Open Access Article
Open Access ArticleCopper-catalyzed cross-dehydrogenative coupling between quinazoline-3-oxides and indoles†
Qin Yang,
Zhijian Yin,
Lifang Zheng,
Jianjun Yuan,
Song Wei,
Qiuping Ding * and
Yiyuan Peng
* and
Yiyuan Peng *
*
Key Laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Province's Key Laboratory of Green Chemistry, Jiangxi Normal University, Nanchang, Jiangxi 330022, China. E-mail: yypeng@jxnu.edu.cn; dqpjxnu@gmail.com
First published on 18th February 2019
Abstract
A novel and simple protocol for the synthesis of 4-(indole-3-yl)quinazolines via cross-dehydrogenative coupling of quinazoline-3-oxides and indoles under an air atmosphere has been developed. A series of biheteroaryl products were obtained in moderate to good yields.
Introduction
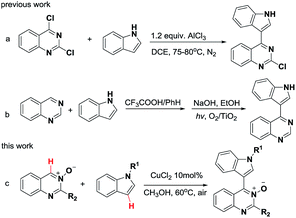
The nitrogen-containing heterocycles occupy an important position in pharmaceuticals and natural products.1 Futhermore, they exhibit remarkable biological activities, such as anticancer, antileukemic, antiviral, and antifungal properties.2 The complexity and diversity of N-heterocycles have been employed broadly in the studies of advanced materials and ligands for transition metal catalysis.3 Among reported studies, quinazoline4 and indole5 derivatives are useful nitrogen-containing heterocyclic compounds, and are core structural motifs of many natural products and pharmaceuticals. Thus heterocyclic compounds bearing these two skeletons most likely possess interesting biological and physicochemical properties.6 For example, indoloquinazoline derivatives have been reported to be protein kinase CK2 inhibitors6a and poly(ADP-ribose) polymerase-1 (PARP-1) inhibitors.6b 4-(Indole-3-yl)quinazolines have been reported to be potent epidermal growth factor receptor tyrosine kinase inhibitors.6cOver the past decade, substantial effort has been devoted to the development of reaction conditions for the coupling between quinazoline and indole compounds.7 One elegant methodology relies on the nucleophilic substitution of quinazoline chlorides or their analogous with indoles. However, this method cannot be widely employed due to the need for harsh reaction conditions and/or operationally complex protocols. For instance, the substitution reaction between 2,4-dichloroquinazolines and indoles relies on 1.2 equiv. of AlCl3 (Scheme 1a).7a Additionally, the reaction of quinazoline with indole required Brønsted acid mediator, followed by photocatalyzed aromatization to give the corresponding 4-(indol-3-yl)-quinazoline product in 70% yield (Scheme 1b).7d
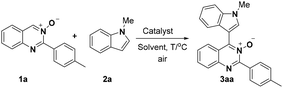
On the other hand, we have noticed that cross-dehydrogenative-coupling (CDC) reactions8 have emerged as an excellent alternative for the formation of C–C bonds. Furthermore, the N-oxide moiety in aza-heteroarene compounds has been recognized as a powerful and removable directing group for ortho C–H bond activation.9 In the past decade, pyridine N-oxides,10 pyrimidine N-oxides,11 quinoline N-oxides and isoquinoline N-oxides12 have been extensively employed as useful building block for the preparation of a diverse range of N-heterocycles. However, the C4–H functionalization of quinazoline N-oxides remains rare.13 Therefore, the development of a general and practical strategy for the synthesis of 4-(indole-3-yl)quinazolines via CDC reactions of quinazoline N-oxides and indoles under mild conditions, is highly desired. Recently, our group has directed its studies to that of the structural elaboration of quinazoline core, with the aim of constructing a quinazoline-based molecular library for bioactivity assays.14 Herein, we report a copper-catalyzed CDC reaction between the Csp2–H of quinazoline-3-oxide and the Csp2–H of various indoles for the synthesis of biheteroaryl structures using a mild and operationally simple procedure (Scheme 1c).
Results and discussion
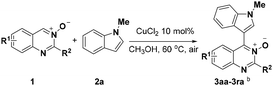
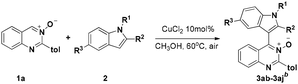
We initiated our investigation on the model reaction of quinazoline-3-oxide (1a) with N-methyl-indole (2a) in order to optimize the reaction parameters (Table 1). First, the reaction was performed in the presence of 20 mol% CuCl2 at 60 °C in toluene (Entry 1). To our delight, the desired product 3a was isolated in a moderate yield of 47%. Encouraged by this preliminary result, we then screened various solvents. The desired product was obtained in moderate or low yields when the reaction was carried out in THF, CH3CN and DMF (Entries 2–4). Further studies showed that CH3OH was the best solvent for this transformation, affording the desired product 3a in 77% yield (Entry 7). Incrementally reducing the catalyst loading from 20 to 5 mol%, showed that 10 mol% catalyst was the optimal amount (Entries 7–9). No transformation took place in the absence of metal catalyst in CH3OH (Entry 10). Next, further screening of other metal salts was conducted, but no better conditions were discovered (Entries 11–15). Several copper salts were utilized to promote the reaction (Entries 16–20); however, it was found that copper(II) chloride was the most effective. No better result was obtained even at a higher or lower reaction temperature (Entries 21 and 22).| Entry | Solvent | Catalyst | T/°C | Yieldc/% |
|---|---|---|---|---|
| a Optimized conditions are denoted in bold.b Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), catalyst, solvent (2.0 mL), 16 h, under air.c Isolated yield. | ||||
| 1 | Toluene | CuCl2 (20 mol%) | 60 | 47 |
| 2 | THF | CuCl2 (20 mol%) | 60 | 57 |
| 3 | CH3CN | CuCl2 (20 mol%) | 60 | 40 |
| 4 | DMF | CuCl2 (20 mol%) | 60 | 32 |
| 5 | PhCl | CuCl2 (20 mol%) | 60 | 70 |
| 6 | DCE | CuCl2 (20 mol%) | 60 | 75 |
| 7 | CH3OH | CuCl2 (20 mol%) | 60 | 77 |
| 8 | CH3OH | CuCl2 (10 mol%) | 60 | 78 |
| 9 | CH3OH | CuCl2 (5 mol%) | 60 | 67 |
| 10 | CH3OH | — | 60 | ND |
| 11 | CH3OH | In(OTf)3 (10 mol%) | 60 | Trace |
| 12 | CH3OH | FeCl2 (10 mol%) | 60 | 37 |
| 13 | CH3OH | FeCl3 (10 mol%) | 60 | 40 |
| 14 | CH3OH | CoCl2 (10 mol%) | 60 | 20 |
| 15 | CH3OH | Ni(OTf)2 (10 mol%) | 60 | 17 |
| 16 | CH3OH | Cu(OTf)2 (10 mol%) | 60 | 73 |
| 17 | CH3OH | Cu(acac)2 (10 mol%) | 60 | Trace |
| 18 | CH3OH | CuCl (10 mol%) | 60 | 62 |
| 19 | CH3OH | CuBr (10 mol%) | 60 | 73 |
| 20 | CH3OH | Cu(OAc)2 (10 mol%) | 60 | 40 |
| 21 | CH3OH | CuCl2 (10 mol%) | 80 | 53 |
| 22 | CH3OH | CuCl2 (10 mol%) | 40 | 50 |
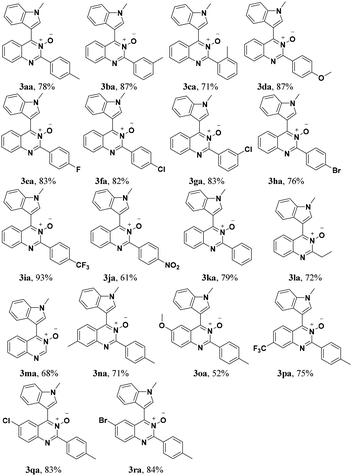
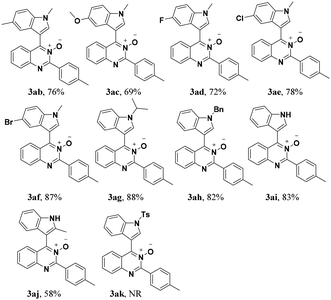
Under the optimized reaction conditions, the reaction scope was explored, and the results of which are shown in Table 2. The CDC reaction of quinazoline-3-oxides 1 and N-methylindole 2a occurred smoothly to generate the desired products 3 in good yields. When R2 was an aryl group, both electron-donating and electron-withdrawing substituents on the aryl ring had a slight effect on the reaction (3aa–3ka), albeit strongly electron-withdrawing substituents, such as the nitro group, greatly retarded the reaction, forming 3ja in 61% yield. It was observed that an R2 aliphatic substituent was also tolerated to give the corresponding product 3la in 72% yield. When the parent quinazoline ring (R2 = H) was used, the desired product 3ma was obtained in 68% yield. Subsequently, R1 substituents on the phenyl ring moiety of the quinazoline 3-oxide were investigated. Methyl, methoxy, trifluoromethyl, chloro and bromo functionalities were all tolerated, providing the desired products 3na–3ra in 52–84% yields.
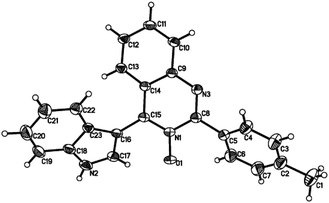
Subsequently, the indole scope was examined under the optimized reaction conditions. As shown in Table 3, quinazoline-3-oxide underwent smooth coupling with a variety of substituted indoles. N-Methylindoles possessing alkyl, halide, and alkoxy substituents at the 5-position are amenable to the reaction conditions (3ab–3af), and the expected products were isolated in moderate to good yields. The coupled products were isolated in high yields for N-iPr and N-Bn substrates (3ag and 3ah). To our surprise, indole itself also proved to be a good coupling partner, providing the corresponding product (3ai) in 83% yield. Single-crystal X-ray analysis of 3ai confirmed its structure and demonstrated the high regioselectivity of the reaction (Fig. 1). In contrast, no desired coupling product was observed for 3-methylindole, which is consistent with selectivity for the C–H activation at the 3-position of the indole (this result is not shown in Table 3). When a CH3 group is present at the 2-position of the indole, the steric hindrance arising from this substituent influences the reaction; the cross-coupling product 3aj was obtained in lower yield. However, indoles containing electron-withdrawing N-protecting groups, for example, N-tosyl-indole, were not amenable to this transformation, and none of the desired product (3ak) was isolated.
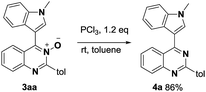
The coupled quinazoline-3-oxides products were easily deoxygenated to give the corresponding 4-(indole-3-yl)quinazolines. For example, when 3aa was treated with PCl3 (rt, 30 min), clean reduction occurred and 4a was obtained in 86% yield (Scheme 2).15 The combination of the described C–H/C–H cross-dehydrogenative-coupling and subsequent reduction provides an attractive and simple procedure for the synthesis of indole-functionalized quinazoline derivatives.
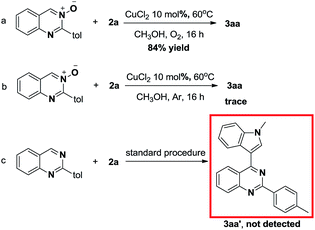
In order to further understand the mechanism of this copper-catalyzed reaction of quinazoline-3-oxide 1 with indole 2, three control experiments were conducted (Scheme 3). The desired product was obtained in 84% yield when the reaction was conducted under oxygen atmosphere (Scheme 3a). However, only a trace amount of product 3aa was obtained when the reaction was carried out under argon, which indicates that oxygen plays a crucial role in this transformation (Scheme 3b). When 1a was replaced by 2-(p-tolyl)quinazoline, the desired product was not obtained (Scheme 3c).
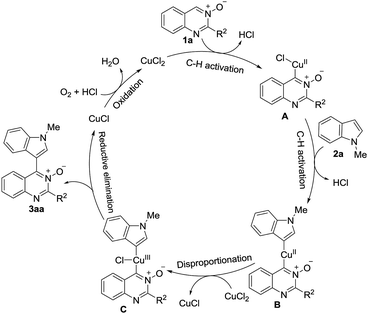
On the basis of these observations and related reports,16 we have proposed a plausible mechanism for this reaction, as shown in Scheme 4. The CuCl2 reacts with quinazoline-3-oxide 1a to form intermediate A via C–H activation of 1a. Next, A undergoes an insertion into the 3-position of the C–H bond of 2a to afford B. Oxidation of B to Cu(III) complex C occurs via disproportionation with a second equivalent of CuCl2, liberating CuCl.17 C undergoes reductive elimination to give the product 3aa together with Cu(I), which is reoxidized to CuCl2 by O2 and HCl, to complete the cycle.
In conclusion, a novel, simple and efficient protocol for the synthesis of 4-(indole-3-yl)quinazolines via a cross-dehydrogenative coupling of quinazoline-3-oxides and indoles under an air atmosphere has been successfully developed. A series of biheteroaryl products were obtained in moderate to good yields. The unique reactivity and selectivity observed in the CDC reaction prompted us to initiate further studies on the reaction mechanism.
Experimental section
Unless otherwise stated, all commercial reagents were used as received. All solvents were dried and distilled according to standard procedures. Flash column chromatography was performed using silica gel (60 Å pore size, 32–63 mm, standard grade). Analytical thin-layer chromatography was performed using glass plates pre-coated with 0.25 mm 230–400 mesh silica gel impregnated with a fluorescent indicator (254 nm). Thin layer chromatography plates were visualized by exposure to ultraviolet light. Organic solutions were concentrated on rotary evaporators at ∼20 torr at 25–35 °C. Nuclear magnetic resonance (NMR) spectra are recorded in parts per million from internal tetramethylsilane on the δ scale. 1H and 13C NMR spectra were recorded in CDCl3 on a Bruker DRX-400 spectrometer operating at 400 MHz and 100 MHz, respectively. All chemical shift values are quoted in ppm and coupling constants quoted in Hz.General experimental procedure for synthesis of 3
The quinazoline-3-oxide (0.2 mmol), indole (0.3 mmol), CuCl2 (0.02 mmol) and 2.0 mL CH3OH were mixed in a dry reaction tube. The mixture was stirred at 60 °C under air for 12–16 hours. After completion of the reaction (monitored by TLC), the mixture was concentrated in vacuum and the residue was purified by flash column chromatography on silica gel with petroleum ether–ethyl acetate as eluent to give the desired product.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from National Natural Science Foundation of China (no. 21762020 and 21362014), Jiangxi Provincial Department of Science and Technology (no. 20171BAB203006), and Key Laboratory of Functional Small Organic Molecule, Ministry of Education, Jiangxi Normal University (no. KLFS-KF-201623) is gratefully acknowledged.Notes and references
- (a) A. F. Pozharskii, A. T. Soldartenkov, and A. R. Katrizky, Heterocycles in Life and Society, John Wiley & Sons, Weinheim, 2nd edn, 2011 CrossRef; (b) W. R. Pitt, D. M. Parry, B. G. Perry and C. R. Groom, J. Med. Chem., 2009, 52, 2952 CrossRef CAS PubMed; (c) S. Bongarzone and M. L. Bolognesi, Expert Opin. Drug Discovery, 2011, 6, 251 CrossRef CAS PubMed; (d) J. Polanski, A. Kurczyk, A. Bak and R. Musiol, Curr. Med. Chem., 2012, 19, 1921 CrossRef CAS PubMed.
- (a) P. Y. Chung, Z. X. Bian, H. Y. Pun, D. Chan, A. S. C. Chan, C. H. Chui and K. H. Lam, Future Med. Chem., 2015, 7, 947 CrossRef CAS PubMed; (b) A. M. Mfuh and O. V. Larionov, Curr. Med. Chem., 2015, 22, 2819 CrossRef CAS PubMed.
- (a) G. Hughes and M. R. Bryce, J. Mater. Chem., 2005, 15, 94 RSC; (b) A. Kimyonok, X. Y. Wang and M. Weck, J. Macromol. Sci., Polym. Rev., 2006, 46, 47 CrossRef CAS.
- For selected reviews, see: (a) D. Connolly, D. Cusack, T. O'Sullivan and P. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef CAS; (b) J. Michael, Nat. Prod. Rep., 2007, 24, 223 RSC; (c) S. Mhaske and N. Argade, Tetrahedron, 2006, 62, 9787 CrossRef CAS.
- (a) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (b) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (c) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed.
- (a) E. Vangrevelinghe, K. Zimmermann, J. Schoepfer, R. Portmann, D. Fabbro and P. Furet, J. Med. Chem., 2003, 46, 2656 CrossRef CAS PubMed; (b) D. V. Ferraris, J. Med. Chem., 2010, 53, 4561 CrossRef CAS PubMed; (c) A. Lüth and W. Löwe, Eur. J. Med. Chem., 2008, 43, 1478 CrossRef PubMed; (d) Y. Rao, H. Liu, L. Gao, H. Yu, J. H. Tan, T. M. Ou, S. L. Huang, L. Q. Gu, J. M. Ye and Z. S. Huang, Bioorg. Med. Chem., 2015, 23, 4719 CrossRef CAS PubMed.
- (a) S. Kumar and D. P. Sahu, J. Heterocycl. Chem., 2009, 46, 748 CrossRef CAS; (b) Y. A. Azev, S. V. Shorshnev and B. V. Golomolzin, Tetrahedron Lett., 2009, 50, 2899 CrossRef CAS; (c) M. Staderini, M. L. Bolognesi and J. C. Menéndez, Adv. Synth. Catal., 2015, 357, 185 CrossRef CAS; (d) I. A. Utepova, M. A. Trestsova, O. N. Chupakhin, V. N. Charushin and A. A. Rempel, Green Chem., 2015, 17, 4401 RSC; (e) O. N. Chupakhin, A. V. Shchepochkin and V. N. Charushin, Green Chem., 2017, 19, 2931 RSC.
- (a) C.-J. LI, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef CAS PubMed; (c) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Rev., 2011, 111, 1293 CrossRef CAS PubMed; (d) Y. Yang, J. Lan and J. You, Chem. Rev., 2017, 117, 8787 CrossRef CAS PubMed; (e) W. Hu and Y. Long, Chin. J. Org. Chem., 2017, 37, 2850 CrossRef CAS.
- For recent reviews: (a) G. Yan, A. J. Borah and M. Yang, Adv. Synth. Catal., 2014, 356, 2375 CrossRef CAS; (b) L. Yali, Z. Yunhui, F. Hongyan and J. Xinhua, Chin. J. Org. Chem., 2013, 33, 267 CrossRef; (c) W. Ma, P. Gandeepan, J. Li and L. Ackermann, Org. Chem. Front., 2017, 4, 1435 RSC.
- (a) X. Gong, G. Song, H. Zhang and X. Li, Org. Lett., 2011, 13, 1766 CrossRef CAS PubMed; (b) M. Li, X. Li, H. Chang, W. Gao and W. Wei, Org. Biomol. Chem., 2016, 14, 2421 RSC; (c) A. Lehecq, K. Rousée, C. Schneider, V. Levacher, C. Hoarau, X. Pannecoucke and S. Couve-Bonnaire, Eur. J. Org. Chem., 2017, 21, 3049 CrossRef.
- (a) J. P. Leclerc and K. Fagnou, Angew. Chem., Int. Ed., 2006, 45, 7781 CrossRef CAS PubMed; (b) D. J. Schipper, M. El-salfiti, C. J. Whipp and K. Fagnou, Tetrahedron, 2009, 65, 4977 CrossRef CAS; (c) J. M. Keith, J. Org. Chem., 2010, 75, 2722 CrossRef CAS PubMed; (d) J. M. Keith, J. Org. Chem., 2012, 77, 11313 CrossRef CAS PubMed; (e) L. A. Galliamova, M. V. Varaksin, O. N. Chupakhin, P. A. Slepukhin and V. N. Charushin, Organometallics, 2015, 34, 5285 CrossRef CAS; (f) A. P. Colleville, R. A. Horan, S. Olazabal and N. C. Tomkinson, Org. Process Res. Dev., 2016, 20, 1283 CrossRef CAS; (g) F. Roudesly, L. F. Veiros, J. Oble and G. Poli, Org. Lett., 2018, 20, 2346 CrossRef CAS PubMed.
- (a) T. Nishida, H. Ida, Y. Kuninobu and M. Kanai, Nat. Commun., 2014, 5, 3387 CrossRef PubMed; (b) Y. Kuninobu, M. Nagase and M. Kanai, Angew. Chem., Int. Ed., 2015, 54, 10263 CrossRef CAS PubMed; (c) X. Y. Zhang, Z. S. Qi and X. W. Li, Angew. Chem., Int. Ed., 2014, 53, 10794 CrossRef CAS PubMed; (d) H. Hwang, J. Kim, J. Jeong and S. Chang, J. Am. Chem. Soc., 2014, 136, 10770 CrossRef CAS PubMed; (e) Q. Xiao, J. Sheng, Q. Ding and J. Wu, Eur. J. Org. Chem., 2014, 1, 217 CrossRef; (f) K. Shin, S. W. Park and S. Chang, J. Am. Chem. Soc., 2015, 137, 8584 CrossRef CAS PubMed; (g) X. Chen, X. Cui and Y. Wu, Org. Lett., 2016, 18, 3722 CrossRef CAS PubMed; (h) D. E. Stephens, J. Lakey-Beitia, A. C. Atesin, T. A. Ateşin, G. Chavez, H. D. Arman and O. V. Larionov, ACS Catal., 2015, 5, 167 CrossRef CAS; (i) B. Yao, C. L. Deng, Y. Liu, R. Y. Tang, X. G. Zhang and J. H. Li, Chem. Commun., 2015, 51, 4097 RSC; (j) N. Barsu, M. Sen, J. R. Premkumar and B. Sundararaju, Chem. Commun., 2016, 52, 1338 RSC; (k) D. Y. Li, Z. L. Huang and P. N. Liu, Org. Lett., 2018, 20, 2028 CrossRef CAS PubMed.
- (a) L. Fan, T. Wang, Y. Tian, F. Xiong, S. Wu, Q. Liang and J. Zhao, Chem. Commun., 2016, 52, 5375 RSC; (b) Q. Yang, M. Lou, Z. Yin, Z. Deng, Q. Ding and Y. Peng, Org. Biomol. Chem., 2018, 16, 8724 RSC.
- (a) Y. Y. Peng, G. Y. S. Qiu, Q. Yang, J. J. Yuan and Z. H. Deng, Synthesis, 2012, 44, 1237 CrossRef CAS; (b) G. Y. S. Qiu, P. Huang, Q. Yang, H. Lu, J. Xu, Z. Deng, M. Zhang and Y. Y. Peng, Synthesis, 2013, 45, 3131 CrossRef CAS; (c) X. Chen, Q. Yang, Y. R. Zhou, Z. H. Deng, X. C. Mao and Y. Y. Peng, Synthesis, 2015, 47, 2055 CrossRef CAS; (d) X. Zhao, Y. Zhou, Y. Xie, Q. Ding, Z. Deng, M. Zhang, J. Xu and Y. Y. Peng, Synthesis, 2013, 45, 3245 CrossRef CAS; (e) Y. Y. Peng, P. Huang, Y. Wang, Y. R. Zhou, J. J. Yuan, Q. Yang, X. Jiang, Z. Deng and J. S. Xu, Org. Biomol. Chem., 2014, 12, 5922 RSC; (f) C. Zhang, Y. Zhou, Z. Deng, X. Chen and Y. Y. Peng, Eur. J. Org. Chem., 2015, 8, 1735 CrossRef; (g) X. L. Ye, Z. H. Yuan, Y. R. Zhou, Q. Yang, Y. P. Xie, Z. H. Deng and Y. Y. Peng, J. Heterocycl. Chem., 2016, 53, 1956 CrossRef CAS; (h) X. L. Ye, J. J. Yuan, Y. R. Zhou, Z. H. Deng, X. C. Mao and Y. Y. Peng, Synthesis, 2016, 48, 3976 CrossRef CAS; (i) M. Lou, Z. Deng, X. Mao, Y. Fu, Q. Yang and Y. Peng, Org. Biomol. Chem., 2018, 16, 1851 RSC.
- S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS PubMed.
- (a) M. Kitahara, N. Umeda, K. Hirano, T. Satoh and M. Miura, J. Am. Chem. Soc., 2011, 133, 2160 CrossRef CAS PubMed; (b) S. Fan, Z. Chen and X. Zhang, Org. Lett., 2012, 14, 4950 CrossRef CAS PubMed; (c) R. Odani, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2013, 78, 11045 CrossRef CAS PubMed.
- (a) A. E. King, T. C. Brunold and S. S. Stahl, J. Am. Chem. Soc., 2009, 131, 5044 CrossRef CAS PubMed; (b) A. M. Suess, M. Z. Ertem, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 9797 CrossRef CAS PubMed; (c) J. C. Vantourout, H. N. Miras, A. Isidro-Llobet, S. Sproules and A. J. Watson, J. Am. Chem. Soc., 2017, 139, 4769 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, characterization data, 1H and 13C NMR spectra of compounds 3. CCDC 1813925 (compound 3ai). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra09864f |
| This journal is © The Royal Society of Chemistry 2019 |