Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Retracted Article: Structural characterization of peptides from Locusta migratoria manilensis (Meyen, 1835) and anti-aging effect in Caenorhabditis elegans
Hui Cao†
a,
Qiang Luo†a,
Huailing Wangab,
Zhigang Liu ab,
Guoqiang Li*c and
Jie Liu
ab,
Guoqiang Li*c and
Jie Liu *ab
*ab
aThe Research Center of Allergy & Immunology, Shenzhen University School of Medicine, Shenzhen, 518060, PR China. E-mail: jnuoligopeptide@sina.com; Fax: +86 0755-86671907; Tel: +86 0755-86671907
bDepartment of Allergy, The Third Affiliated Hospital of Shenzhen University, Shenzhen, 518020, PR China
cCollege of Food Science and Engineering, Foshan University, Foshan 528000, PR China. E-mail: lzgszuniversity@sina.com; Fax: +86 0755-86671903; Tel: +86 0755-86671903
First published on 21st March 2019
Abstract
Locusts are a kind of agricultural pest rich in protein and widely eaten by people, yet, the nutritional and antioxidant activities of locust peptide have never been explored. In the current study, the locust peptides (LPs) were isolated from the Locusta migratoria manilensis (Meyen, 1835) and the anti-aging effects on Caenorhabditis elegans (C. elegans) were evaluated. The mean lifespan of C. elegans was significantly extended using LPs with a concentration of 1.0 mg mL−1. Out of the 23 peptides, LP-1, a pentapeptide with The-Phe-Lys-His-Gly sequence, with a concentration of 2.5 mg mL−1 significantly extended the lifespan of the worms by 23.5%. Additionally, LP-1 was observed to be a strong free radical-scavenger which can improve the survival of the C. elegans under oxidative stress, thermal stress and UV radiation. Furthermore, the LP-1 can up-regulate the expression of the transcription factor DAF-16 and jnk-1, suggesting that LP-1 may promote the C. elegans lifespan and stress resistance through a JNK-1-DAF-16 pathway. This study will be significant for the development of locusts and improvement of functional insect peptide production.
1 Introduction
Aging is an inevitable biological process, which can be defined as a progressive decline in physiological capacities accompanied by an increased vulnerability to environmental challenges and aging-related diseases.1 Reactive oxygen species (ROS) are generated during mitochondrial oxidative metabolism as well as in cellular response to destructive stimuli.2 Oxidative stress refers to the imbalance due to excess ROS or oxidants over the capability of the cell to mount an effective antioxidant response.3 Although the exact biological and cellular mechanisms of the aging process are not well understood, a large body of evidence indicates that oxidative stress plays a key role in aging and aging-related diseases. Production of free radicals is an unavoidable process in the course of cellular metabolism.4 There is evidence that oxidative stress, exerting downstream effects such as lipid peroxidation, DNA damage and mitochondrial impairment, may play a fatal role in senility.5 Indeed, there are many factors to which the organism is exposed like smoke, microorganisms, or UV radiation, that can induce anti stress responses, leading to the generation of ROS. Some agents such as paraquat, UV-radiation and lipopolysaccharide are usually used to induce oxidative damage and premature aging.6,7 In recent years studies have shown that oxidative stress and aging can be counteracted and delayed by protective antioxidant compounds: vitamins, polyphenols and amino acids. As such, the scavenging of detrimental free radicals by antioxidants may alleviate oxidative damage of cells, therefore promoting longevity and preventing aging-related disorders.8,9The Caenorhabditis elegans (C. elegans) is a popular model in aging research because these nematodes decline behaviorally and physiologically with age in a manner similar to that of higher mammals, including humans. At the gene and protein level, C. elegans share up to 80% homology with human genes and a conserved protein network involved in aging. Besides being a biologically relevant aging model, these nematodes are easy to culture and have a short life cycle, allowing for rapid replication of experimental treatments.10 Moreover, the evolutionary conserved insulin/insulin-like growth factor (IGF-1) signaling (IIS) pathway is one of the well-understood longevity-regulating pathways in animals. In this pathway, the Forkhead box O (FOXO) transcription factors are key players, which are under control of IGF-1 receptors.11 In C. elegans, the DAF-2 activation would recruit and activate the phosphoinositide 3-kinase/protein kinase B signaling cascade and results in the phosphorylation of DAF-16. Being phosphorylated, DAF-16 is prevented from its nuclear translocation and the induction of gene expression of downstream longevity-promoting genes.12,13 Besides the IIS pathway, the Jun-N-terminal kinase-1 (JNK-1) signaling pathway is another important upstream pathway that can regulate the DAF-16 nuclear localization. Once in the nucleus, several proteins cooperate with DAF-16 to modulate downstream gene transcription. Among these co-regulators, the heat-shock-factor-1 (HSF-1) acts together with DAF-16 to activate expression of specific genes, such as genes encoding small heat shock proteins, which enhance stress resistance and longevity in C. elegans.14 Additionally, the p38 mitogen-activated protein kinase (PMK-1), a parallel pathway to DAF-16, also contributes to longevity of C. elegans.15
There are more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 species of locusts have been identified and are widely distributed in tropical and subtropical regions. And locusts are notorious as pests harming crops.16 Although locusts are regarded as a mortal malady of the agriculture. The locust are high nutritional insects which are rich in protein. According to the records of traditional Chinese medicine, the locusts can relieve cough and asthma, and enhance immunity. It is also widely used to treat asthma, sore throat and other diseases.17 Studies revealed that nutrition components, such as protein, amino acids, mineral elements and vitamins are abundant in locusts. Furthermore, the locusts are a good source of edible protein, which can provide abundant protein and meet the nutritional needs of essential amino acids. Studies reported that the locust is also rich in functional proteins, such as antifreeze protein, storage protein, antimicrobial and antioxidant peptides.18,19
000 species of locusts have been identified and are widely distributed in tropical and subtropical regions. And locusts are notorious as pests harming crops.16 Although locusts are regarded as a mortal malady of the agriculture. The locust are high nutritional insects which are rich in protein. According to the records of traditional Chinese medicine, the locusts can relieve cough and asthma, and enhance immunity. It is also widely used to treat asthma, sore throat and other diseases.17 Studies revealed that nutrition components, such as protein, amino acids, mineral elements and vitamins are abundant in locusts. Furthermore, the locusts are a good source of edible protein, which can provide abundant protein and meet the nutritional needs of essential amino acids. Studies reported that the locust is also rich in functional proteins, such as antifreeze protein, storage protein, antimicrobial and antioxidant peptides.18,19
Although the antioxidant activities of peptides have been extensively studied, up to now there have been no reports concerning the protein components and identification of locust. Consequently, identifying the bioactive peptides in locust and exploring their effects on human health will be of great value and significance. To illustrate the health benefits of the locust peptides and expand the use in the functional protein production. In this study, a serious of antioxidant peptides were isolated and identified from the locust. We then explored their anti-aging potential using C. elegans. We report here for the first time that the peptides from locust can significantly extend the lifespan of C. elegans under normal conditions and promote the survival rates of the C. elegans under oxidative stress. The possible mechanism of lifespan-promoting effects of the locust peptides on C. elegans has also been investigated. This study reports novel anti-aging capabilities of insect peptides isolated from locust and suggests a novel resource for functional peptide development.
2 Materials and methods
2.1 Chemicals and materials
Locusta migratoria manilensis (Meyen, 1835) was acquired from Institute of Plant Protection, Chinese Academy of Agricultural Sciences (Beijing). H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate) (Sigma-Aldrich, Shanghai, China) was used as a fluorescent probe. Paraquat, was used to induce oxidative stress in nematodes (Sigma-Aldrich, Shanghai, China). All other chemicals and agents used in this study were of analytical grade and were obtained from Sigma-Aldrich (Shanghai, China).2.2 Amino acid analysis
An amino acid analysis system for the quantification of locust protein was programmed on a Hitachi High Speed Amino Acid Analyzer Model 835-50. A ninhydrin post-column detecting system with a Hitachi 2619-F column (2.6 × 150 mm) was programmed. Areas of amino acid standards were used to calculate quantity of each amino acid in samples with the amino acid composition of protein.202.3 Isolation and identification of the locust peptide
All extraction and separation procedures were carried out at 4 °C. The locusts were minced to a homogenate and defatted as follow. The homogenate and iso-propanol were mixed in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (w/v) and stirred uninterrupted for 3 h at room temperature. The iso-propanol was replaced every 0.5 h. The supernatant was removed, and the sediment was freeze-dried and stored at −20 °C.
7 (w/v) and stirred uninterrupted for 3 h at room temperature. The iso-propanol was replaced every 0.5 h. The supernatant was removed, and the sediment was freeze-dried and stored at −20 °C.
The defatted precipitate (10 g) was dissolved (5%, w/v) in 0.2 M phosphate buffer solution (PBS, pH 7.2) then a KQ-250B ultrasonic cleaner (Shanghai, China) with straight probe and continuous pulse was used to ultrasound for 2 h. After centrifugation (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 10 min), the supernatant was collected as total protein and then was fractionated by salting-out with increasing concentrations of ammonium sulfate, and the resulting supernatant was freeze-dried and stored at −20 °C for further analysis.
000 × g, 10 min), the supernatant was collected as total protein and then was fractionated by salting-out with increasing concentrations of ammonium sulfate, and the resulting supernatant was freeze-dried and stored at −20 °C for further analysis.
Hydrophobic chromatography: the LP-α was dissolved in 1.40 M (NH4)2SO4 prepared with 30 mM phosphate buffer (pH 7.5) and loaded onto a Phenyl Sepharose CL-4B hydrophobic chromatography column (2.0 cm × 100 cm) which had previously been equilibrated with the above buffer. A stepwise elution was carried out with decreasing concentrations of (NH4)2SO4 (1.40, 0.70 and 0 M) dissolved in 30 mM phosphate buffer (pH 7.5) at a flow rate of 2.0 mL min−1. Each fraction was collected at a volume of 100 mL and was monitored at 280 nm. Five fractions were lyophilized and anti-aging activity in N2 nematodes was detected. The fraction having the strongest anti-aging activity was collected and prepared for anion-exchange chromatography.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v) and methanol–trifluoroacetic acid (solvent B; 100
0.1, v/v) and methanol–trifluoroacetic acid (solvent B; 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v). The peptides were separated using a gradient elution from 20% to 70% of solvent B for 50 min at a flow rate of 1.0 mL min−1. Detection wavelength was set at 280 nm. Here, the retention time of the LP-1 is 35 min. (The retention times for the other LPs are not list in the current study).
0.1, v/v). The peptides were separated using a gradient elution from 20% to 70% of solvent B for 50 min at a flow rate of 1.0 mL min−1. Detection wavelength was set at 280 nm. Here, the retention time of the LP-1 is 35 min. (The retention times for the other LPs are not list in the current study).HPLC-Q-TOF-MS was carried out on a SCIEX X500R Q-TOF mass spectrometer (Framingham, U.S.A.). And the MS conditions were as follows: ESI-MS analysis was performed using a SCIEX X500R Q-TOF mass spectrometer equipped with an ESI source. The mass range was set at m/z 50–1500. The Q-TOF MS data were acquired in positive mode and conditions of MS analysis were as follows: CAD gas flow-rate, 7 L min−1; drying gas temperature, 550 °C; ion spray voltage, 5500 V; declustering potential, 80 V. Software generated data file: SCIEX OS 1.0.
After the amino acid sequences of the peptides were determined, the peptides were synthesized using a high-efficiency solid-phase peptide synthesizer (Protein Technologies, Inc., Tucson, AZ, U.S.A.) in Chen's lab (Jinan University, Guangzhou, China).20 The purity of the synthesized peptides were verified by HPLC and the purity ≥ 99%. The synthesized peptide was stored at −20 °C until use.
2.4 Nematode strains and maintenance
Bristol N2 and the transgenic strains of mev-1 (TK22), and daf-16 (CF1038) and age-1 (hx546) were also obtained from the Caenorhabditis Genetics Center. Nematodes were maintained at 20 °C on plates containing Nematode Growth Medium (NGM) seeded with a live Escherichia coli strain OP50 as the food source according to the general procedures.212.5 Life span and stress resistance assays
2.6 Intracellular ROS, motility and brood size assays
2.7 Lipofuscin
Nematodes were treated as described Life span assay, and on day 11, mounted onto 2% agarose pads and immobilized in 20 μM sodium azide. Slides were visualized using the Leica DM6000B Microscope (Bannock burn, USA) (excitation 450/490 nm, emission 510 nm).29 Image quantification of fluorescence intensity was checked by tracing the nematode's intestine and determining mean pixel intensity.2.8 Quantitative real-time PCR
Nematodes were treated as described ROS measurement. Total RNA was extracted with Trizol reagent (Invitrogen), and cDNA was produced by oligo (dT) priming. The RT-PCR primers were list in Table 1. mRNA expression was performed with the method reported before. The gene expression data were analyzed using the comparative 2−ΔΔCT method, taking Actin-1 mRNA as the normalizer.30| Gene | Type | Sequence |
|---|---|---|
| daf-16 | Fwd | 5′-CCAGACGGAAGGCTTAAAACT-3′ |
| Rev | 5′-ATTCGCATGAAACGAGAATG-3′ | |
| sod-3 | Fwd | 5′-AGCATCATGCCACCTACGTGA-3′ |
| Rev | 5′-CACCACCATTGAATTTCAGCG-3′ | |
| daf-2 | Fwd | 5′-GGCCGATGGACGTTATTTTG-3′ |
| Rev | 5′-TTCCACAGTGAAGAAGCCTGG-3′ | |
| hsf-1 | Fwd | 5′-TTGACGACGACAAGCTTCCAGT-3′ |
| Rev | 5′-AAAGCTTGCACCAGAATCATCCC-3′ | |
| age-1 | Fwd | 5′-GAAATTAGAGCTCCACGGC-3′ |
| Rev | 5′-TACCCTCAAGTCCACGTGTC-3′ | |
| akt-1 | Fwd | 5′-TCAAGGATGCAGCGACAATG-3′ |
| Rev | 5′-TTGGCTGCTGATTGGTTTCC-3′ | |
| jnk-1 | Fwd | 5′-AGCGTGGAAGAGGATCACAA-3′ |
| Rev | 5′-CCTCCTCCTGTTCCACTGTT-3′ | |
| pmk-1 | Fwd | 5′-GGAACTGTTTGTGCTGCTGA-3′ |
| Rev | 5′-CGATATGTACGACGGGCATG-3′ | |
| actin-1 | Fwd | 5′-CCAGGAATTGCTGATCGTATGCAGAA-3′ |
| Rev | 5′-TGGAGAGGGAAGCGAGGATAGA-3′ |
2.9 Statistical analysis
The mean lifespan and survival data were analyzed with SPSS 18 (Chicago, USA) Kaplan-Meier Survival function and log-rank test. N: total number of nematodes in each individual experiment. p values were calculated for individual experiments, each consisting of control and experimental nematodes as the same time. All the other analyses were done using Graphpad Prism 5 (San Diego, USA). Lipofuscin accumulation was analyzed using one-way ANOVA. All values are expressed as mean ± SEM (n = 3). p < 0.05 was considered to be statistically significant.3 Results
3.1. Amino acid analysis
The result of essential amino acid contents in locust are summarized in the Tables 2 and 3. The essential amino acid compositions of locust protein are compared to those of the FAO/WHO reference pattern.31 Locust protein contains various amino acid compositions in which the content of human essential amino acids (45.6%) is more preferable than (compared to) FAO/WHO standard (35.0%). Contents of isoleucine, tyrosine, lysine and tryptophan are higher than FAO/WHO standard. Isoleucine, tyrosine, lysine and tryptophan are important biological constituents of numerous proteins in animals and plants, which are essential amino acid in human and animal diets.32 These results indicate that locust provides sufficient amount of the essential amino acids required for the body to function. Therefore, locust possesses great functional value, which might be a valuable source of high quality protein.| Amino acid | Locust protein | Amino acid | Locust protein |
|---|---|---|---|
| Asp | 1.92 | Ile | 2.38 |
| Thr | 1.58 | Leu | 4.52 |
| Ser | 4.84 | Tyr | 8.01 |
| Glu | 8.41 | Phe | 5.28 |
| Gly | 2.34 | Lys | 7.11 |
| Ala | 3.15 | His | 1.03 |
| Cys | 0.56 | Arg | 2.42 |
| Val | 4.16 | Pro | 3.08 |
| Met | 3.92 | Trp | 3.21 |
| Amino acid | Locust protein | WHO/FAO standard |
|---|---|---|
| Thr | 243 | 250 |
| Val | 267 | 310 |
| Met + Cys | 219 | 220 |
| Ile | 276 | 250 |
| Leu | 383 | 440 |
| Tyr | 855 | 380 |
| Lys | 356 | 340 |
| Trp | 70 | 60 |
| Total | 2669 | 2190 |
| Percentage in total (%) | 45.58 | 35.00 |
3.2 Isolation and identification of locust peptides
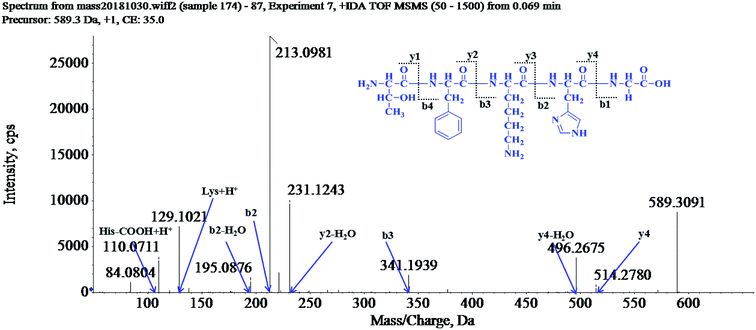
The peptide is usually protonated under ESI-MS/MS conditions, and fragmentations mostly occur at the amide bonds because it is difficult to break the chemical bonds of the side chains at such low energy. Therefore, the b and y ions are the main fragment ions when the collision energy is <200 eV.33 After a series of chromatographic separation and anti-aging activity detection, here, twenty-three peptides were isolated. Their structures were identified by HPLC-Q-TOF-MS analysis, and the amino acid sequences are list in Table 4.| Compounds | Amino acid sequences | Mean lifespan (days) |
|---|---|---|
| a All values are expressed as mean ± SEM (n = 3). | ||
| LP-1 | TFKHG | 30.2 ± 2.42 |
| LP-2 | YKHGRW | 27.5 ± 2.16 |
| LP-3 | LEHGSH | 29.1 ± 2.18 |
| LP-4 | FKGHRL | 27.2 ± 1.83 |
| LP-5 | FGEHLH | 24.2 ± 1.29 |
| LP-6 | YESHGA | 27.4 ± 1.43 |
| LP-7 | WERHFG | 24.7 ± 2.11 |
| LP-8 | HRLWSYG | 29.3 ± 2.24 |
| LP-9 | GEYHSHE | 24.5 ± 2.01 |
| LP-10 | TKFSYE | 28.4 ± 2.17 |
| LP-11 | YKHEWR | 25.6 ± 1.92 |
| LP-12 | KHGEL | 26.3 ± 1.80 |
| LP-13 | EGHGF | 26.7 ± 1.72 |
| LP-14 | YEEGAH | 28.5 ± 1.91 |
| LP-15 | AHEFEL | 26.3 ± 1.92 |
| LP-16 | DSHTS | 29.0 ± 1.97 |
| LP-17 | EAHGHSF | 26.6 ± 2.41 |
| LP-18 | EHGEYF | 19.2 ± 2.31 |
| LP-19 | EGFHL | 29.5 ± 2.14 |
| LP-20 | TFKHG | 26.2 ± 2.11 |
| LP-21 | WEGRGHG | 26.3 ± 2.03 |
| LP-22 | YEHSHG | 26.1 ± 2.16 |
| LP-23 | YSLHLHG | 29.3 ± 2.20 |
| Control | — | 25.5 ± 1.62 |
The molecular mass of the LP-1 was determined to be 589.3091 Da. The ion fragment m/z 514.2780 was regarded as the y4 ion, while m/z 496.2675 was regarded as the y4-H2O ion and m/z 341.1939 was regarded as the b3 ion. The ion (m/z 231.1243) was y2-H2O ion, m/z 213.0981 was the b2 ion, m/z 195.0876 was the b2-H2O ion and m/z 129.1021 was the [Lys + H]+ ion, and m/z 110.0711 was the typical fragment of [His-COOH + H]+ ion. On the basis of this, we concluded that the sequence of the peptide was Thr-Phe-Lys-His-Gly (TFKHG).
Furthermore, after administering the peptides 1.0 mg mL−1, starting at the L4 larvae stage, mean lifespan was list in Table 4. All the peptides extend the lifespan of the N2 nematodes. Interestingly, with LP-1 treatment, the lifespan was increased by 27.7% (27.2 ± 1.56 days) compared to the control group (21.3 ± 1.44 days). LP-1 showed the best lifespan extending activity in N2 nematodes under normal culture conditions, and this is why we chose LP-1 for further experiment.
3.3 LP-1 significantly extend the life span of N2 nematodes
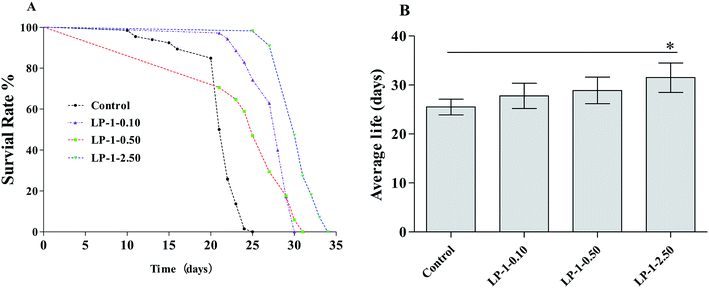
The growth of the bacteria may affect the diet of the nematodes, and then affect the lifespan.34 However, we observed that the LPs did not infect the growth cycle of the Escherichia coli strain OP50, and the minimal inhibitory concentration (MIC) values of the LPs are higher than 4.25 mg mL−1. So, the possibility that the bacteria may have an effect on the lifespan of nematodes was ruled out (Fig. 1).The wild-type N2 nematodes have a mean lifespan of 14–28 days at 20 °C.10 Under our standard laboratory conditions, wild-type N2 nematodes lived an average of 25.5 ± 1.62 days (maximum of 27 days). After administering with LP-1 (0.10, 0.50 and 2.50 mg mL−1), starting at the L4 larvae stage, mean lifespan increased to 27.8 ± 2.57 days (maximum of 29 days), 28.9 ± 2.71 days (maximum of 31 days) and 31.5 ± 3.02 days (maximum of 34 days), respectively, in a statistically significant, dose-dependent manner (p < 0.05; Table 5, Fig. 2). These changes represent lifespan increases of 9.21%, 13.3% and 23.5%, respectively.
| Groups | N | Mean lifespan (days) | % of control | Maximum lifespans (days) |
|---|---|---|---|---|
| a All values are expressed as mean ± SEM (n = 3). | ||||
| Control 0 mg mL−1 | 112 | 25.5 ± 1.62 | 100.0 | 27 |
| LP-1 0.10 mg mL−1 | 111 | 27.8 ± 2.57 | 120.9 | 29 |
| LP-1 0.50 mg mL−1 | 121 | 28.9 ± 2.71 | 124.4 | 31 |
| LP-1 2.50 mg mL−1 | 115 | 31.5 ± 3.02 | 133.3 | 34 |
3.4 LP-1 improves motility and attenuates lipofuscin accumulation
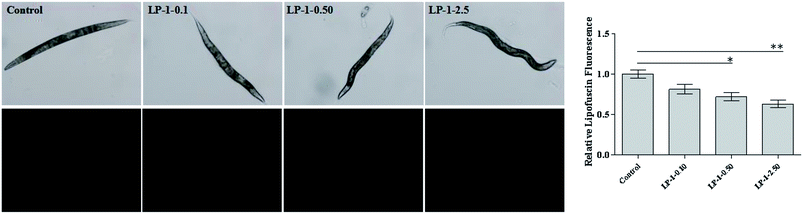
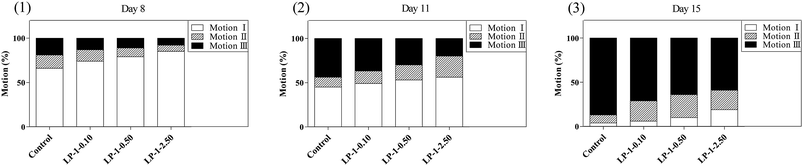
Lipofuscin is a byproduct of lysosomal degradation that accumulates with age in most organisms, including nematodes.35 Wild-type N2 nematodes treated with LP-1 as experimental design. As expected, lipofuscin content in LP-1 treated groups was significantly less than the control group. N2 nematodes treated with LP-1 decreased about 18.7%, 27.9%, 36.6% compared with the control group (p < 0.05, Fig. 3).Next, we examined whether the increase in lifespan was accompanied by an overall improvement in health and vitality. The decline in motility on day 8 was delayed significantly in nematodes treated with the samples (Fig. 4A). On day 11, most nematodes in the groups continued to move spontaneously, but there was a gentle difference in spontaneous (class I) motility among the control and treatment groups (p < 0.05; Fig. 4B). By day 11, nematodes treated with LP-1 2.50 mg mL−1 had significantly (p < 0.05) more high-motility (class I) individuals (56.2%) than the control group (45.1%).
By day 15, the dose-dependent effects on motility were clearly evident (Fig. 4C), such that 87.4% of the control group would barely move their heads with a gentle touch (class III), while 19.1% of the LP-1 2.50 mg mL−1 treatment group still moved spontaneously (class I).
We continued to follow the nematodes that remained alive to day 17; interestingly, treatment groups still displayed highly significant dose-dependent differences in motility while most of the control nematodes had died (p < 0.01, data not shown).
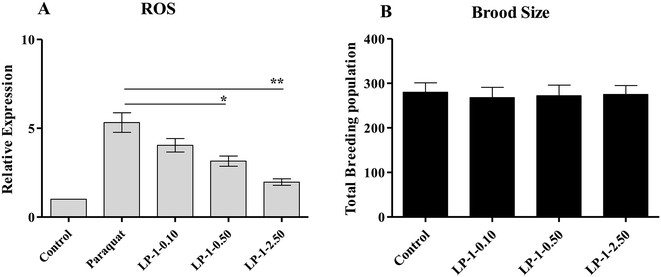
3.5 LP-1 decreases the intracellular ROS level and does not affect brood size
The free radical theory of aging hypothesized that free radical species caused deterioration of cells and organism. Oxidants are involved in many human diseases and aging processes. In chronic damage associated with the development of ageing, destructive oxidants and oxygen free radicals can be generated, which are very toxic to tissues and may result in further tissue necrosis and cellular damage. The antioxidants constitute a major cell defense against acute oxygen toxicity and protect membrane components against damage caused by free radicals.36,37 Does LP-1 enhance the stress resistance of nematodes under stress to prolong its longevity by removing ROS? To explore how LP-1 could enhance the stress resistance of nematodes under environmental stress, the free radical scavenging abilities of LP-1 were evaluated in the experiment.The ROS levels were detected in paraquat (1.0 mM) treated wild-type N2 nematodes. As shown in Fig. 5A, the ROS level was increased by 432%, compared to the control groups. Interestingly, with 0.10, 0.50 and 2.50 mg mL−1 LP-1 treatments, the ROS levels were reduced by 24.1%, 40.8% and 62.9%, respectively (Fig. 5A). Therefore, we conclude that LP-1 is a versatile free radical scavenger in vivo.
An increase in lifespan is often correlated with a decrease in reproductive capacity.38 To test whether the LP-1 adversely affect reproductive capacity, we measured brood sizes of N2 nematodes under LP-1 treatments. Results showed that there were no significant differences between LP-1 treated groups and the control groups (Fig. 5B).
3.6 LP-1 enhances the stress resistance of nematodes under stress conditions
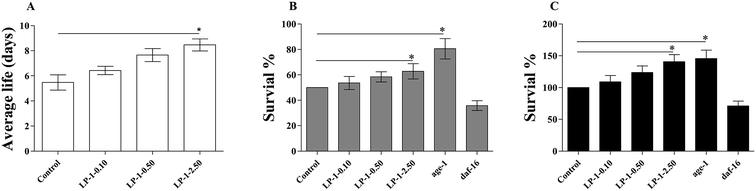
Studies demonstrated that increased lifespan often correlates with increased anti-oxidant stress resistance.39 We treated adult N2 nematodes with LP-1 for three days, and examined their response to a variety of fatal stressors, including oxidative stress, heat shock and UV radiation.To test the antioxidant capability of LP-1 in vivo, we put nematodes on NGM plates containing 1.0 mM paraquat. While control nematodes survived an average of 5.53 ± 0.65 days (maximum of 8 days), those treated with LP-1 0.10, 0.50 and 2.50 mg mL−1 survived for an average of 6.76 ± 0.64 (maximum of 9 days), 7.84 ± 0.92 (maximum of 11 days), and 8.83 ± 0.82 days (maximum of 13 days), respectively. These represented statistically significant increases in mean life span of 22.2%, 41.7% and 59.7% over the control (p < 0.05; Table 6 and Fig. 6A). Interestingly, the lifespan of TK22 and CF1038 under 2.50 mg mL−1 LP-1 treatments were down-regulated. We speculated that the excessive enzymatic hydrolysates of LP-1 may induce the injury damage to nematodes.
| Strains | Treatments | N | Mean lifespan (days) | Maximum lifespans (days) | % of control |
|---|---|---|---|---|---|
| a The mean lifespan was calculated by a log-rank (Kaplan–Meier) statistical test. N: total number of nematodes in each individual experiment. p values were calculated for individual experiments, each consisting of control and experimental nematodes as the same time. All statistical were calculated by using SPSS package. All values are expressed as mean ± SEM (n = 3). | |||||
| TK22 | Control 0 mg mL−1 | 125 | 9.56 ± 0.82 | 11 | 100.0 |
| TK22 | LP-1 0.10 mg mL−1 | 129 | 10.7 ± 0.88 | 14 | 111.9 |
| TK22 | LP-1 0.50 mg mL−1 | 127 | 10.8 ± 0.75 | 14 | 113.0 |
| TK22 | LP-1 2.50 mg mL−1 | 133 | 10.4 ± 0.83 | 13 | 108.8 |
| CF1038 | Control 0 mg mL−1 | 116 | 10.5 ± 0.92 | 12 | 100.0 |
| CF1038 | LP-1 0.10 mg mL−1 | 118 | 11.2 ± 0.99 | 14 | 106.7 |
| CF1038 | LP-1 0.50 mg mL−1 | 119 | 11.7 ± 0.95 | 14 | 111.4 |
| CF1038 | LP-1 2.50 mg mL−1 | 115 | 10.9 ± 0.92 | 13 | 103.8 |
| N2 | Control 0 mg mL−1 | 113 | 5.53 ± 0.65 | 8 | 100.0 |
| N2 | LP-1 0.10 mg mL−1 | 121 | 6.76 ± 0.64 | 9 | 122.2 |
| N2 | LP-1 0.50 mg mL−1 | 118 | 7.84 ± 0.92 | 11 | 141.7 |
| N2 | LP-1 2.50 mg mL−1 | 116 | 8.83 ± 0.82 | 13 | 159.7 |
Nematodes were placed in a 35 °C heat shock chamber and monitored until approximately half of the wild-type N2 controls had died (10.2 hours). At that time, 53.6%, 58.4% and 62.8% of LP-1 0.10, 0.50 and 2.50 mg mL−1 treated nematodes were still alive. Nematodes that have a mutation in age-1, the PI3 kinase, are more resistant to heat stress, were used as a negative control and survived at a 80.5% rate; daf-16 nematodes, which are more sensitive to heat stress, were used as a positive control and had a 35.8% survival rate (p < 0.05; Fig. 6B).
Post-irradiation survival time for N2 nematodes treated with LP-1 0.10, 0.50 and 2.50 mg mL−1 was increased by 9.10%, 23.7% and 40.6%, respectively. age-1 nematodes, which are also more resistant to UV stress than N2, were used as a negative control and survived 45.8% longer than the N2 controls. daf-16 nematodes, which are more sensitive to UV stress than N2 nematodes, were used as a positive control and survived 29.0% less than the N2 controls (p < 0.05; Table 7 and Fig. 6C).
| Groups | N | Mean lifespan (d) | % of control | Maximum lifespans |
|---|---|---|---|---|
| a All values are expressed as mean ± SEM (n = 3). | ||||
| Control 0 mg mL−1 | 67 | 3.97 ± 0.65 | 100.0 | 6 |
| LP-1 0.10 mg mL−1 | 76 | 4.33 ± 0.47 | 109.1 | 8 |
| LP-1 0.50 mg mL−1 | 69 | 4.91 ± 0.65 | 123.7 | 9 |
| LP-1 2.50 mg mL−1 | 68 | 5.58 ± 0.71 | 140.6 | 11 |
| age-1 | 75 | 5.79 ± 0.68 | 145.8 | 12 |
| daf-16 | 69 | 2.82 ± 0.31 | 71.0 | 4 |
Apparently, LP-1 could enhance the stress-resistance of nematodes under heat stress, UV irradiation, and especially under oxidative stress, suggesting that LP-1 might obtain this effect by scavenging free radicals.
3.7 LP-1 regulates the aging associated gene expressions
To explore the possible mechanism of LP-1 extending the C. elegans lifespan, we tested the effects on mutant nematodes, which are short-lived due to the oxidative stress. Results listed in Table 6, the LP-1 failed to extend the life span of TK22 and CF1038 nematodes, indicating that endogenous signaling pathways rather than the anti-oxidative activity alone are required to promote lifespan.The transcription factors of the FOXO family play a central role in regulating diverse traits, such as metabolics, stress resistance or longevity in nematodes. In the C. elegans, the best studied member of these transcription factors is DAF-16, which is one of the central targets of the insulin-like signaling cascade, characterised by the insulin-like receptor DAF-2.15 In nematodes, activation of DAF-2, the nematodes homolog of IGF-1 receptor, recruits and activates the phosphoinositide 3-kinase/protein kinase B signaling cascade, which in turn results in phosphorylation of DAF-16.29 DAF-16 activity is further modulated by the MAPK c-Jun N-terminal kinase-1 (JNK-1). Once in the nucleus, the heat-shock-factor-1 (HSF-1) acts together with DAF-16 to activate expression of stress-response genes, such as genes encoding small heat shock proteins, which enhance stress resistance and longevity in nematodes. Additionally, the p38 mitogen-activated protein kinase (PMK-1), a parallel pathway to DAF-16, also contributes to longevity of nematodes.23
We then focused on the insulin/insulin-like growth factor-1 (IGF1) signaling pathway, in which the translocation of the FOXO transcription factor DAF-16 into the nucleus is believed to modulate stress response and longevity of the nematodes. Due to the important role of DAF-16 in this pathway, we assessed the effects of the LP-1 on the life span of a mutant CF1038 strain, which lacks of daf-16. As a result, LP-1 failed to extend the life span of this mutant (Table 6). Taken together with the results on the N2 and TK22 nematodes, our data clearly suggest that signaling pathways are definitely required to promote longevity, and DAF-16 is indispensable for the life span-extending effects of the LP-1.
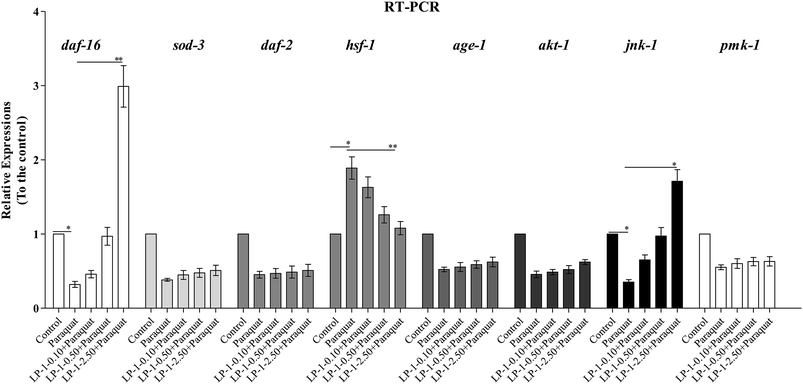
To investigate the possible underlying mechanisms of LP-1 extending C. elegans lifespan, we performed quantitative real-time PCR experiments to assess the expression of daf-2, age-1, akt-1, daf-16, sod-3, jnk-1, hsf-1 and pmk-1. As seen in Fig. 7, LP-1 can significantly up-regulate the expression of daf-16, whereas the expression of age-1, akt-1, daf-2 and sod-3 remained unchanged. The result indicates that the LP-1 promotes the longevity of C. elegans via modulation of DAF-16. Intriguingly, it was observed that LP-1 significantly up-regulated the expression of jnk-1. In addition, LP-1 also significantly up-regulate the expression of hsf-1 which cooperates with DAF-16 in regulating stress-response genes. Furthermore, we found that the expression of pmk-1 was not changed after LP-1 treatment. Collectively, our results suggest that the LP-1 may promote the C. elegans lifespan and stress resistance through a JNK-1-DAF16 pathway. This result suggests that the LP-1 are apparently taken up and exhibit in vivo antioxidant activities to reduce the burden of oxidative stress.
4 Discussion
Natural products represent an extraordinary source of novel chemical entities for potential anti-aging agents. Furthermore, studies showed that natural products performed excellent activities in delaying age-related decline and extending lifespan of the nematodes.40,41 Here, we reported that the antioxidant peptides isolated from Locusta migratoria manilensis (Meyen, 1835) possess promising anti-aging activities observed in C. elegans, a widely used model animal in aging research. To our knowledge, this is the first report describing the anti-aging effects of locust peptides in C. elegans, adding a novel source to the anti-aging natural products. We found that the locust peptides can significantly increase the lifespan of the wild type N2 nematodes under normal culture conditions at concentrations of 1.0 mg mL−1 (Table 4). Particularly, LP-1, a pentapeptide, exhibited the strongest longevity-promoting effect. At the concentration of 2.50 mg mL−1, LP-1 significantly extended the mean lifespan of C. elegans by 23.5%.Numerous studies have shown that longevity-promoting activity of natural products is correlated to their antioxidant activities.41 Interestingly, the LP-1 show potent in vivo antioxidant potentials in C. elegans. We found that the LP-1 significantly decreased endogenous ROS levels in the N2 nematodes (Fig. 5A), indicating that the LP-1 is indeed in vivo antioxidants in C. elegans. More intriguingly, the LP-1 were able to markedly increase the lifespan of C. elegans under oxidative stress induced by the strong pro-oxidant paraquat (Fig. 6A), suggesting that LP-1 protects the C. elegans from severe oxidative stress.
Hormesis mechanisms have been found in lifespan extension and stress resistance of C. elegans upon treatments with some natural compounds.41,42 As such, we speculate that the protective effects of the LP-1 in C. elegans under oxidative stress are likely due to stress hormesis, in which endogenous antioxidant response pathways are activated by treatment of antioxidant natural products. However, we observed that LP-1 failed to extend the mean life span of TK22 and CF1038 mutant C. elegans (Table 6), which are short-lived due to a high endogenous oxidative flux. This finding highly suggests that endogenous signaling pathways other than only direct antioxidant mechanism are involved in the life span extension of C. elegans induced by the LP-1.
To further explore the underlying mechanism of action of the LP-1, we firstly focused on the IIS pathway, which is a pivotal longevity-regulating pathway conserved in both C. elegans and mammals. In C. elegans the major longevity promoting transcription factor DAF-16 is a central player in the IIS pathway. In contrast to the effects observed in N2 C. elegans, LP-1 failed to extend the life span of the mutant strain lacking DAF-16, implying that the LP-1 extend life span of C. elegans through a mechanism that is mediated by DAF-16. The nuclear translocation of DAF-16 is a key step in the IIS pathway, as it regulates the transcription of a number of longevity- and stress tolerance-associated genes.15,22 However, we did not observe alterations of expression of daf-2, age-1 and akt-1 which are key upstream elements of IIS pathway in the C. elegans treated by the LP-1. It suggests that IIS pathway may not contribute to the longevity promotion effect of LP-1. In addition, by using quantitative RT-PCR we found that the transcriptional level of the daf-16 in N2 C. elegans can be markedly up-regulated by LP-1 about 1.99-fold, suggesting that DAF-16 may be involved in the action of the LP-1. Furthermore, the RT-PCR results showed that LP-1 strongly up-regulate the expression of jnk-1, thus suggesting that the anti-aging activity of LP-1 is possibly mediated by the jnk-1 signaling pathway.
It is reported that HSF-1 not only cooperates with DAF-16 to activate target genes but also itself can promote longevity in C. elegans.23 We found that LP-1 significantly up-regulated the expression of hsf-1, which indicted that hsf-1 is also involved in the longevity extension effect of LP-1. Considering the observation that LP-1 promotes the hsf-1 expression, it is possible that heat shock proteins (such as hsp-16.2) that are under the regulation of DAF-16 or HSF-1 may play a role in the stress resistance and longevity promotion of C. elegans treated by the LP-1.
5 Conclusion
In the present study, we have shown for the first time that peptide isolated from locust for potential anti-aging properties. Our data have clearly shown promising anti-aging activities of these peptides, thus adding a new functional bio-activity to this insect. Within this study, we could identify locust peptides as powerful longevity-promoting antioxidants that are worthy to be further evaluated for anti-aging applications in other animal systems. Furthermore, the locust peptides are interesting not only for their potent antioxidant activities to increase the nematode's resistance to fatal stress, but also for their efficacy to regulate the longevity-promoting transcription factor DAF-16 through the JNK-1 pathway. Due to the complexity and interactions of pathways involved in the regulation of aging, other mechanisms that possibly contribute to the anti-aging effects of the locust peptides cannot be excluded. We believe that further evaluations of the anti-aging potentials of the locust peptides would be worthy.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We thank Professors Heru Chen (Jinan University, Guangzhou) for technical assistance as well as critical editing of the manuscript. We thank professors Zehua Zhang and Guangjun Wang (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing) for providing the Locusta migratoria manilensis (Meyen, 1835). We thank Miao Gong (Shenzhen University) for transporting the locust from Beijing to Shenzhen University.References
- R. Martin, The biology of aging, Z. Gerontol. Geriatr., 2001, 34(6), 427–428 CrossRef CAS PubMed.
- P. D. Ray, B.-W. Huang and Y. Tsuji, Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling, Cell. Signalling, 2012, 24(5), 981–990 CrossRef CAS PubMed.
- S. Sajadimajd and M. Khazaei, Oxidative Stress and Cancer: The role of Nrf2, Curr. Cancer Drug Targets, 2018, 17, 538–557 CrossRef PubMed.
- G. D. Florida-James, R. Simpson, G. Davison and G. Close, Exercise, Free Radical Metabolism, and Aging: Cellular and Molecular Processes, Oxid. Med. Cell. Longevity, 2016, 2016, 1–2 CrossRef PubMed.
- M. Ogawa, T. Matsuda, A. Ogata, T. Hamasaki, A. Kumanogoh, T. Toyofuku and T. Tanaka, DNA damage in rheumatoid arthritis: an age-dependent increase in the lipid peroxidation-derived DNA adduct, heptanone-etheno-2'-deoxycytidine, Autoimmune Dis., 2013, 2013(23), 183487 Search PubMed.
- K. Yao, L. Zhang, Y. D. Zhang, P. P. Ye and N. Zhu, The flavonoid, fisetin, inhibits UV radiation-induced oxidative stress and the activation of NF-kappaB and MAPK signaling in human lens epithelial cells, Mol. Vision, 2008, 14(219–223), 1865–1871 CAS.
- H. A. Yamamoto and P. V. Mohanan, Effects of melatonin on paraquat or ultraviolet light exposure-induced DNA damage, J. Pineal Res., 2010, 31(4), 308–313 CrossRef.
- R. V. Sekhar, S. G. Patel, A. P. Guthikonda, R. Marvin, B. Ashok, G. E. Taffet and J. Farook, Deficient synthesis of glutathione underlies oxidative stress in aging and can be corrected by dietary cysteine and glycine supplementation, Am. J. Clin. Nutr., 2011, 94(3), 847–853 CrossRef CAS PubMed.
- K. Chatzianagnostou, S. D. Turco, A. Pingitore, L. Sabatino and C. Vassalle, The Mediterranean Lifestyle as a Non-Pharmacological and Natural Antioxidant for Healthy Aging, Antioxidants, 2015, 4(4), 719–736 CrossRef CAS PubMed.
- P. Shen, Y. Yue and Y. Park, A Living Model for Obesity and Aging Research: Caenorhabditis elegans, Crit. Rev. Food Technol., 2018, 5, 1 CAS.
- D. K. Kim, T. H. Kim and S. J. Lee, Mechanisms of aging-related proteinopathies in Caenorhabditis elegans, Exp. Mol. Med., 2016, 48(10), e263 CrossRef CAS.
- H. Ma, Y. Ma, Z. Zhang, Z. Zhao, R. Lin, J. Zhu, Y. Guo and X. Li, l-Arginine Enhances Resistance against Oxidative Stress and Heat Stress in Caenorhabditis elegans, Int. J. Environ. Res. Public Health, 2016, 13(10), 969 CrossRef PubMed.
- D. B. Danilo, A. Giulia, V. Claudia, C. Giuseppina and C. Calogero, Association between genetic variations in the insulin/insulin-like growth factor (Igf-1) signaling pathway and longevity: a systematic review and meta-analysis, Curr. Vasc. Pharmacol., 2013, 12(5), 4443 Search PubMed.
- D. S. Patel, Analysis of the DAF-2 insulin/IGF-1 receptor gene in Caenorhabditis elegans, University of London, 2006 Search PubMed.
- S. Gottlieb and G. Ruvkun, daf-2, daf-16 and daf-23: genetically interacting genes controlling Dauer formation in Caenorhabditis elegans, Genetics, 1994, 137(1), 107 CAS.
- K. Swayamjot, W. Fang, V. Manickam and A. Marcello, X-linked inhibitor of apoptosis (XIAP) inhibits c-Jun N-terminal kinase 1 (JNK1) activation by transforming growth factor beta1 (TGF-beta1) through ubiquitin-mediated proteosomal degradation of the TGF-beta1-activated kinase 1 (TAK1), J. Biol. Chem., 2005, 280(46), 38599–38608 CrossRef PubMed.
- O. Seung Wook, M. Arnab, S. Nenad, J. Feng, D. Roger J and T. Heidi A, JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16, Proc. Natl. Acad. Sci. U. S. A., 2005, 102(12), 4494–4499 CrossRef PubMed.
- H. Xiao, Extraction, Enzymatic Hydrolysis and Antioxidant Peptides of Oxya chinensis Protein, Shaanxi Normal University, 2006 Search PubMed.
- L. W. Wen, Exploitation and utilization of locust protein resources in China, Food Nutr., 2007, 8, 23–24 Search PubMed.
- V. K. Sarin, S. B. H. Kent, A. R. Mitchell and R. B. Merrifield, A general approach to the quantitation of synthetic efficiency in solid-phase peptide synthesis as a function of chain length, Chem. Informationsdienst, 1985, 16(25), 7845–7850 Search PubMed.
- W. Shi, J. Qin, N. Ye and B. Lin, Droplet-based microfluidic system for individual Caenorhabditis elegans assay, Lab Chip, 2008, 8(9), 1432–1435 RSC.
- M. Kurauchi, H. Morise and T. Eki, Using the nematode Caenorhabditis elegans daf-16 mutant to assess the lifespan toxicity of prolonged exposure to ecotoxic agents, J. Health Sci., 2009, 55(55), 796–804 Search PubMed.
- H. J. Joo, S. Park, K. Y. Kim, M. Y. Kim, H. Kim, D. Park and Y. K. Paik, HSF-1 is involved in regulation of ascaroside pheromone biosynthesis by heat stress in Caenorhabditis elegans, Biochem. J., 2016, 473(6), 789 CrossRef CAS PubMed.
- S. Kim, J. Noguera, J. Morales and A. Velando, Heritability of resistance to oxidative stress in early life, J. Evol. Biol., 2010, 23(4), 769–775 CrossRef PubMed.
- W. Dayong and X. Xiaojuan, Pre-treatment with mild UV irradiation suppresses reproductive toxicity induced by subsequent cadmium exposure in nematodes, Ecotoxicol. Environ. Saf., 2010, 73(3), 423–429 CrossRef PubMed.
- P. Hanbyeol, S. J. Sup, K. Hyo Geun, L. Hyejung and O. Myung Sook, Ampelopsis Radix Protects Dopaminergic Neurons against 1-Methyl-4-phenylpyridinium/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Toxicity in Parkinson's Disease Models In Vitro and In Vivo, Evid.-Based Complementary Altern. Med., 2013, 2013, 346438 Search PubMed.
- C. A. Easom and D. J. Clarke, Motility is required for the competitive fitness of entomopathogenic Photorhabdus luminescens during insect infection, BMC Microbiol., 2008, 8(1), 168 CrossRef PubMed.
- B. Anna, B. Esther, R. Fabio, D. Brion and M. Emilio, Phenotypic comparison of clinical and plant-beneficial strains of Pantoea agglomerans, Int. Microbiol., 2014, 17(2), 81 Search PubMed.
- S. HW, C. SM, L. MH, K. HJ, J. H and C. DS, Catalpol Modulates Lifespan via DAF-16/FOXO and SKN-1/Nrf2 Activation in Caenorhabditis elegans, Evid.-Based Complementary Altern. Med., 2015, 2015, 524878 Search PubMed.
- T. R. Maier, T. Hewezi, J. Peng and T. J. Baum, Isolation of whole esophageal gland cells from plant-parasitic nematodes for transcriptome analyses and effector identification, Mol. Plant-Microbe Interact., 2013, 26(1), 31–35 CrossRef CAS.
- H. K. Maehre, G. K. Edvinsen, K. E. Eilertsen and E. O. Elvevoll, Heat treatment increases the protein bioaccessibility in the red seaweed dulse (Palmaria palmata), but not in the brown seaweed winged kelp (Alaria esculenta), J. Appl. Phycol., 2016, 28(1), 581–590 CrossRef CAS.
- D. J. Millward, The nutritional value of plant-based diets in relation to human amino acid and protein requirements, Proc. Nutr. Soc., 1999, 58(2), 249 CrossRef CAS PubMed.
- M. J. Raftery and C. L. Geczy, Electrospray low energy CID and MALDI PSD fragmentations of protonated sulfinamide cross-linked peptides, J. Am. Soc. Mass Spectrom., 2002, 13(6), 709–718 CrossRef CAS PubMed.
- Y. Li, X. Yan, C. Ye, H. Zhao, X. Chen, F. Hu and H. Li, Bacterial Respiration and Growth Rates Affect the Feeding Preferences, Brood Size and Lifespan of Caenorhabditis elegans, PLoS One, 2015, 10(7), e0134401 CrossRef PubMed.
- G. Douglas A and W. John, Lipofuscin and aging: a matter of toxic waste, Sci. Aging Knowledge Environ., 2005, 2005(5), re1 Search PubMed.
- T. H. Hung, S. F. Chen, J. D. Liou, J. J. Hsu, M. J. Li, Y. L. Yeh and T. T. Hsieh, Bax, Bak and Mitochondrial Oxidants are Involved in Hypoxia-reoxygenation-induced Apoptosis in Human Placenta, Placenta, 2008, 29(7), 565–583 CrossRef CAS PubMed.
- J. Ali, M. H. Dziegiel, L. Rasmus, L. K. Nielsen, S. Subhash and P. L. Druilhe, A novel antibody-dependent cellular cytotoxicity mechanism involved in defense against malaria requires costimulation of monocytes FcgammaRII and FcgammaRIII, J. Immunol., 2007, 178(5), 3099 CrossRef.
- I. M. A. Ernst, K. Pallauf, J. K. Bendall, L. Paulsen, S. Nikolai, P. Huebbe, T. Roeder and G. Rimbach, Vitamin E supplementation and lifespan in model organisms, Ageing Res. Rev., 2013, 12(1), 365–375 CrossRef CAS PubMed.
- C. J. Vermeulen, L. V. D. Zande and R. Bijlsma, Resistance to Oxidative Stress Induced by Paraquat Correlates Well with Both Decreased and Increased Lifespan in Drosophila melanogaster, Biogerontology, 2005, 6(6), 387 CrossRef CAS PubMed.
- J. L. Wolfender, E. F. Queiroz and K. Hostettmann, Phytochemistry in the microgram domain - a LC-NMR perspective, Magn. Reson. Chem., 2005, 43(9), 697–709 CrossRef CAS PubMed.
- H. Yoko, F. Yasunori, M. Hiroe, A. Yoko, I. Kenji, S. Akira, K. Toshio, T. Masashi, N. Yoshinori and I. Masafumi, Lifespan-Extending Effects of Royal Jelly and Its Related Substances on the Nematode Caenorhabditis elegans, PLoS One, 2011, 6(8), e23527 CrossRef PubMed.
- J. Cypser and T. E. Johnson, Hormesis extends the correlation between stress resistance and life span in long-lived mutants of Caenorhabditis elegans, Hum. Exp. Toxicol., 2001, 20(6), 295–296 CrossRef CAS.
Footnote |
| † Equal contributions to the article. |
| This journal is © The Royal Society of Chemistry 2019 |