.77 | 0.20 | 182.7 | 6.79 | 0.18 | 178.0 | 6.97 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| 1B1u ← 1A1g | |||||||||||
| 610 | 212.7 | 5.83 | 0.24 | 207.0 | 5.99 | 0.08 | 207.0 | 5.99 | 0.08 | 204.2 | 6.07 |
| 610110 | 208.8 | 5.94 | 0.25 | 203.4 | 6.10 | 0.09 | 203.6 | 6.09 | 0.10 | 200.3 | 6.19 |
| 610120 | 204.9 | 6.05 | 0.26 | 200.2 | 6.19 | 0.12 | 200.1 | 6.20 | 0.11 | 196.6 | 6.31 |
| 610130 | 201.2 | 6.16 | 0.26 | 196.8 | 6.30 | 0.12 | 196.5 | 6.31 | 0.11 | 193.0 | 6.42 |
| 610140 | 193.4 | 6.41 | 0.12 | 193.3 | 6.41 | 0.12 | 189.8 | 6.53 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) CO2 mixture annealed to 80, 90 and 140 K. Fig. 2 shows the VUV photoabsorption spectra of the 1
CO2 mixture annealed to 80, 90 and 140 K. Fig. 2 shows the VUV photoabsorption spectra of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture as it is annealed to 80, 90 and 140 K corresponding to the temperature at which pure CO2 is crystalline (>80 K), pure benzene begins to crystallise and pure CO2 desorbs (75–90 K), and, pure benzene desorbs (140 K). At 80 K the CO2
CO2 mixture as it is annealed to 80, 90 and 140 K corresponding to the temperature at which pure CO2 is crystalline (>80 K), pure benzene begins to crystallise and pure CO2 desorbs (75–90 K), and, pure benzene desorbs (140 K). At 80 K the CO2  and
and  transition bands are still present in the same position as that of spectra taken at 20 K, albeit with lower intensity, and by 90 K they have disappeared due to desorption of CO2. At 80 K the 1E1u ← 1A1g transition of benzene has lowered in intensity and the band profile is showing signs of the splitting characteristic of that of crystallisation of benzene. The 1B1u ← 1A1g vibronic structure appears less well defined than at 20 K and may be due to desorption of CO2 which is also occurring at 80 K. When the CO2 is fully desorbed at 90 K the 1E1u ← 1A1g has shifted from 183.4 nm to 192.8 nm with a well defined splitting in the band (190.6 nm and 194.6 nm) observed. Additionally the 1B1u ← 1A1g vibronic features have sharpened compared to that at 80 K and shifted to pure, crystalline benzene positions.24 At 140 K all the benzene has been removed from the substrate.
transition bands are still present in the same position as that of spectra taken at 20 K, albeit with lower intensity, and by 90 K they have disappeared due to desorption of CO2. At 80 K the 1E1u ← 1A1g transition of benzene has lowered in intensity and the band profile is showing signs of the splitting characteristic of that of crystallisation of benzene. The 1B1u ← 1A1g vibronic structure appears less well defined than at 20 K and may be due to desorption of CO2 which is also occurring at 80 K. When the CO2 is fully desorbed at 90 K the 1E1u ← 1A1g has shifted from 183.4 nm to 192.8 nm with a well defined splitting in the band (190.6 nm and 194.6 nm) observed. Additionally the 1B1u ← 1A1g vibronic features have sharpened compared to that at 80 K and shifted to pure, crystalline benzene positions.24 At 140 K all the benzene has been removed from the substrate.
3.2 Electron irradiation and subsequent annealing of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) CO2 mixture
CO2 mixture
After irradiating with 1 keV electrons there is a noticeable difference in the spectrum compared to that of the unirradiated spectrum as shown in Fig. 3. The irradiation product carbon monoxide (CO) is observed via the vibronic structure of the A1Π ← X1∑+ transition, the assignments are shown in Table 2 and compared with pure CO and CO formed via VUV irradiation of CO2. Davydov splitting observed in the lower energy bands (0,0–3,0) of pure CO are not observed in CO formed from irradiation of electrons, consistent with that of the CO formed from VUV photons in a study by Cruz-Diaz et al.31 Additionally the vibronic bands have blueshifted compared to that of pure CO, which is also consistent with that of Cruz-Diaz et al.31 The  transition of CO2 is also present after irradiation but at lower intensity, which might be due to a combination of dissociation from electron irradiation and sputtering. The
transition of CO2 is also present after irradiation but at lower intensity, which might be due to a combination of dissociation from electron irradiation and sputtering. The 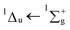 transition of CO2 is not observable due to the overlapping A1Π ← X1∑+ CO band which has a much higher cross section. The 1E1u ← 1A1g and 1B1u ← 1A1g transitions of benzene are also present in the same position as the unirradiated benzene, albeit with lower intensity due to formation of products or sputtering.
transition of CO2 is not observable due to the overlapping A1Π ← X1∑+ CO band which has a much higher cross section. The 1E1u ← 1A1g and 1B1u ← 1A1g transitions of benzene are also present in the same position as the unirradiated benzene, albeit with lower intensity due to formation of products or sputtering.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture irradiated by 1 keV electrons for 180 min and compared with pure CO taken from Mason et al.25and CO formed from VUV photons in a CO2 matrix.31 Band splitting is indicated by numbers in parenthesis
CO2 mixture irradiated by 1 keV electrons for 180 min and compared with pure CO taken from Mason et al.25and CO formed from VUV photons in a CO2 matrix.31 Band splitting is indicated by numbers in parenthesis
| Band assignment (v′, v′′) | Pure CO25 | This work | CO in CO2 matrix31 | ||
|---|---|---|---|---|---|
| eV | nm | eV | nm | eV | |
| 7, 0 | 9.16 | 134.75 | 9.20 | 134.8 | 9.20 |
| 6, 0 | 9.01 | 137.10 | 9.04 | 137.0 | 9.05 |
| 5, 0 | 8.88 | 139.60 | 8.88 | 139.4 | 8.89 |
| 4, 0 | 8.68 | 142.10 | 8.73 | 142.1 | 8.73 |
| 3, 0 | 8.48 (8.51) | 144.95 | 8.55 | 144.8 | 8.56 |
| 2, 0 | 8.29 (8.33) | 148.00 | 8.38 | 147.9 | 8.38 |
| 1, 0 | 8.09 (8.14) | 151.35 | 8.19 | 151.1 | 8.21 |
| 0, 0 | 7.90 (7.96) | 155.00 | 8.00 | 154.4 | 8.03 |
Upon annealing to 80 K there is a further decrease in intensity of the 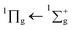 transition of CO2 as it is close to the desorption temperature of CO2. At 80 K it is expected that the CO would have desorbed, as pure CO desorbs between 28–36 K depending on the original deposition temperature (a lower deposition temperature will allow H2 to vapour deposit on the surface which can stimulate desorption at lower temperatures).32 However, the A1Π ← X1∑+ transition is still clearly observable although the intensity is lowered somewhat, with the (7,0) band no longer visible and the (6,0) band less well defined. Like the unirradiated sample discussed in Section 3.1.2 the benzene transition bands are present with a lower intensity for both electronic transitions and the vibronic structure of the 1B1u ← 1A1g transition are less well defined compared to that of spectrum taken at 20 K. At 90 K CO2 and CO have desorbed and interestingly the benzene band has increased in intensity. The 1E1u ← 1A1g transition shows signs of splitting similar to that observed in the unirradiated sample indicating crystallisation of benzene, however, this is less well defined compared to that of the unirradiated sample and may be due to the bands in the sample being saturated as the sample is thicker than the unirradiated sample. At 140 K the spectrum looks relatively flat; however, when zoomed in the 1E1u ← 1A1g transition is present, indicating that benzene is still desorbing at this temperature.
transition of CO2 as it is close to the desorption temperature of CO2. At 80 K it is expected that the CO would have desorbed, as pure CO desorbs between 28–36 K depending on the original deposition temperature (a lower deposition temperature will allow H2 to vapour deposit on the surface which can stimulate desorption at lower temperatures).32 However, the A1Π ← X1∑+ transition is still clearly observable although the intensity is lowered somewhat, with the (7,0) band no longer visible and the (6,0) band less well defined. Like the unirradiated sample discussed in Section 3.1.2 the benzene transition bands are present with a lower intensity for both electronic transitions and the vibronic structure of the 1B1u ← 1A1g transition are less well defined compared to that of spectrum taken at 20 K. At 90 K CO2 and CO have desorbed and interestingly the benzene band has increased in intensity. The 1E1u ← 1A1g transition shows signs of splitting similar to that observed in the unirradiated sample indicating crystallisation of benzene, however, this is less well defined compared to that of the unirradiated sample and may be due to the bands in the sample being saturated as the sample is thicker than the unirradiated sample. At 140 K the spectrum looks relatively flat; however, when zoomed in the 1E1u ← 1A1g transition is present, indicating that benzene is still desorbing at this temperature.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) CO2 electron irradiated mixture. By 200 K all of the benzene has desorbed and a residue is left as shown in Fig. 4 and while it displays spectral characteristics similar to that of benzene it has additional structure around 230 nm not typical of pure benzene. From our work, yet to be published,33 on toluene, which is a derivative of benzene, we know that the electronic transitions of toluene redshift compared to that of benzene. Additionally, a study by Hashimoto and Akimoto34 showed that when UV spectra of several derivatives of benzene (toluene, p-xylene, mesitylene and durene) in oxygen matrices were taken the absorption maxima of the absorption bands shift from 238 nm for benzene to 266 nm for durene (the most substituted ring). This may suggest that the additional feature at 230 nm is due to the addition of a functional group on the benzene ring, in this case a carboxyl group. Furthermore, the mid-IR results obtained in the study by McMurtry et al.15 at 180 K show evidence of benzoic acid, isophthalic acid and terephthalic acid present, which also leads us to believe that our residue at 200 K contains benzene derivatives. However, further studies on benzene derivatives in the VUV are still required for more definitive identification of products as such data, to our knowledge, is not available in the literature.
CO2 electron irradiated mixture. By 200 K all of the benzene has desorbed and a residue is left as shown in Fig. 4 and while it displays spectral characteristics similar to that of benzene it has additional structure around 230 nm not typical of pure benzene. From our work, yet to be published,33 on toluene, which is a derivative of benzene, we know that the electronic transitions of toluene redshift compared to that of benzene. Additionally, a study by Hashimoto and Akimoto34 showed that when UV spectra of several derivatives of benzene (toluene, p-xylene, mesitylene and durene) in oxygen matrices were taken the absorption maxima of the absorption bands shift from 238 nm for benzene to 266 nm for durene (the most substituted ring). This may suggest that the additional feature at 230 nm is due to the addition of a functional group on the benzene ring, in this case a carboxyl group. Furthermore, the mid-IR results obtained in the study by McMurtry et al.15 at 180 K show evidence of benzoic acid, isophthalic acid and terephthalic acid present, which also leads us to believe that our residue at 200 K contains benzene derivatives. However, further studies on benzene derivatives in the VUV are still required for more definitive identification of products as such data, to our knowledge, is not available in the literature.
4 Conclusion
We have presented the results of the first vacuum ultraviolet spectroscopic study of the interstellar ice analogue of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 C6H6
100 C6H6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2 mixture. Benzene is a prototypical, aromatic molecule believed to be present in the icy mantles of interstellar dust where simple molecules such as H2O, CO, CO2, CH4 and NH3 are known to reside. These icy mantles will be subjected to energetic processing which can alter the composition of the icy mantles and possibly form benzene derivatives which may later be released into the gas-phase through thermal or photo-induced desorption. Benzene has a relatively high photoabsorption cross section in the vacuum ultraviolet part of the electromagnetic spectrum making it an attractive spectroscopic technique to use for the study of interstellar ice analogues containing dilute mixtures of benzene. Characterisation of the interstellar ice analogue before irradiation suggests that when benzene is diluted into CO2 a matrix-isolation effect takes place. This is evidenced through the shift in the 1E1u ← 1A1g and 1B1u ← 1A1g electronic transition towards gas-phase values of benzene compared to that of pure, solid benzene and the change in band profile to resemble a more gas-like character. Benzene derivatives are predicted to form after irradiation of the interstellar ice analogue15 and the residue at 200 K observed in this study shows that a benzene-like structure has formed. However, definitive assignment begs the need for further VUV spectroscopic work of condensed molecular films of potential irradiation products formed as such data has not yet been published.
CO2 mixture. Benzene is a prototypical, aromatic molecule believed to be present in the icy mantles of interstellar dust where simple molecules such as H2O, CO, CO2, CH4 and NH3 are known to reside. These icy mantles will be subjected to energetic processing which can alter the composition of the icy mantles and possibly form benzene derivatives which may later be released into the gas-phase through thermal or photo-induced desorption. Benzene has a relatively high photoabsorption cross section in the vacuum ultraviolet part of the electromagnetic spectrum making it an attractive spectroscopic technique to use for the study of interstellar ice analogues containing dilute mixtures of benzene. Characterisation of the interstellar ice analogue before irradiation suggests that when benzene is diluted into CO2 a matrix-isolation effect takes place. This is evidenced through the shift in the 1E1u ← 1A1g and 1B1u ← 1A1g electronic transition towards gas-phase values of benzene compared to that of pure, solid benzene and the change in band profile to resemble a more gas-like character. Benzene derivatives are predicted to form after irradiation of the interstellar ice analogue15 and the residue at 200 K observed in this study shows that a benzene-like structure has formed. However, definitive assignment begs the need for further VUV spectroscopic work of condensed molecular films of potential irradiation products formed as such data has not yet been published.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research leading to this result has been supported by the project CALIPSO plus under the Grant Agreement 730872 from the EU Framework Programme for Research and Innovation HORIZON 2020. RLJ acknowledges the STFC for her PhD Studentship under grant no. ST/N50421X/1. AD acknowledges the Leverhulme Trust for her Fellowship under grant no. ECF/2016-842. RLJ and AD acknowledge the Open University for financial support. We gratefully acknowledge Prof. Nigel J. Mason for his contribution to resources and transportation of the Portable Astrochemistry Chamber. All data is provided in full in the Results and discussion section of this paper.Notes and references
- J. Chiar, A. G. G. M. Tielens, A. Adamson and A. Ricca, Astrophys. J., 2013, 770, 78 CrossRef.
- J. V. Keane, A. G. G. M. Tielens, A. C. A. Boogert, W. A. Schutte and D. C. B. Whittet, Astron. Astrophys., 2001, 376, 254–270 CrossRef CAS.
- J. Cernicharo, A. M. Heras, A. G. G. M. Tielens, J. R. Pardo, F. Herpin, M. Guélin and L. B. F. M. Waters, Astrophys. J., 2001, 546, L123–L126 CrossRef CAS.
- S. E. Malek, J. Cami and J. Bernard-Salas, Astrophys. J., 2011, 744, 16 CrossRef.
- B. A. McGuire, A. M. Burkhardt, S. Kalenskii, C. N. Shingledecker, A. J. Remijan, E. Herbst and M. C. McCarthy, Science, 2018, 359, 202–205 CrossRef CAS PubMed.
- P. Merino, M. Švec, J. Martinez, P. Jelinek, P. Lacovig, M. Dalmiglio, S. Lizzit, P. Soukiassian, J. Cernicharo and J. Martin-Gago, Nat. Commun., 2014, 5, 3054 CrossRef CAS PubMed.
- T. Q. Zhao, Q. Li, B. S. Liu, R. K. E. Gover, P. J. Sarre and A. S.-C. Cheung, Phys. Chem. Chem. Phys., 2016, 18, 3489–3496 RSC.
- D. S. N. Parker, F. Zhang, Y. S. Kim, R. I. Kaiser, A. Lander, V. V. Kislov, A. M. Mebel and A. G. G. M. Tielens, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 53–58 CrossRef CAS PubMed.
- D. P. Cruikshank, C. M. D. Ore, R. N. Clark and Y. J. Pendleton, Icarus, 2014, 233, 306–315 CrossRef CAS.
- S. J. Clemett, C. R. Maechling, R. N. Zare, P. D. Swan and R. M. Walker, Science, 1993, 262, 721–724 CrossRef CAS PubMed.
- S. A. Sandford, Planet. Space Sci., 2002, 50, 1145–1154 CrossRef CAS.
- V. C. Geers, E. F. van Dishoeck, K. M. Pontoppidan, F. Lahuis, A. Crapsi, C. P. Dullemond and G. A. Blake, Astron. Astrophys., 2009, 495, 837–846 CrossRef CAS.
- K. E. Smith, M. P. Callahan, P. A. Gerakines, J. P. Dworkin and C. H. House, Geochim. Cosmochim. Acta, 2014, 136, 1–12 CrossRef CAS.
- C. K. Materese, M. Nuevo and S. A. Sandford, Astrophys. J., 2015, 800, 116 CrossRef.
- B. M. McMurtry, S. E. J. Saito, A. M. Turner, H. K. Chakravarty and R. I. Kaiser, Astrophys. J., 2016, 831, 174 CrossRef.
- B. Zuckerman, J. Ball and C. A. Gottlieb, Astrophs. J., 1971, 163, L41–L45 CrossRef CAS.
- D. M. Mehringer, L. E. Snyder, Y. Miao and F. J. Lovas, Astrophs. J., 1997, 480, L71–L74 CrossRef CAS.
- S. Pizzarello, Y. Huang, L. Becker, R. J. Poreda, R. A. Nieman and G. C. M. Williams, Science, 2001, 293, 2236–2239 CrossRef CAS PubMed.
- S. Pizzarello and Y. Huang, Meteorit. Planet. Sci., 2002, 37, 687–696 CrossRef CAS.
- R. W. Hilts, C. D. K. Herd, D. N. Simkus and G. F. Slater, Meteorit. Planet. Sci., 2014, 49, 526–549 CrossRef CAS.
- S. Pizzarello and Y. Huang, Geochim. Cosmochim. Acta, 2005, 69, 599–605 CrossRef CAS.
- Z. Martins, J. S. Watson, M. A. Sephton, O. Botta, P. Ehrenfreund and I. Gilmour, Meteorit. Planet. Sci., 2006, 41, 1073–1080 CrossRef CAS.
- L. Remusat, S. Derenne and F. Robert, Geochim. Cosmochim. Acta, 2005, 69, 4377–4386 CrossRef CAS.
- A. Dawes, N. Pascual, S. V. Hoffmann, N. C. Jones and N. J. Mason, Phys. Chem. Chem. Phys., 2017, 19, 27544–27555 RSC.
- N. J. Mason, A. Dawes, P. D. Holtom, R. J. Mukerji, M. P. Davis, B. Sivaraman, R. I. Kaiser, S. V. Hoffmann and D. A. Shaw, Faraday Discuss., 2006, 133, 311–329 RSC.
- L. Zhou, W. Zheng, R. I. Kaiser, A. Landera, A. M. Mebel, M.-C. Liang and Y. L. Yung, Astrophs. J., 2010, 718, 1243–1251 CrossRef CAS.
- M. Bouilloud, N. Fray, Y. Bénilan, H. Cottin, M.-C. Gazeau and A. Jolly, Mon. Not. R. Aston. Soc., 2015, 451, 2145–2160 CrossRef CAS.
- M. H. Palmer, T. Ridley, S. V. Hoffmann, N. C. Jones, M. Coreno, M. de Simone, C. Grazioli, M. Biczysko, A. Baiardi and P. Limão-Vieira, J. Chem. Phys., 2015, 142, 134302 CrossRef PubMed.
- D. Drouin, A. R. Couture, D. Joly, X. Tastet, V. Aimez and R. Gauvin, Scanning, 2007, 29, 92–101 CrossRef CAS PubMed.
- A. Dawes, N. Pascual, N. J. Mason, S. Gärtner, S. V. Hoffmann and N. C. Jones, Phys. Chem. Chem. Phys., 2018, 20, 15273–15287 RSC.
- G. Cruz-Diaz, G. M. M. Caro, T.-J. Chen and T.-S. Yih, Astron. Astrophys., 2014, 562, A120 CrossRef.
- G. M. M. Caro, A. Jiménez-Escobar, J. Á. Martín-Gago, C. Rogero, C. Atienza, S. Puertas, J. M. Sobrado and J. Torres-Redondo, Astron. Astrophys., 2010, 522, A108 CrossRef.
- R. L. James, J. Dezaley, N. C. Jones, S. V. Hoffmann and A. Dawes, unpublished work.
- S. Hashimoto and H. Akimoto, J. Phys. Chem., 1989, 93, 571–577 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |



