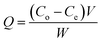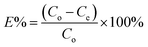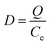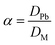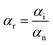Open Access Article
Open Access ArticleSynthesis and characterization of a surface-grafted Pb(II)-imprinted polymer based on activated carbon for selective separation and pre-concentration of Pb(II) ions from environmental water samples
Zhenhua Li *,
Lihua Chen,
Qiong Su,
Lan Wu,
Xiaohong Wei*,
Liang Zeng and
Muchen Li
*,
Lihua Chen,
Qiong Su,
Lan Wu,
Xiaohong Wei*,
Liang Zeng and
Muchen Li
Key Laboratory of Environmental Friendly Composite Materials and Biomass Utilization, Chemical Engineering Institute, Northwest Minzu University, Lanzhou, China. E-mail: lizhh02006@163.com; weixh12@lzu.edu.cn; Fax: +86 931 4512932; Tel: +86 931 4512932
First published on 11th February 2019
Abstract
Even the lowest concentration level of lead (Pb) in the human body is dangerous to health due to its bioaccumulation and high toxicity. Therefore, it is very important to develop selective and fast adsorption methods for the removal of Pb(II) from various samples. In this paper, a new Pb(II) ion-imprinted polymer (Pb(II)-IIP) was prepared with surface imprinting technology by using lead nitrate as a template, for the solid-phase extraction of trace Pb(II) ions in environmental water samples. The imprinted polymer was characterized by X-ray diffraction, Fourier transform infrared spectroscopy, Raman spectroscopy, scanning electron microscopy and N2 adsorption–desorption isotherms. The separation/pre-concentration conditions for Pb(II) were investigated, including the effects of pH, shaking time, sample flow rate, elution conditions and interfering ions. Compared with non-imprinted particles, the ion-imprinted polymer had a higher selectivity and adsorption capacity for Pb(II). The pseudo-second-order kinetics model and Langmuir isotherm model fitted well with the adsorption data. The relative selectivity factor values (αr) of Pb(II)/Zn(II), Pb(II)/Ni(II), Pb(II)/Co(II) and Pb(II)/Cu(II) were 168.20, 192.71, 126.13 and 229.39, respectively, which were all much greater than 1. The prepared Pb(II)-imprinted polymer was shown to be promising for the separation/pre-concentration of trace Pb(II) from natural water samples. The adsorption and desorption mechanisms were also proposed.
Introduction
In recent years, water pollution caused by heavy metals has been one of the major economic and environmental problems all over the world. Among heavy metal ions, lead (Pb) is one of the most toxic for animals and humans. Through the food chain system of soil-plant-animal-human, Pb(II) is transferred into animals and human beings, causing severe contamination.1 Therefore, the accurate and sensitive determination of Pb(II) in real samples is an important part of analytical chemistry. Reproducible, accurate and sensitive analytical methods are required for the determination of trace Pb in environmental samples. Among the spectral methods, inductively coupled plasma atomic emission spectrometry (ICP-OES) and atomic absorption spectrometry (AAS) are simple and rapid methods for the determination of metal ion concentrations,2 however they are usually insufficiently sensitive due to matrix interferences and the very low concentration of metal ions present. Therefore, a separation/pre-concentration step is required.Nowadays, the solid-phase extraction (SPE) technique is widely used for the pre-concentration of heavy metals due to its high enrichment factors, absence of emulsion formation, safety with respect to hazardous samples, minimal costs due to low consumption of reagents, flexibility and ease of automation.3
The choice of sorbent is an important factor in SPE because it can control the analytical parameters such as affinity, capacity and selectivity.4 Activated carbon is known as the most traditional adsorbent for removal of organic and inorganic pollutants from aqueous as well as gaseous environments.5 It is a widely used adsorbent in water and wastewater treatments due to its high surface area, environmentally friendly nature and because the methods are well developed. However, without any surface treatment, activated carbon presents an adsorption capacity for metal ions ranging from fair to zero, due to the fact that metal ions often exist in solution either as ions or as hydrous ionic complexes. On the other hand, chelating-agent modified activated carbons have been reported for the enrichment of metal ions.6–9 Although remarkable adsorption capacities and adsorption rates may be achieved by using these adsorbents, the adsorption selectivity for target metal ions remains unsatisfactory, which means that the adsorbents are unlikely to remove or enrich target metal ions from wastewater in the presence of many competitive ions. Recently, however, more and more extensive studies describing highly selective molecularly imprinted materials have been reported.10–12
Molecular imprinting is a method for the tailor-made preparation of highly selective synthetic polymer receptors for given molecules. The principle of molecular imprinting is that a target molecule (template) and functional monomers are polymerized with a crosslinking reagent. After removal of the template, the functional groups in the resulting binding sites should be arranged in positions suitable for subsequent interactions with template molecules13 and so molecular imprinting polymers (MIPs) are capable of recognizing and binding the desired molecular target with a high affinity and selectivity.14 An ion-imprinted polymer (IIP) is obtained when a metal ion is used as the template in the above-described synthesis. In most cases, specific ligands capable of forming a stable complex with the metal ion (or metal ion complexes with such specific ligands) are used in the polymerization process. The high selectivity of IIP can be explained by the polymer memory effect toward the metal ion interaction with a specific ligand, coordination geometry, metal ion coordination number, charge and size.15 One potential application of IIPs that has recently attracted widespread interest is the clean-up and enrichment of analytes present at low concentrations in complex matrices.16,17 Many studies on IIPs and their use for selective separation and pre-concentration of metal ions have been reported.18–21 However, as far as we know, there has been no report on using a surface-imprinted activated carbon sorbent for Pb(II) enrichment.
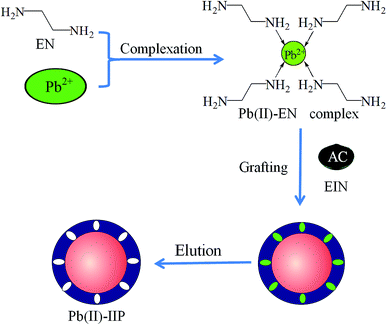
Ethylenediamine has stable physical and chemical properties, and is commonly used as complexing agent. Based on this, in this work, ethylenediamine was selected as the functional monomer, epichlorohydrin was selected as the cross-linker, and a new and green Pb(II)-IIP was prepared by the surface molecular imprinting technique for the removal of Pb(II) ions from aqueous solutions. The proposed method presented high adsorption capacity and selectivity for Pb(II), and offered a convenient, accurate and simple route to the clean-up of water samples.
Experimental
Apparatus
An Iris Advantage ER/S inductively coupled plasma emission spectrometer, (Thermo Jarrel Ash, Franklin, MA, USA) was used for the determination of all metal ions. The instrumental parameters were those recommended by the manufacturer. The wavelength selected for Pb was 216.999 nm. Infrared spectra (4000–400 cm−1) in KBr were recorded on a Nicolet NEXUS 670 FT-IR apparatus (USA). Raman spectra were measured with a 532 nm edge by using a LabRAM HR Evolution spectrometer (HORIBA Jobin Yvon S.A.S.) A JSM-6701F (JEOL, Japan) was used to obtain scanning electron microscopy (SEM) images. A Philips X'pert diffractometer with Cu Kα radiation (Holland) was used to acquire the X-ray diffraction (XRD) patterns of powdered samples. N2 adsorption–desorption isotherms (Brunauer–Emmett–Teller; BET) were measured using a TriStar II 3020 V1.04 apparatus (USA). The pH values were controlled with a pHs-3C digital pH meter (Shanghai Lei Ci Device Works, China). A YL-110 peristaltic pump (General Research Institute for Non-ferrous Metals, Beijing, China) was used in the separation/pre-concentration process. A polytetrafluoroethylene (PTFE) column (50 mm × 9.0 mm i.d.) (Tianjin Jinteng Instrument Factory, Tianjin, China) was used.Chemicals and reagents
Standard stock solutions of Pb(II), Zn(II), Ni(II), Co(II) and Cu(II) (1 mg mL−1) were prepared by dissolving appropriate amounts of analytical grade salts in double distilled water (DDW) with the addition of 1.0% HNO3, and they were further diluted daily prior to use. Activated carbon (AC, 40–60 mesh) was provided by Tianjin Guangfu Fine Chemical Research Institute, Tianjin, China. It was dried in a vacuum at 110 °C for 48 h before use. Ethylenediamine (EN), thiourea and N,N′-dicyclohexylcarbodiimide (DCC) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Epichlorohydrin (EIN) was provided by Shenyang Xinxing Reagent Factory (Shenyang, China). The certified reference materials (GBW 08301: river sediment; GBW 08303: polluted farming soil) were obtained from the National Research Center for Certified Reference Materials (Beijing, China).Sample preparation
The river water samples were collected from Yellow River (Chengguan district of Lanzhou, China) and Huangshui River (Centre Square of Xining, China). Lake water was collected from South Lake, Lanzhou, China. The water samples were filtered through a 0.45 μm PTFE millipore filter, and acidified to a pH of about 2 with concentrated HCl prior to storage for use. Tap water samples taken from our research laboratory were analyzed without any pretreatment. The pH value was adjusted to 2 with 0.1 mol L−1 HCl prior to use.The certified reference materials (GBW 08301: river sediment; GBW 08303: polluted farming soil) were digested according to the literature methods.22
Synthesis
Procedures
where Q represents the adsorption capacity (mg g−1), Co and Ce represent the initial and equilibrium concentrations of Pb(II) (μg mL−1), W is the mass of Pb(II)-imprinted amino-functionalized AC polymer (g), V is the volume of metal ion solution (L), E% is the extraction percentage, D is the distribution ratio (mL g−1), DPb and DM represent the distribution ratios of Pb(II) and Ni(II), Co(II), Zn(II), or Cu(II) respectively, α is the selectivity coefficient, αr is the relative selectivity coefficient, and αi and αn represent the selectivity factors of Pb(II)-IIP and Pb(II)-NIP, respectively.
Results and discussion
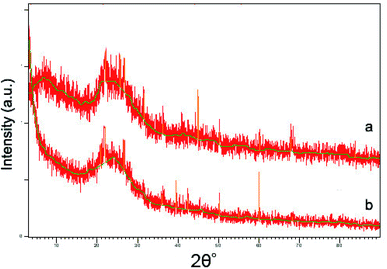
Characteristics of the imprinted polymer
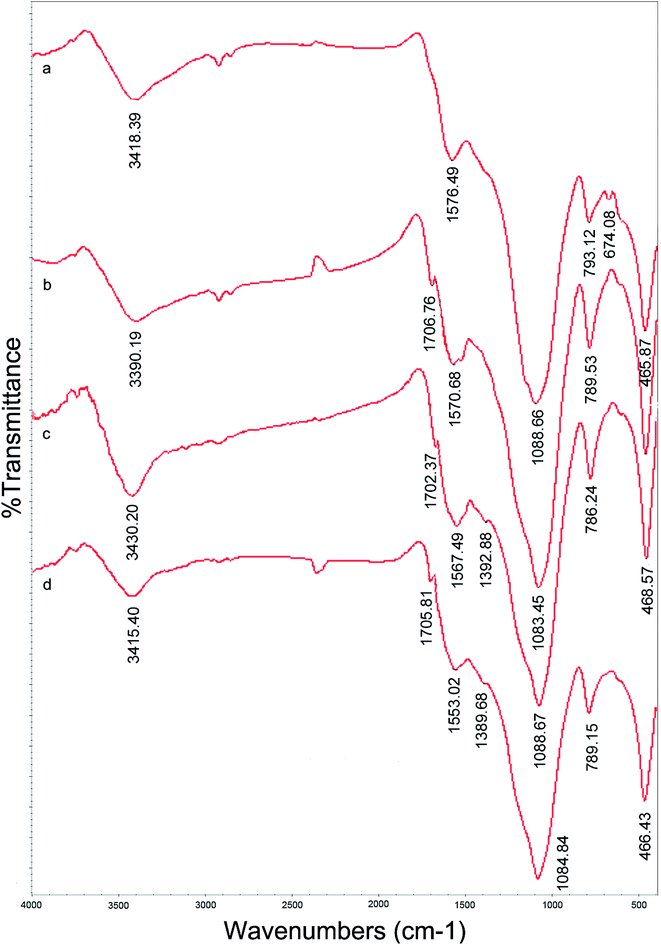
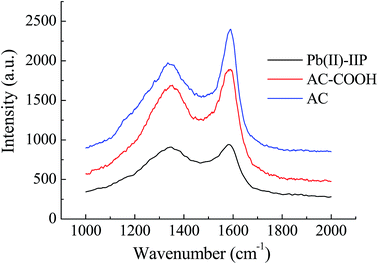
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the carboxylic acid group, which indicated that the carboxylic derivative of AC had been prepared successfully. Several new peaks also appeared in the spectrum of Pb(II)-IIP. According to the literature,23,24 the new peaks could be assigned as follows: the peak at 1702 cm−1 was due to C
O stretching vibration of the carboxylic acid group, which indicated that the carboxylic derivative of AC had been prepared successfully. Several new peaks also appeared in the spectrum of Pb(II)-IIP. According to the literature,23,24 the new peaks could be assigned as follows: the peak at 1702 cm−1 was due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations; the peak at 1567 cm−1 was caused by N–H bending vibrations; and the bands around 3430 cm−1 could be assigned to N–H stretching vibrations. The main bands of the spectra of Pb(II)-IIP and Pb(II)-NIP showed very similar locations and appearances. This indicated that N–H was recovered after removal of Pb(II) in the imprinted sorbent. These results suggested that –NH2 had been grafted onto the surface of the AC after modification.
O stretching vibrations; the peak at 1567 cm−1 was caused by N–H bending vibrations; and the bands around 3430 cm−1 could be assigned to N–H stretching vibrations. The main bands of the spectra of Pb(II)-IIP and Pb(II)-NIP showed very similar locations and appearances. This indicated that N–H was recovered after removal of Pb(II) in the imprinted sorbent. These results suggested that –NH2 had been grafted onto the surface of the AC after modification.
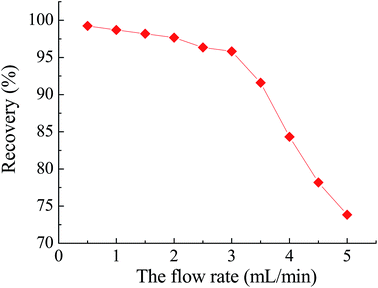
From the data given in Table 1, it is obvious that quantitative elution was not obtained by using thiourea alone as the desorption reagent (recovery < 60%). When HCl was used as the desorption agent, the coordination spheres of chelated Pb(II) were disrupted and subsequently Pb(II) ions were released from the lead templates into the desorption medium. Quantitative recoveries (>95%) of Pb(II) could be obtained using 2 mL of 0.04 mol L−1 HCl solution as the eluent. Therefore, 2 mL of 0.04 mol L−1 HCl was used as the eluent in subsequent experiments.
| Optimization of eluent type and concentration (V = 10 mL) | ||
|---|---|---|
| Eluent type | Concentration of eluent | Recovery (%) |
| Thiourea | 0.01 M | 42.47 |
| 0.05 M | 58.09 | |
| HCl | 0.01 M | 90.74 |
| 0.02 M | 94.82 | |
| 0.03 M | 98.35 | |
| 0.04 M | 99.42 | |
| 0.05 M | 99.11 | |
| Optimization of eluent volume (C = 0.04 mol L−1 HCl) | ||||||
| Eluent volume (mL) | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 4.0 |
| Recovery (%) | 86.65 | 96.16 | 100 | 100 | 100 | 100 |
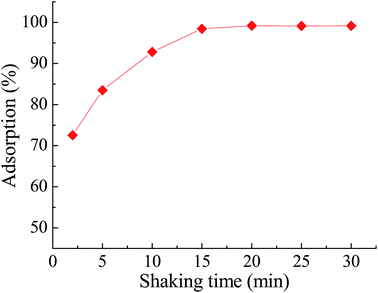 | ||
| Fig. 7 Effect of shaking time on the adsorption of Pb(II) on Pb(II)-IIP. Other conditions: pH 4.0, temperature 25 °C. | ||
In order to investigate the factors influencing the adsorption rate, it is necessary to study the kinetics of the adsorption process. Pb(II)-IIP (30 mg) was allowed to interact with 10 mL of Pb(II) solution (400 ug mL−1) for periods of 15–120 min.
Therefore, the kinetic changes in this study were evaluated using the pseudo-first-order kinetic model (1) and pseudo-second-order kinetic model (2):
ln(Qt − Qe) = −K1t + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qe Qe
| (1) |
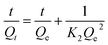 | (2) |
The kinetics parameters for Pb(II) adsorption are listed in Table 2. The correlation coefficients (R2) of the pseudo-second-order equation were higher than those of the pseudo-first-order equation. Also, the calculated Qe values were in better agreement with the experimental results for the pseudo-second-order kinetic model, indicating its better suitability for describing the adsorption kinetics of Pb(II) onto Pb(II)-IIP. The good fit to the pseudo-second-order kinetic model could imply that the predominant adsorption process was chemisorption. The results also suggested that Pb(II) ions could combine with active sites on the Pb(II)-IIP via covalent chemical bonding.28 The results could be expected because the polymerization reactions occurred only on the AC.
| First-order model | Second-order model | ||||
|---|---|---|---|---|---|
| K1 (min−1) | Qe (mg g−1) | R2 | K2 (g mg−1 min−1) | Q2 (mg g−1) | R2 |
| 0.041 | 3.64 | 0.779 | 0.036 | 90.90 | 0.999 |
| Ion | Concentration (μg mL−1) | Added as | Recovery (%) |
|---|---|---|---|
| K+ | 2000 | KCl | 100 |
| Na+ | 2000 | NaCl | 100 |
| Mg2+ | 1000 | MgSO4 | 96.45 |
| Ca2+ | 1000 | CaCl2 | 97.24 |
| Cd2+ | 100 | Cd(NO3)2·4H2O | 98.18 |
| Cr3+ | 100 | CrCl3 | 96.38 |
| Co2+ | 100 | CoCl2 | 96.35 |
| Fe3+ | 100 | FeCl3 | 94.23 |
| Mn2+ | 100 | MnSO4·H2O | 91.90 |
| Ni2+ | 100 | NiCl2·6H2O | 93.82 |
| Zn2+ | 100 | ZnCl2 | 97.01 |
| Hg2+ | 100 | HgCl2 | 96.87 |
| SO42− | 3000 | Na2SO4 | 96.75 |
| NO3− | 3000 | NaNO3 | 99.18 |
| Cl− | 3000 | NaCl | 92.42 |
| F− | 3000 | NaF | 99.17 |
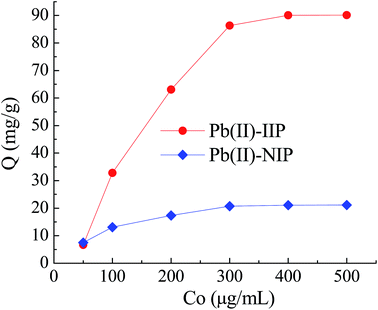 | ||
| Fig. 9 Effect of initial concentration (Co) of Pb(II) on the adsorption quantity (Q) of Pb(II)-IIP and Pb(II)-NIP. Other conditions: pH 4.0, temperature 25 °C. | ||
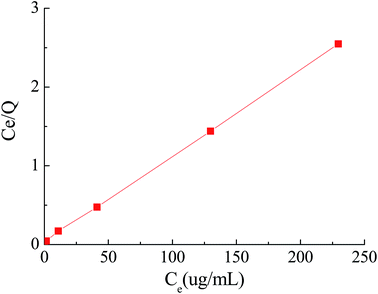
The adsorption process is normally described by the Langmuir isotherm. The Langmuir isotherm is valid for monolayer adsorption onto a surface containing a finite number of homogeneous sites. The Langmuir equation is expressed as follows (3):31
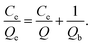 | (3) |
The plot of Ce/Qe versus Ce gave a straight line (R2 = 0.999) with a slope of 1/Q and an intercept of 1/Qb which confirmed the validity of the Langmuir model for this process (Fig. 10).
| Metal ions | E (%) | D (mL g−1) | α | αr | |||
|---|---|---|---|---|---|---|---|
| Imprinted | Non-imprinted | Imprinted | Non-imprinted | αi | αn | ||
| Pb(II) | 99.70 | 59.77 | 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213.24 213.24 |
495.18 | |||
| Zn(II) | 52.56 | 42.97 | 368.44 | 251.11 | 331.70 | 1.972 | 168.20 |
| Ni(II) | 55.90 | 49.74 | 422.78 | 329.95 | 289.07 | 1.500 | 192.71 |
| Co(II) | 56.35 | 39.75 | 430.25 | 219.92 | 284.05 | 2.252 | 126.13 |
| Cu(II) | 59.61 | 57.84 | 491.95 | 457.33 | 248.43 | 1.083 | 229.39 |
The presented method was applied to the determination of trace Pb(II) in standard materials (GBW 08301: river sediment; GBW 08303: polluted farming soil) for validation. The analytical results for the certified reference materials (Table 5) were in good agreement with the certified values.
| Analytes | Measured (μg g−1) | Certified (μg g−1) |
|---|---|---|
| GBW 08301 | 79.2 ± 6.7 | 79 ± 12.0 |
| GBW 08303 | 73.4 ± 1.3 | 73 ± 2.0 |
For the analysis of Yellow River water, Huangshui water, South Lake water and tap water samples, the standard addition method was used. The results are given in Table 6. It was found that the recoveries of analyte were in the range of 98–102%. Evidently, the obtained results indicated that the method was reliable, feasible and satisfactory for the analysis of water samples.
| Ion | Added | Found a | Recovery (%) |
|---|---|---|---|
| a The value following “±” is the standard deviation (N = 3). | |||
| Pb(II) (μg mL−1) | |||
| Yellow River water | 0 | 6.49 ± 0.22 | |
| 5 | 11.65 ± 0.05 | 101.4 | |
| 10 | 16.56 ± 0.12 | 100.6 | |
| Huangshui River | 0 | 2.07 ± 0.09 | |
| 5 | 7.13 ± 0.11 | 100.5 | |
| 10 | 12.04 ± 0.15 | 99.7 | |
| South Lake | 0 | 3.86 ± 0.08 | |
| 5 | 8.69 ± 0.07 | 98.1 | |
| 10 | 13.74 ± 0.13 | 99.1 | |
| Tap water | 0 | 4.14 ± 0.08 | |
| 5 | 9.12 ± 0.21 | 99.8 | |
| 10 | 14.33 ± 0.14 | 101.3 | |
| Sorbent | ETa (min) | FRb (mL min−1) | Q (mg g−1) | EFa | LOD (ng mL−1) | Number of cycles | RSD (%) | Selectivity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a Equilibrium time.b Flow rate.c Enrichment factor. | |||||||||
| Pb(II)-imprinted silica gel | 30 | 1 | 19.66 | 100 | 0.004 | — | <2.0 | Pb | 32 |
| Co3O4 | — | — | 35.5 | 175 | 0.72 | — | 1.5 | Pb | 33 |
| Pb(II)-imprinted polymer in nano-TiO2 matrix | 10 | — | 22.7 | — | 47 | 4 | 4.1 | Pb | 34 |
| Functionalized halloysite nanotubes | 7 | 0.5 | 23.58 | — | 0.32 | — | 3.4 | Pb | 2 |
| Diethylenetriamine-functionalized carbon nanotubes | 30 | 1.5 | 5.4 | 75 | 0.16 | — | 3 | Cr, Fe, Pb and Mn | 35 |
| A new chelating resin | — | 4 | 18.3 | 200 | 1.9 | — | — | Fe, Zn, Cu, Cr, Co and Mn | 36 |
| 4-Aminoantipyrine immobilized bentonite | — | 2 | 38.8 | 100 | 0.12 | — | 2.7 | Cr, Hg and Pb | 37 |
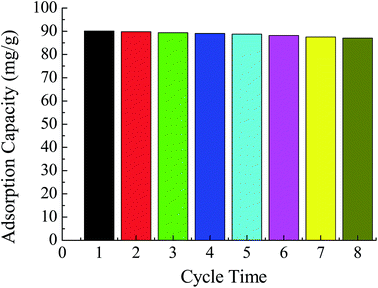
| The new surface-imprinted activated carbon sorbent for Pb(II) | 15 | 3 | 35.33 | 200 | 0.18 | 8 | 2.2 | Pb | This work |
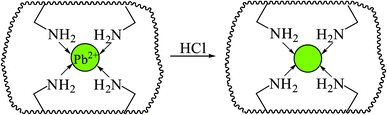
A plausible adsorption mechanism of Pb(II) on Pb(II)-IIP is shown in Fig. 12 and is proposed as follows. Nitrogen atoms of Pb(II)-IIP have a lone pair of electrons that could bind with the electron deficient Pb(II) ions via chelation. Therefore, each divalent Pb(II) ion could bind to four nitrogen atoms to form a compound with a coordination number of four. The IIP was tailored to the template Pb(II). Other metal ions could chelate with the nitrogen atoms of IIP, but they did not fit the tailored material. Therefore, IIP had a selectivity only for Pb(II). According to the selectivity experiment, the selectivity was controlled by the shape of the cavities (the size of the metal ions) and the coordination-geometry.34
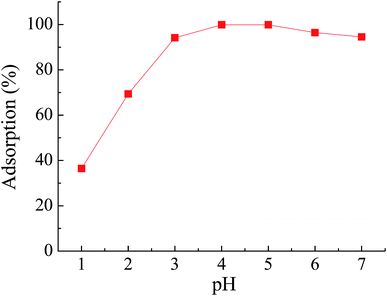
The influences of pH and the initial concentration of Pb(II) on the adsorption rate suggested that there was a competitive adsorption of Pb(II) and H+ to the nitrogen atoms. At low pH, the nitrogen atoms would be protonated, which would repel the positively charged Pb(II) ions. Therefore, the chelation performance decreased with the decreasing of pH and this is the reason why HCl could achieve effective elution in this experiment.
Conclusions
Ion-imprinted sorbents have attracted widespread attention as highly selective sorbents for the selective removal of target metal ions in the presence of other metal ions. In this study, we have firstly synthesized a new surface-modified Pb(II)-IIP, incorporating ethylenediamine as the chelating unit. The test data from FT-IR, XRD, SEM and BET proved that the synthesis of Pb(II)-IIP was successful. The prepared Pb(II)-IIP could be applied to selectively extract or separate Pb(II) ions from environmental samples containing foreign coexisting ions. The adsorption process could be described well by the pseudo-second-order model and Langmuir isotherm model. In addition, this adsorbent showed a number of good characteristics such as high chemical stability, excellent reproducibility, high adsorption capacity, high enrichment capability, high selectivity, and fast adsorption and desorption kinetics. As a conclusion, the Pb(II)-IIP prepared in this work can be considered to be a suitable and reliable sorbent for the separation and pre-concentration of Pb(II) ions in environmental water samples.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities, Northwest Minzu University (No. 31920180040; No. zyz2012065) and the National Natural Science Foundation of China (No. 31760608; No. 21762038).References
- B. Zargar and A. Khazaeifar, Microchim. Acta, 2017, 184, 4521–4529 CrossRef CAS.
- Q. He, D. Yang, X. Deng, Q. Wu, R. Li, Y. Zhai and L. Zhang, Water Res., 2013, 47, 3976–3983 CrossRef CAS PubMed.
- N. Li, H. L. Jiang, X. Wang, X. Wang, G. Xu, B. Zhang, L. Wang, R. S. Zhao and J. M. Lin, TrAC, Trends Anal. Chem., 2018, 102, 60–74 CrossRef CAS.
- C. F. Poole, TrAC, Trends Anal. Chem., 2003, 22, 362–373 CrossRef CAS.
- Y. Matsui, S. Nakao, A. Sakamoto, T. Taniguchi, L. Pan, T. Matsushita and N. Shirasaki, Water Res., 2015, 85, 95–102 CrossRef CAS PubMed.
- D. Li, X. Chang, Z. Hu, Q. Wang, Z. Tu and R. Li, Microchim. Acta, 2011, 174, 131–136 CrossRef CAS.
- M. Song, Y. Wei, S. Cai, L. Yu, Z. Zhong and B. Jin, Sci. Total Environ., 2018, 618, 1416–1422 CrossRef CAS PubMed.
- A. Macías-García, M. Gómez Corzo, M. Alfaro Domínguez, M. Alexandre Franco and J. Martínez Naharro, J. Hazard. Mater., 2017, 328, 46–55 CrossRef PubMed.
- M. Ghaedi, H. R. Noormohamadi, A. Asfaram, M. Montazerozohori and M. Soylak, J. Mol. Liq., 2016, 221, 748–754 CrossRef CAS.
- X. F. Zheng, Q. Lian and H. Yang, RSC Adv., 2014, 4, 42478–42485 RSC.
- W. Han, L. Gao, X. Li, L. Wang, Y. Yan, G. Che, B. Hu, X. Lin and M. Song, RSC Adv., 2016, 6, 81346–81353 RSC.
- J. Tan, Z. Jiang, R. Li and X. Yan, TrAC, Trends Anal. Chem., 2012, 39, 207–217 CrossRef CAS.
- H. H. Yang, S. Q. Zhang, W. Yang, X. L. Chen, Z. X. Zhang, J. G. Xu and X. R. Wang, J. Am. Chem. Soc., 2004, 126, 4054–4055 CrossRef CAS PubMed.
- K. Haupt, Analyst, 2001, 126, 747–756 RSC.
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812–1832 CrossRef CAS.
- K. Ensing and T. Boer, TrAC, Trends Anal. Chem., 1999, 18, 138–145 CrossRef CAS.
- Y. Liu, X. Chang and S. Wang, Anal. Chim. Acta, 2004, 519, 173–179 CrossRef CAS.
- J. Qian, S. Zhang, Y. Zhou, P. Dong and D. Hua, RSC Adv., 2015, 5, 4153–4161 RSC.
- F. Shakerian, K. H. Kim, E. Kwon, J. E. Szulejko, P. Kumar, S. Dadfarnia, A. Mohammad and H. Shabani, TrAC, Trends Anal. Chem., 2016, 83, 55–69 CrossRef CAS.
- H. He, Q. Gan and C. Feng, RSC Adv., 2017, 7, 15102–15111 RSC.
- J. Wang, C. Jiang, X. Wang, L. Wang, A. Chen, J. Hu and Z. Luo, Analyst, 2016, 141, 5886–5892 RSC.
- R. Garcia, C. Pinel, C. Madic and M. Lemaire, Tetrahedron Lett., 1998, 39, 8651–8654 CrossRef CAS.
- H. T. Tang, Organic Compound Spectra Determination, Publishing House of Bei-jing University, Beijing, 1992, pp. 124–59 Search PubMed.
- Q. N. Dong, IR Spectrum Method, Publishing House of the Chemical Industry, Beijing, 1979, pp. 104–168 Search PubMed.
- Y. Liu, L. H. Zhu, Y. Y. Zhang and H. Q. Tang, Sens. Actuators, B, 2012, 171–172, 1151–1158 CrossRef CAS.
- H. Meng, Z. Li, F. Ma, X. Wang, W. Zhou and L. Zhang, RSC Adv., 2015, 5, 67662–67668 RSC.
- P. Yuan, P. D. Southon, Z. W. Liu, M. E. R. Green, J. M. Hook, S. J. Antill and C. J. Kepert, J. Phys. Chem. C, 2008, 112, 15742–15751 CrossRef CAS.
- J. J. Wang and X. Li, Ind. Eng. Chem. Res., 2013, 52, 572–577 CrossRef CAS.
- G. Z. Fang, J. Tan and X. P. Yan, Anal. Chem., 2005, 77, 1734–1739 CrossRef CAS PubMed.
- A. Maquieira, H. Elmahadi and R. Puchades, Anal. Chem., 1994, 66, 3632–3638 CrossRef CAS.
- R. S. A. Machado, J. M. G. Fonseca, L. N. H. Arakaki, J. G. P. Espinola and S. F. Oliveira, Talanta, 2004, 63, 317–322 CrossRef CAS PubMed.
- X. Zhu, Y. Cui, X. Chang, X. Zou and Z. Li, Microchim. Acta, 2009, 164, 125–132 CrossRef CAS.
- E. Yavuz, Ş. Tokalıoğlu, H. Şahan and Ş. Patat, Talanta, 2013, 115, 724–729 CrossRef CAS PubMed.
- C. Li, J. Gao, J. Pan, Z. Zhang and Y. Yan, J. Environ. Sci., 2009, 21, 1722–1729 CrossRef CAS.
- X. Zhu, Y. Cui, X. Chang and H. Wang, Talanta, 2016, 146, 58–363 Search PubMed.
- Ş. T. Daşbaş, N. Çankaya and C. Soykan, Food Chem., 2016, 211, 68–73 CrossRef PubMed.
- Q. Wang, X. Chang, D. Li, Z. Hu, R. Li and Q. He, J. Hazard. Mater., 2011, 186, 1076–1081 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |