Open Access Article
Open Access ArticleHigh-contrast mechanochromic benzothiadiazole derivatives based on a triphenylamine or a carbazole unit†
Yuan-yuan Zhu,
Hong-ying Xia,
Li-feng Yao,
Dan-ping Huang,
Jun-yan Song,
Hai-feng He *,
Liang Shen* and
Feng Zhao*
*,
Liang Shen* and
Feng Zhao*
School of Chemistry and Chemical Engineering, Jiangxi Science and Technology Normal University, Nanchang 330013, PR China. E-mail: hehf0427@jxstnu.com.cn; liangshen@jxstnu.com.cn; zhf19752003@163.com
First published on 4th March 2019
Abstract
Four triphenylamine or carbazole-based benzothiadiazole fluorescent molecules have been successfully synthesized and characterized. Interestingly, the donor–acceptor (D–A) type luminogens 1, 2, 3 and 4 showed different solid-state fluorescence. Furthermore, the four compounds exhibited reversible high-contrast mechanochromism characteristics.
Stimuli-responsive materials receive much attention currently due to their academic importance and potential applications in optoelectronic devices and fluorescent sensors,1–7 especially organic smart materials whose solid-state luminescence can be tuned by external stimuli.8–13 Mechanochromic fluorescence materials, as a class of smart materials, are also receiving increasing attention.14–20 To date, a number of mechanofluorochromic organic molecules have been reported.21–24 In contrast, examples of high-contrast mechanochromic luminescence materials are still inadequate. Indeed, many traditional organic materials are aggregation caused quenching (ACQ)-active, and these materials are weakly emissive or nonluminescent in the solid state due to the presence of strong intermolecular electronic interactions in their aggregated state, which promotes the formation of exciplexes and excimers.25–27 Obviously, the ACQ effect is unbeneficial to gain high-contrast mechanofluorochromic materials.28–30 It is no doubt that mechanochromic molecules with a bright solid-state fluorescence emission are easier to achieve high-contrast mechanofluorochromic phenomenon. Therefore, the corresponding highly emissive smart luminophors have attracted considerable attention.31,32
In general, the emission characteristics of mechanochromic luminescence materials depend strongly on their molecular structures and intermolecular interactions.33–35 Therefore, it is an effective method for the realization of mechanofluorochromic materials to change the morphological structures by means of external mechanical stimulus.36
Benzothiadiazole-based derivatives are regarded as attractive candidates for the organic π-conjugated fluorescent dyes owing to their strongly electron-withdrawing feature.37–41 Meanwhile, the benzothiadiazole unit is also advantageous to the construction of donor–acceptor (D–A) type molecules, which have emerged as a significative class of optical materials finding potential value in some areas such as in fluorescent sensors and displays.42,43 Motivated by the fact that triphenylamine or carbazole fluorogen has been broadly applied in the field of emissive materials,44,45 we attempted to link one triphenylamine or carbazole group to one benzothiadiazole moiety. As a result, we have obtained four D–A type fluorescent molecules on the basis of a combination of the electron-donating triphenylamine or carbazole unit and the electron-accepting benzothiadiazole unit (Fig. 1). Compound 1, 2, 3 or 4 contains rotatable aromatic rings, and thus their molecular structures are nonplanar, which is advantageous to the radiative decay in the aggregated state. Indeed, compounds 1, 2, 3 and 4 showed bright solid-state fluorescence with different emission colors. In addition, we found that the D–A type luminogens 1, 2, 3 and 4 applying the triphenylamine or carbazole moiety as an electron donor and the benzothiadiazole moiety as an electron acceptor exhibited various mechanochromic fluorescence characteristics with good reversibility. Furthermore, luminogen 1 showed mechanofluorochromic behavior involving color change from orange to rare red.
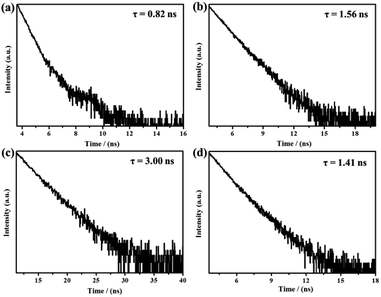
To investigate the solid-state fluorescence behaviors of compounds 1, 2, 3 and 4 in detail, the corresponding solid-state emission spectra were studied initially. As shown in Fig. 2, the fluorescence spectrum of triphenylamine-containing benzothiadiazole derivative 1 exhibited one emission band with the λmax at 575 nm, and the fluorescent molecule exhibited strong orange luminescence with the fluorescence quantum yield (Φ) of 7.13%, and triphenylamine-containing compound 2 exhibited strong yellow luminescence (Φ = 7.43%) with the λmax at 567 nm. In contrast, the emission spectrum of carbazole-based benzothiadiazole derivative 3 exhibited one emission band with the λmax at 504 nm, and the luminogen exhibited bright green fluorescence with the quantum yield of 16.10%, and carbazole-based compound 4 also exhibited bright green fluorescence (Φ = 16.53%) with the λmax at 498 nm. Therefore, the photoluminescence (PL) behaviors of compounds 1, 2, 3 and 4 could be adjusted via introducing various fluorogens containing triphenylamine and carbazole. In addition, the fluorescence lifetimes of 1, 2, 3 and 4 were also measured. As shown in Fig. 3, the average lifetime of fluorescent molecule 1 was 0.82 ns, the average lifetime of 2 was 1.56 ns, the average lifetime of 3 was 3.00 ns, and the average lifetime of 4 was 1.41 ns.
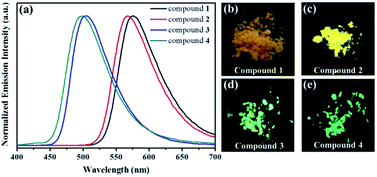 | ||
| Fig. 2 Solid-state emissive spectra of the compounds 1, 2, 3 and 4, and the related fluorescence images under 365 nm UV light. | ||
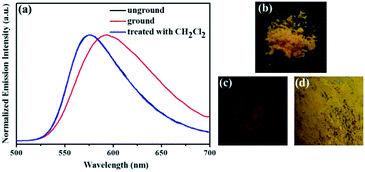
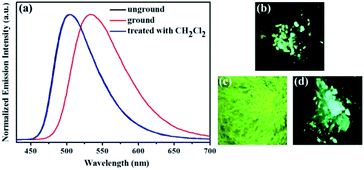
Subsequently, the mechanochromic fluorescence characteristics of compounds 1, 2, 3 and 4 were investigated. As shown in Fig. 4, the solid sample of luminogen 1 showed a bright orange fluorescence. Interestingly, the orange luminescence was changed to the red luminescence with the λmax at 593 nm upon treating with mechanical force stimulus. Furthermore, the initial orange emission could be restored after treatment of the ground compound 1 with fuming dichloromethane for 1 min. Therefore, 1 showed reversible high-contrast mechanofluorochromic behavior with color change from orange to red, which is a relatively rare color conversion among all mechanochromic fluorescence phenomena.
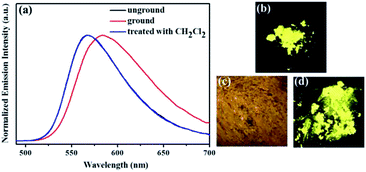
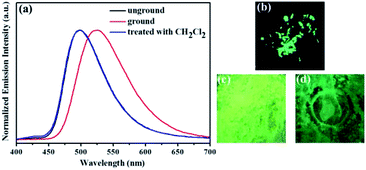
Similarly, as shown in Fig. 5, compound 2 also showed reversible high-contrast mechanochromic fluorescence behavior. Moreover, the reversible mechanochromic fluorescence of 1 or 2 could be repeated four times between the orange or yellow and red or orange emissions without obvious changes by alternating grinding and dichloromethane treatments. To date, this mechanochromic luminescence conversion of some reported mechanochromism compounds with superior performance is also repeated three or four times,46–48 and thus the reversibility of the mechanochromic fluorescence effect of 1 or 2 is good (Fig. 6). On the other hand, as shown in Fig. 7, when sample 3 were ground in an agate mortar with a pestle, the green emission was changed to the yellow-green fluorescence with the λmax at 533 nm. Moreover, the yellow-green emission could also revert to the original green emission after a 1 min treatment of the ground powder with fuming dichloromethane vapor. Furthermore, as shown in Fig. 8, compound 4 also showed similar mechanochromic fluorescence behavior.
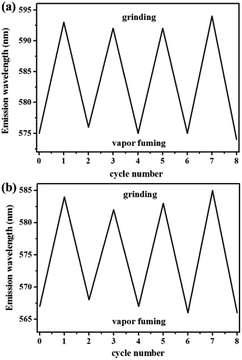 | ||
| Fig. 6 (a) Repetitive experiment of mechanofluorochromic effect for compound 1. (b) Repetitive experiment of mechanofluorochromic effect for compound 2. | ||
As can be seen in Fig. 9, the reversibility of the mechanofluorochromic behavior of compound 3 or 4 is also excellent. Next, the powder X-ray diffraction (XRD) patterns were studied in order to ensure the morphological characteristics. As can be seen in Fig. 10, the XRD patterns of compound 1 or 2 exhibited a number of sharp reflection peaks, suggesting that the unground compound 1 or 2 was crystalline in nature. However, the ground powder sample became amorphous, with a lack of sharp diffraction peaks. Therefore, the change in fluorescence of compound 1 or 2 could be attributed to the conversion from a crystalline state to an amorphous state. On the other hand, when the ground sample was exposed to dichloromethane vapor for 1 min, the sharp and intense peaks reappeared, indicative of the recovery of the crystalline nature. As presented in Fig. 11, the structural transition of the powder sample of compound 3 or 4 was similar to that of 1 or 2. Based on the above mentioned analysis, the powder XRD results demonstrated that the interesting mechanochromic fluorescence characteristics of compounds 1, 2, 3 and 4 were ascribed to the switchable morphology transition between the crystalline state and the amorphous state.
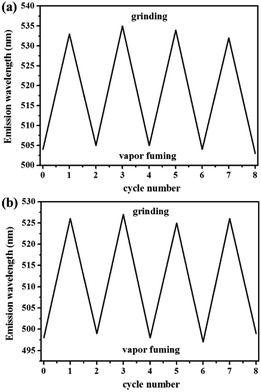 | ||
| Fig. 9 (a) Repetitive experiment of mechanofluorochromic effect for compound 3. (b) Repetitive experiment of mechanofluorochromic effect for compound 4. | ||
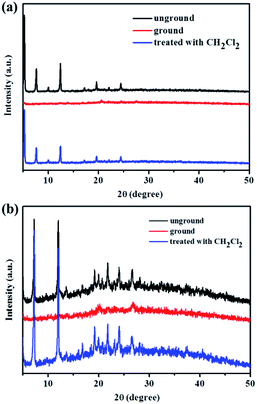 | ||
| Fig. 10 (a) Powder XRD patterns of compound 1 in different solid states. (b) Powder XRD patterns of compound 2 in different solid states. | ||
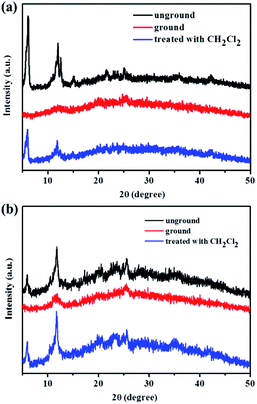 | ||
| Fig. 11 (a) Powder XRD patterns of compound 3 in different solid states. (b) Powder XRD patterns of compound 4 in different solid states. | ||
In conclusion, in this work, four triphenylamine or carbazole-based benzothiadiazole fluorescent molecules were successfully synthesized. The compounds 1, 2, 3 and 4 belonged to the highly solid-state emissive donor–acceptor (D–A) type luminescent molecules. It is noteworthy that the four D–A type luminogens exhibited high-contrast mechanofluorochromic characteristics. Furthermore, the reversibility of their mechanochromic phenomena is good. The results of powder XRD experiments confirmed that this switchable morphology transformation is responsible for the reversible mechanochromic fluorescence characteristics of 1, 2, 3 and 4. This work is valuable for designing high-contrast mechanochromic materials involving red light-emitting feature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (21867011, 51563011) and the Project of the Science Fund of Jiangxi Education Office (GJJ170672).Notes and references
- C. Dou, L. Han, S. Zhao, H. Zhang and Y. Wang, J. Phys. Chem. Lett., 2011, 2, 666–670 CrossRef CAS.
- N. V. Lakshmi, D. Mandal, S. Ghosh and E. Prasad, Chem.–Eur. J., 2014, 20, 9002–9011 CAS.
- B. Xu, Z. Chi, X. Zhang, H. Li, C. Chen, S. Liu, Y. Zhang and J. Xu, Chem. Commun., 2011, 47, 11080–11082 RSC.
- K.-P. Wang, Y. Chen and Y. Liu, Chem. Commun., 2015, 51, 1647–1649 CAS.
- X. Zhu, R. Liu, Y. Li, H. Huang, Q. Wang, D. Wang, X. Zhu, S. Liu and H. Zhu, Chem. Commun., 2014, 50, 12951–12954 CAS.
- X. Cui, J. Zhao, Y. Zhou, J. Ma and Y. Zhao, J. Am. Chem. Soc., 2014, 136, 9256–9259 CAS.
- Z. Chen, G. Liu, S. Pu and S. H. Liu, Dyes Pigm., 2017, 143, 409–415 CrossRef CAS.
- Z. Chen, D. Wu, X. Han, Y. Nie, J. Yin, G.-A. Yu and S. H. Liu, RSC Adv., 2014, 4, 63985–63988 RSC.
- Y. Dong, B. Xu, J. Zhang, X. Tan, L. Wang, J. Chen, H. Lv, S. Wen, B. Li, L. Ye, B. Zou and W. Tian, Angew. Chem., Int. Ed., 2012, 51, 10782–10785 CAS.
- W. Chen, S. Wang, G. Yang, S. Chen, K. Ye, Z. Hu, Z. Zhang and Y. Wang, J. Phys. Chem. C, 2016, 120, 587–597 CrossRef CAS.
- J. Wei, B. Liang, X. Cheng, Z. Zhang, H. Zhang and Y. Wang, RSC Adv., 2015, 5, 71903–71910 RSC.
- F. Zhao, C. Fan, Z. Chen, G. Liu and S. Pu, RSC Adv., 2017, 7, 43845–43848 RSC.
- J. Zhang, Z. Chen, L. Yang, F. Hu, G.-A. Yu, J. Yin and S.-H. Liu, Dyes Pigm., 2017, 136, 168–174 CrossRef CAS.
- Z. Chi, X. Zhang, B. Xu, X. Zhou, C. Ma, Y. Zhang, S. Liu and J. Xu, Chem. Soc. Rev., 2012, 41, 3878–3896 RSC.
- P. Xue, J. Ding, P. Wang and R. Lu, J. Mater. Chem. C, 2016, 4, 6688–6706 RSC.
- R. Tan, S. Wang, H. Lan and S. Xiao, Curr. Org. Chem., 2017, 21, 236–248 CrossRef CAS.
- H. Ito, T. Saito, N. Oshima, N. Kitamura, S. Ishizaka, Y. Hinatsu, M. Wakeshima, M. Kato, K. Tsuge and M. Sawamura, J. Am. Chem. Soc., 2008, 130, 10044–10045 CrossRef CAS PubMed.
- Z. Chen, J. Zhang, M. Song, J. Yin, G.-A. Yu and S. H. Liu, Chem. Commun., 2015, 51, 326–329 RSC.
- Z. Chen, J. Liang, Y. Nie, X. Xu, G.-A. Yu, J. Yin and S. H. Liu, Dalton Trans., 2015, 44, 17473–17477 RSC.
- T. Hu, B. Yao, X. Chen, W. Li, Z. Song, A. Qin, J. Z. Sun and B. Z. Tang, Chem. Commun., 2015, 51, 8849–8852 RSC.
- S. Xue, X. Qiu, Q. Sun and W. Yang, J. Mater. Chem. C, 2016, 4, 1568–1578 RSC.
- F. Zhao, Z. Chen, G. Liu, C. Fan and S. Pu, Tetrahedron Lett., 2018, 59, 836–840 CrossRef CAS.
- B. Xu, J. He, Y. Mu, Q. Zhu, S. Wu, Y. Wang, Y. Zhang, C. Jin, C. Lo, Z. Chi, A. Lien, S. Liu and J. Xu, Chem. Sci., 2015, 6, 3236–3241 RSC.
- M. Mitani, S. Ogata, S. Yamane, M. Yoshio, M. Hasegawa and T. Kato, J. Mater. Chem. C, 2016, 4, 2752–2760 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361–5388 RSC.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
- Z. Chen, G. Liu, S. Pu and S. H. Liu, Dyes Pigm., 2018, 152, 54–59 CrossRef CAS.
- Z. Chen, G. Liu, S. Pu and S. H. Liu, Dyes Pigm., 2018, 159, 499–505 CrossRef CAS.
- Y. Qi, Y. Wang, Y. Yu, Z. Liu, Y. Zhang, G. Du and Y. Qi, RSC Adv., 2016, 6, 33755–33762 RSC.
- G.-G. Shan, H.-B. Li, H.-T. Cao, D.-X. Zhu, P. Li, Z.-M. Su and Y. Liao, Chem. Commun., 2012, 48, 2000–2002 RSC.
- Z. Chen, D. Wu, X. Han, J. Liang, J. Yin, G.-A. Yu and S. H. Liu, Chem. Commun., 2014, 50, 11033–11035 RSC.
- J. Ni, Y.-G. Wang, H.-H. Wang, L. Xu, Y.-Q. Zhao, Y.-Z. Pan and J.-J. Zhang, Dalton Trans., 2014, 43, 352–360 RSC.
- J. Ni, X. Zhang, Y.-H. Wu, L.-Y. Zhang and Z.-N. Chen, Chem.–Eur. J., 2011, 17, 1171–1183 CrossRef CAS PubMed.
- Z. Chen, Z. Li, F. Hu, G.-A. Yu, J. Yin and S. H. Liu, Dyes Pigm., 2016, 125, 169–178 CAS.
- F. Ciardelli, G. Ruggeri and A. Pucci, Chem. Soc. Rev., 2013, 42, 857–870 RSC.
- S. Ito, T. Taguchi, T. Yamada, T. Ubukata, Y. Yamaguchi and M. Asami, RSC Adv., 2017, 7, 16953–16962 CAS.
- S. Ito, T. Yamada, T. Taguchi, Y. Yamaguchi and M. Asami, Chem.–Asian J., 2016, 11, 1963–1970 CAS.
- X. Song, H. Yu, X. Yan, Y. Zhang, Y. Miao, K. Ye and Y. Wang, Dalton Trans., 2018, 47, 6146–6155 CAS.
- C. Dou, D. Chen, J. Iqbal, Y. Yuan, H. Zhang and Y. Wang, Langmuir, 2011, 27, 6323–6329 CrossRef CAS PubMed.
- K. C. Naeem, K. Neenu and V. C. Nair, ACS Omega, 2017, 2, 9118–9126 CrossRef CAS.
- D. Wu, B. Fang, M. Zhang, W. Du, J. Zhang, X. Tian, Q. Zhang, H. Zhou, J. Wu and Y. Tian, Dyes Pigm., 2018, 159, 142–150 CrossRef CAS.
- J. Sun, X. Lv, P. Wang, Y. Zhang, Y. Dai, Q. Wu, M. Ouyang and C. Zhang, J. Mater. Chem. C, 2014, 2, 5365–5371 RSC.
- B. Wex and B. R. Kaafarani, J. Mater. Chem. C, 2017, 5, 8622–8653 CAS.
- X. Gan, Y. Wang, X. Ge, W. Li, X. Zhang, W. Zhu, H. Zhou, J. Wu and Y. Tian, Dyes Pigm., 2015, 120, 65–73 CrossRef CAS.
- J. Liang, Z. Chen, L. Xu, J. Wang, J. Yin, G.-A. Yu, Z.-N. Chen and S. H. Liu, J. Mater. Chem. C, 2014, 2, 2243–2250 CAS.
- F. Zhao, Z. Chen, C. Fan, G. Liu and S. Pu, Dyes Pigm., 2019, 164, 390–397 CAS.
- Y. Han, H.-T. Cao, H.-Z. Sun, G.-G. Shan, Y. Wu, Z.-M. Su and Y. Liao, J. Mater. Chem. C, 2015, 3, 2341–2349 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, and copies of NMR spectra and mass spectra. See DOI: 10.1039/c9ra00141g |
| This journal is © The Royal Society of Chemistry 2019 |