 Open Access Article
Open Access ArticleMn4+-activated Li3Mg2SbO6 as an ultrabright fluoride-free red-emitting phosphor for warm white light-emitting diodes
Shaoying Wang,
Qi Sun,
Balaji Devakumar,
Jia Liang,
Liangling Sun and
Xiaoyong Huang *
*
College of Physics and Optoelectronics, Taiyuan University of Technology, Taiyuan 030024, P. R. China. E-mail: huangxy04@126.com
First published on 25th January 2019
Abstract
In this paper, we report on highly efficient Mn4+-activated double perovskite Li3Mg2SbO6 (LMS) red-emitting phosphors. These LMS:Mn4+ phosphors can be efficiently excited over a broad wavelength band from 235 nm to 600 nm peaking at 344 nm and 469 nm, and exhibited an intense red emission band with a range from 600 nm to 800 nm centered around 651 nm. The optimal Mn4+ doping concentration of LMS:Mn4+ was 0.6 mol% and its internal quantum efficiency can reach as high as 83%. Besides, the thermal quenching effect on the optical property was also analyzed. Finally, a warm white light-emitting diode (WLED) lamp was fabricated by using a 454 nm InGaN blue LED chip combined with a blend of YAG:Ce3+ yellow phosphors and the as-prepared LMS:0.6% Mn4+ red phosphors, which showed bright white light with CIE chromaticity coordinates (0.4093, 0.3725), correlated color temperature (CCT = 3254 K), color rendering index (CRI = 81) and luminous efficacy (LE = 87 lm/W).
1. Introduction
In the past decade, the research on white light-emitting diodes (WLEDs), which show numerous merits including low energy consumption, long working time, low cost, small volume, and environmental compatibility, is becoming a hotspot in the lighting and display fields.1–9 WLEDs are considered to replace the traditional fluorescent and incandescent lamps as the next-generation solid-state lighting source. Currently, the popular method to fabricate the commercial WLEDs is using the InGaN blue LED chips combined with YAG:Ce3+ yellow phosphors.10–13 Unfortunately, owing to the absence of red phosphors, such WLEDs exhibit cold white light accompanied with low color rendering index (CRI) as well as high correlated color temperature (CCT), which is unfavorable for their further application in indoor lighting.14–16 Hence, it is of great theoretical value and practical significance to seek red-emitting phosphors with good luminescent performance for fabricating warm WLEDs.Up till now, the most studied red-emitting phosphors were activated by rare earth ions Eu3+ and Eu2+ owing to their preferable properties.17 However, there are some limitations in those red phosphors, for example, Eu3+ activated phosphors exhibit sharp excitation peaks in the near-ultraviolet (UV) and blue light region owing to its parity-forbidden 4f–4f transitions,18,19 while the preparation process of Eu2+ doped (oxy)nitrides is quite harsh, and the rare earth ions are normally expensive.20,21 In order to obtain the low-cost and easily available red-emitting phosphors with good luminescent properties, the transition metal ions Mn4+ with 3d3 electronic configuration are regarded as alternative activators because the Mn4+-activated phosphors can be prepared on a mild condition and show broad absorption band in the near-UV or blue light region accompanied with deep red emission due to the spin-forbidden 2Eg → 4A2g transition, the energy of which is highly dependent on the hybridization of Mn4+-ligand.22–24 Mn4+ ions are normally stable by occupying the cation sites in octahedral environments of the host materials such as fluorides and oxides.25,26 Recently, many Mn4+-activated fluorides (i.e., A2MF6:Mn4+, A = Na, K, Rb, and Cs; M = Si, Ti, Ge, and Zr) have been investigated and they present a potential application for warm WLEDs, due to the suitable red emission peak locating at 620–640 nm because of the weak hybridization effect.27,28 Unfortunately, during the preparation process, the excessive use of hydrofluoric acid, which is harmful to the environment and health, limits their economic applications and industrial productions.29 As an alternative, the environmentally friendly and chemically stable Mn4+ doped oxides (such as: Ba2GdSbO6:Mn4+,17 Ca2YSbO6:Mn4+,30 and Mg2TiO4:Mn4+ (ref. 31)) have been discovered to be outstanding red converters for application in warm WLEDs, even if the red emission of Mn4+-doped oxides shifts to the deep-red region (>650 nm) due to the strong hybridization effect.32 As a double perovskite-type oxide, the compound Li3Mg2SbO6 (LMS) possesses [SbO6] octahedron and Mn4+ ions are expected to substitute the cationic sites of Sb5+ ions. However, so far the synthesis and luminescence properties of LMS:Mn4+ phosphors have not been reported.
In this work, a series of novel high-efficiency Mn4+-activated LMS phosphors were successfully synthesized. Under excitations by the near-UV light and blue light, the LMS:Mn4+ can exhibit intense red emission with high internal quantum efficiency (IQE). Furthermore, the crystal structure and optical properties of LMS:Mn4+ samples were investigated in detail. Finally, a prototype warm WLED lamp which generated bright white light was also successfully fabricated.
2. Experimental
Li3Mg2Sb(1−x)O6:xMn4+ (abbreviated as: LMS:xMn4+) phosphors were synthesized by a high-temperature solid-state reaction procedure. As the raw materials, Li2CO3 (analytical reagent, AR), MgO (AR), Sb2O5 (99.9%), and MnCO3 (AR) were weighed in stoichiometric proportions and ground in an agate mortar. Then, the obtained powders were transferred into the alumina crucibles. Finally, the phosphors can be finally obtained after pre-firing at 600 °C for 5 h and sintering again at 1200 °C for 4 h in the air.The phase purity was studied by X-ray diffraction patterns recorded on a diffractometer (Bruker D8) with Cu Kα radiation (λ = 1.54 Å) in the 2θ range of 10–80° at a step rate of 0.02°. Crystal structure was analyzed according to the Rietveld refinement performed using the FullProf program. The photoluminescence (PL) and PL excitation (PLE) spectra were measured with the excitation and emission slit width of 0.9 nm as well as the dwell time of 0.1 s by a spectrometer (Edinburgh FS5) which was designed with plane gratings and equipped with a 150 W Xenon lamp. The decay curves were recorded on the spectrometer (Edinburgh FS5) with a pulsed Xenon lamp. The IQE and EQE were determined by the same spectrometer with an integrating sphere coated with BaSO4. The temperature-dependent PL spectra were also obtained on the spectrometer (Edinburgh FS5) equipped with a temperature controller. WLED devices were fabricated by coating the obtained phosphor-silicone on the surface of the LED chip. The as-fabricated WLEDs were operated by 3 V forward voltage with a driven current of 20 mA, and their electroluminescence (EL) spectra, luminous efficacy (LE), color rendering index (CRI), and correlated color temperature (CCT) were recorded by using an integrating-sphere spectroradiometer system (HAAS2000, Everfine).
3. Results and discussion
The XRD patterns of the LMS:xMn4+ phosphors (x = 0.2%, 0.6%, and 1.2%) agreed well with the standard PDF card (JCPDS #36-1019) of the LMS host, shown in Fig. 1(a), indicating that Mn4+ ions were well incorporated into the LMS without any significant effects on the host crystal structure. In order to characterize the crystal structure of LMS:Mn4+ phosphors, the XRD Rietveld refinement for LMS:0.6% Mn4+ sample was carried out and shown in Fig. 1(b).33,34 The refinement results including the cell parameters of LMS:0.6% Mn4+ determined to be a = 8.6393(5) Å, b = 5.9262(4) Å, c = 17.8170(1) Å, α = β = γ = 90°, and V = 912.20(17) Å3 were achieved with the residual factors of Rp = 9.36%, and Rwp = 13.72%. Accordingly, the crystal structure of the LMS:0.6% Mn4+ was a partially ordered rock salt structure belonging to orthorhombic crystal system with a space group Fddd (see Fig. 1(c)).35 The crystal structure of LMS included four types of octahedrons, where Sb5+ ions completely occupied a group of octahedral sites and Li/Mg distributed non-randomly over other three groups of octahedral sites.36 Here, it was more reasonable for Mn4+ (r = 0.53 Å) to substitute the Sb5+ (r = 0.6 Å) than Li+ (r = 0.76 Å) and Mg2+ (r = 0.72 Å) ions because of the closer radius.37Fig. 2(a) shows the PLE spectrum of LMS:0.6% Mn4+ phosphors. In the range of 235–600 nm, the PLE spectrum consisted of two broad excitation bands peaking at 344 nm attributed to the Mn–O charge transfer band (CTB) and the 4A2g → 4T1g transition, as well as 469 nm owing to 4A2g → 4T2g transition of Mn4+ ions with a monitoring wavelength of 651 nm, respectively,38–40 indicating that the phosphors can be excited by near-UV and blue light. Besides, there were some sharp PLE lines in the range of 450–500 nm due to the Xe lamp excitation source. The PL spectra of LMS:0.6% Mn4+ phosphors under 344 nm and 469 nm excitations were shown in Fig. 2(b). Evidently, the emission spectrum excited at 344 nm had the similar profile but the stronger emission intensity compared with that excited at 469 nm, and the PL spectra were composed of broad red emission bands in the range of 600–800 nm centered at around 651 nm, owing to the 2Eg → 4A2g transition of Mn4+ ions.41,42 Furthermore, the CIE chromaticity coordinates of LMS:0.6% Mn4+ under the excitation 344 nm and 469 nm were both calculated to be (0.724, 0.276), which located in red region. Moreover, the phosphors also exhibited bright red light under 365 nm UV lamp, as shown in the inset in Fig. 2(b).
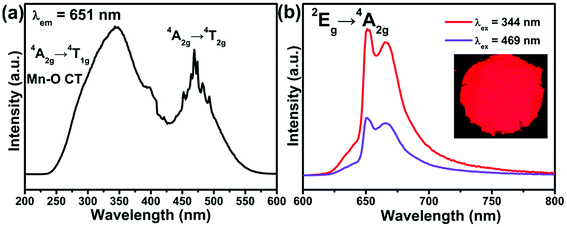 | ||
| Fig. 2 (a) The PLE spectrum of LMS:0.6% Mn4+ phosphors monitored at 651 nm. (b) The PL spectra of LMS:0.6% Mn4+ phosphors (λex = 344 and 469 nm) and the digital photograph under 365 nm near-UV lamp. | ||
Fig. 3(a) exhibits the emission spectra of LMS:xMn4+ phosphors (x = 0.1%, 0.2%, 0.4%, 0.6%, 0.8%, 1.0% and 1.2%) under 344 nm excitation. All the PL spectra exhibited similar emission profiles except that the PL intensities were different. Intuitively, the emission intensity of LMS:xMn4+ as a function of Mn4+ concentration was shown in Fig. 3(b). When x increased from 0.1% to 1.2%, the emission intensity first increased to the maximum with the optimal Mn4+ doping concentration x = 0.6% and then gradually decreased due to the concentration quenching effect.23,43 The concentration quenching effect was caused by the nonradiative energy transfer among Mn4+ ions.44 And the critical distance (Rc) can be evaluated by the following equation to determine the energy transfer mechanism among Mn4+ ions for the concentration quenching:45,46
 | (1) |
The specific type of the energy transfer mechanism can be further deduced using the following formula:47,48
log(I/x) = A − (θ/3)log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x x
| (2) |
Fig. 3(d) illustrates the decay curves of the 651 nm emissions of LMS:xMn4+ phosphors excited at 344 nm. The decay curves can be well fitted by a single-exponential function as below:52
It = I0 + A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ) exp(−t/τ)
| (3) |
Importantly, the IQE and EQE of the LMS:0.6Mn4+ phosphors can be determined by the following expressions:54,55
| IQE = ∫LS/(∫ER − ∫ES) | (4) |
| ε = (∫ER − ∫ES)/∫ER | (5) |
| EQE = ε × IQE | (6) |
Fig. 4(a) shows the temperature-dependent emission spectra of LMS:0.6% Mn4+ phosphor with the temperature range of 303–443 K under the 344 nm excitation. There were no shifts in the emission peaks, while the emission intensity decreased a little rapidly with increasing the temperature, owing to the severe thermal quenching effect. The activation energy (ΔE) can be calculated based on the Arrhenius equation:56
ln(I0/I − 1) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C − ΔE/kT C − ΔE/kT
| (7) |
 | ||
| Fig. 4 (a) The temperature-dependent PL spectra of LMS:0.6% Mn4+ phosphor ranging from 303 to 443 K. (b) The dependence of ln(I0/I − 1) on 1/kT. | ||
Consequently, the potential application of LMS:Mn4+ red phosphors were evaluated by comparing two types of WLED devices. Fig. 5(a) and (b) exhibit the electroluminescence (EL) spectra of the two as-fabricated WLED lamps. Owing to the absence of red phosphors, the LED device fabricated by using a 454 nm InGaN blue chip combined with only YAG:Ce3+ yellow phosphors possessed a lower Ra (i.e., the value of CRI) of 74.8 than that fabricated by using InGaN blue chip combined with a blend of YAG:Ce3+ yellow phosphors and LMS:0.6% Mn4+ red phosphors (Ra = 81). More importantly, the addition of LMS:0.6% Mn4+ red phosphors into the WLED device resulted in warm white light with CIE chromaticity coordinates (0.4093, 0.3725) and CCT = 3254 K, as shown in Fig. 5(c). Normally, the LE decreased when mixing the red components into the LED devices due to the Stokes shifting and the weak sensitivity of human eyes to red light, which can explain that the LE of the cold white LED lamp (LE = 158 lm W−1) was much higher than that of the warm WLED lamp (LE = 87 lm W−1).57 Even so, the LE value was higher than that of some warm WLEDs fabricated using the Mn4+ activated oxides or fluorides red phosphors which were reported before such as Sr2MgGe2O7:Mn4+ (LE = 78.7 lm W−1),58 Ca14Zn6Ga10O35:Mn4+ (LE = 64.83 lm W−1),52 Gd2ZnTiO6:Mn4+ (LE = 1.8 lm W−1),22 Na3AlF6:Mn4+ (LE = 70 lm W−1),59 Na3GaF6:Mn4+ (LE = 56.73 lm W−1),60 and K2LiGaF6:Mn4+ (LE = 53.3 lm W−1).61 These results confirmed that the LMS:Mn4+ red phosphors had a promising application in warm WLEDs.
4. Conclusions
To sum up, in this work, we reported novel highly efficient red-emitting LMS:Mn4+ phosphors. When excited at 344 nm and 469 nm, the LMS:Mn4+ phosphors can generate red emission with the peak locating at around 651 nm in the range of 600–800 nm, corresponding to the 2Eg → 4A2g transition of Mn4+ ions. This LMS:Mn4+ phosphor with the optimal concentration 0.6 mol% possessed high IQE up to 83%. And the impact of temperature on the luminescence property was also investigated. Moreover, the fabricated WLED lamp by using the InGaN blue chip coated with a blend of YAG:Ce3+ yellow phosphors and the as-prepared LMS:0.6% Mn4+ red phosphors showed warm white light with a higher CRI value (∼81) and lower CCT value (∼3254 K) than that fabricated without the red phosphors (Ra = 74.8, CCT = 6496 K). All the results exhibited above indicated that LMS:Mn4+ phosphors were promising red components for warm WLEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51502190), the Program for the Outstanding Innovative Teams of Higher Learning Institutions of Shanxi.References
- X. Huang, Nat. Photonics, 2014, 8, 748–749 CrossRef CAS.
- D. L. Dexter, J. Chem. Phys., 1953, 21, 836–850 CrossRef CAS.
- X. Huang, B. Li and H. Guo, J. Alloys Compd., 2017, 695, 2773–2780 CrossRef CAS.
- N. Narendran, Y. Gu, J. P. Freyssinier, H. Yu and L. Deng, J. Cryst. Growth, 2004, 268, 449–456 CrossRef CAS.
- B. Wang, H. Lin, J. Xu, H. Chen and Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 22905–22913 CrossRef CAS PubMed.
- B. Li, X. Huang, H. Guo and Y. Zeng, Dyes Pigm., 2018, 150, 67–72 CrossRef CAS.
- H. Guo, X. Huang and Y. Zeng, J. Alloys Compd., 2018, 741, 300–306 CrossRef CAS.
- F. Baur and T. Jüstel, J. Lumin., 2016, 177, 354–360 CrossRef CAS.
- L. Meng, L. Liang and Y. Wen, J. Mater. Sci.: Mater. Electron., 2014, 25, 2676–2681 CrossRef CAS.
- P. Du, X. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef CAS.
- S. Li, X. Wei, K. Deng, X. Tian, Y. Qin, Y. Chen and M. Yin, Curr. Appl. Phys., 2013, 13, 1288–1291 CrossRef.
- W. Lü, Y. Jia, Q. Zhao, W. Lv and H. You, Chem. Commun., 2014, 50, 2635–2637 RSC.
- P. Pust, P. J. Schmidt and W. Schnick, Nat. Mater., 2015, 14, 454–458 CrossRef CAS PubMed.
- Y. Liang, H. M. Noh, W. Ran, S. H. Park, B. C. Choi, J. H. Jeong and K. H. Kim, J. Alloys Compd., 2017, 716, 56–64 CrossRef CAS.
- X. Huang, S. Wang, B. Li, Q. Sun and H. Guo, Opt. Lett., 2018, 43, 1307–1310 CrossRef CAS PubMed.
- A. A. Setlur, E. V. Radkov, C. S. Henderson, J.-H. Her, A. M. Srivastava, N. Karkada, M. S. Kishore, N. P. Kumar, D. Aesram, A. Deshpande, B. Kolodin, L. S. Grigorov and U. Happek, Chem. Mater., 2010, 22, 4076–4082 CrossRef CAS.
- J. Zhong, S. Zhou, D. Chen, J. Li, Y. Zhu, X. Li, L. Chen and Z. Jia, Dalton Trans., 2018, 47, 8248–8256 RSC.
- X. Zhang, L. Zhou and M. Gong, Opt. Mater., 2013, 35, 993–997 CrossRef.
- Y. Shi, B. Liu, B. Liu, C. Li and Z. Wang, RSC Adv., 2015, 5, 95953–95959 RSC.
- P. F. Smet, A. B. Parmentier and D. Poelman, J. Electrochem. Soc., 2011, 158, R37–R54 CrossRef CAS.
- T. Takeda, R.-J. Xie, T. Suehiro and N. Hirosaki, Prog. Solid State Chem., 2018, 51, 41–51 CrossRef CAS.
- H. Chen, H. Lin, Q. Huang, F. Huang, J. Xu, B. Wang, Z. Lin, J. Zhou and Y. Wang, J. Mater. Chem. C, 2016, 4, 2374–2381 RSC.
- M. G. Brik and A. M. Srivastava, J. Lumin., 2013, 133, 69–72 CrossRef CAS.
- S. Zhang, Y. Hu, H. Duan, L. Chen, Y. Fu, G. Ju, T. Wang and M. He, RSC Adv., 2015, 5, 90499–90507 RSC.
- M. H. Du, J. Mater. Chem. C, 2014, 2, 2475–2481 RSC.
- Q. Peng, R. Cao, Y. Ye, S. Guo, Z. Hu, T. Chen and G. Zheng, J. Alloys Compd., 2017, 725, 139–144 CrossRef CAS.
- A. G. Paulusz, J. Electrochem. Soc., 1973, 120, 942–947 CrossRef CAS.
- H.-D. Nguyen and R.-S. Liu, J. Mater. Chem. C, 2016, 4, 10759–10775 RSC.
- Y. Arai and S. Adachi, J. Lumin., 2011, 131, 2652–2660 CrossRef CAS.
- J. Zhong, D. Chen, X. Chen, K. Wang, X. Li, Y. Zhua and Z. Jia, Dalton Trans., 2018, 47, 6528–6537 RSC.
- C.-S. Huang, C.-L. Huang, Y.-c. Liu, S.-k. Lin, T.-S. Chan and H.-W. Tu, Chem. Mater., 2018, 30, 1769–1775 CrossRef CAS.
- T. S. Sreena, P. P. Rao, A. K. V. Raj and T. R. A. Thara, J. Alloys Compd., 2018, 751, 148–158 CrossRef CAS.
- K. Mommaa and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef.
- K. Mommaa and F. Izumi, J. Appl. Crystallogr., 2008, 41, 653–658 CrossRef.
- G. C. Mather, R. I. Smith, J. M. S. Skakle, J. G. Fletcher, M. A. R. Castellanos, M. P. Gutierrez and A. R. West, J. Mater. Chem., 1995, 5, 1177–1182 RSC.
- J. M. S. Skakle, M. A. Castellanos R., S. T. Tovar and A. R. West, J. Solid State Chem., 1997, 131, 115–120 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., 1976, 32, 751–767 CrossRef.
- Q. Sun, B. Li, S. Wang, H. Guo and X. Huang, J. Mater. Sci.: Mater. Electron., 2018, 29, 12972–12977 CrossRef CAS.
- S. J. Kim, H. S. Jang, S. Unithrattil, Y. H. Kim and W. B. Im, J. Lumin., 2016, 172, 99–104 CrossRef CAS.
- L. Qin, S. Bi, P. Cai, C. Chen, J. Wang, S. I. Kim, Y. Huang and H. J. Seo, J. Alloys Compd., 2018, 755, 61–66 CrossRef CAS.
- X. Huang and H. Guo, Dyes Pigm., 2018, 152, 36–42 CrossRef CAS.
- J. Liang, L. Sun, B. Devakumar, S. Wang, Q. Sun, H. Guo, B. Li and X. Huang, RSC Adv., 2018, 8, 27144–27151 RSC.
- A. Fu, L. Zhou, S. Wang and Y. Li, Dyes Pigm., 2018, 148, 9–15 CrossRef CAS.
- U. B. Humayoun, S. N. Tiruneh and D.-H. Yoon, Dyes Pigm., 2018, 152, 127–130 CrossRef.
- X. Huang, H. Guo and B. Li, J. Alloys Compd., 2017, 720, 29–38 CrossRef CAS.
- X. Ding, G. Zhu, W. Geng, Q. Wang and Y. Wang, Inorg. Chem., 2016, 55, 154–162 CrossRef CAS.
- Y. Jin, Y. Hu, H. Wu, H. Duan, L. Chen, Y. Fu, G. Ju, Z. Mu and M. He, Chem. Eng. J., 2016, 288, 596–607 CrossRef CAS.
- L. G. V. Uitert, J. Electrochem. Soc., 1967, 114, 1048–1053 CrossRef.
- R. Yu, H. M. Noh, B. K. Moon, B. C. Choi, J. H. Jeong, K. Jang, S. S. Yi and J. K. Jang, J. Alloys Compd., 2013, 576, 236–241 CrossRef CAS.
- D. Deng, H. Yu, Y. Li, Y. Hua, G. Jia, S. Zhao, H. Wang, L. Huang, Y. Li, C. Li and S. Xu, J. Mater. Chem. C, 2013, 1, 3194–3199 RSC.
- G. Blasse, Phys. Lett. A, 1968, 28, 444–445 CrossRef CAS.
- C. Yang, Z. Zhang, G. Hu, R. Cao, X. Liang and W. Xiang, J. Alloys Compd., 2017, 694, 1201–1208 CrossRef CAS.
- K. Li, H. Lian and R. V. Deun, Dalton Trans., 2017, 47, 2501–2505 RSC.
- H. Ji, L. Wang, Y. Cho, N. Hirosaki, M. S. Molokeev, Z. Xia, Z. Huang and R.-J. Xie, J. Mater. Chem. C, 2016, 4, 9872–9878 RSC.
- X. Ji, J. Zhang, Y. Li, S. Liao, X. Zhang, Z. Yang, Z. Wang, Z. Qiu, W. Zhou, L. Yu and S. Lian, Chem. Mater., 2018, 30, 5137–5147 CrossRef CAS.
- X. Huang, J. Liang, B. Li, L. Sun and J. Lin, Opt. Lett., 2018, 43, 3305–3308 CrossRef CAS.
- Y. Jin, M. H. Fang, M. Grinberg, S. Mahlik, T. Lesniewski, M. G. Brik, G. Y. Luo, J. G. Lin and R. S. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 11194–11203 CrossRef CAS.
- W. Chen, Y. Cheng, L. Shen, C. Shen, X. Liang and W. Xiang, J. Alloys Compd., 2018, 762, 688–696 CrossRef CAS.
- E. H. Song, J. Q. Wang, S. Ye, X. F. Jiang, M. Y. Peng and Q. Y. Zhang, J. Mater. Chem. C, 2016, 4, 2480–2487 RSC.
- T. T. Deng, E. H. Song, J. Sun, L. Y. Wang, Y. Deng, S. Ye, J. Wang and Q. Y. Zhang, J. Mater. Chem. C, 2017, 5, 2910–2918 RSC.
- Y. Zhu, J. Yu, Y. Liu, M. G. Brik, L. Huang, T. Xuan and J. Wang, RSC Adv., 2017, 7, 30588–30593 RSC.
| This journal is © The Royal Society of Chemistry 2019 |



