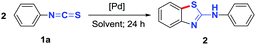Open Access Article
Open Access ArticleDMSO-mediated palladium-catalyzed cyclization of two isothiocyanates via C–H sulfurization: a new route to 2-aminobenzothiazoles†
Guangkai Yaoac,
Bing-Feng Wangab,
Shuai Yangac,
Zhi-Xiang Zhangac,
Han-Hong Xu *ac and
Ri-Yuan Tang
*ac and
Ri-Yuan Tang *ab
*ab
aKey Laboratory of Natural Pesticide and Chemical Biology, Ministry of Education, South China Agricultural University, Guangzhou 510642, China. E-mail: hhxu@scau.edu.cn; rytang@scau.edu.cn
bDepartment of Applied Chemistry, College of Materials and Energy, South China Agricultural University, Guangzhou 510642, China
cState Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, South China Agricultural University, Guangzhou 510642, China
First published on 25th January 2019
Abstract
DMSO was found to activate arylisothiocyanates for self-nucleophilic addition. A subsequent intramolecular C–H sulfurization catalyzed by PdBr2 enables access to a wide range of 2-aminobenzothiazole derivatives in moderate to good yields. This is the first example of a DMSO-mediated Pd-catalyzed synthesis of 2-aminobenzothiazoles through cyclization/C–H sulfurization of two isothiocyanates.
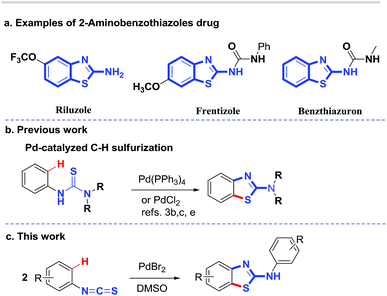
2-Aminobenzothiazole derivatives are privileged scaffolds which have been widely used in medicine and agricultural chemicals (Scheme 1a).1 Significant effort has been devoted to the development of synthetic methodologies for the preparation of such species.2 Thioureas,3,4 isothiocyanates,5,6 and benzothiazoles7 are effective reaction partners for the synthesis of 2-aminobenzothiazole derivatives via cross-coupling reactions or C–H functionalization. Intramolecular C–H sulfurization of N-arylthioureas is an effective strategy for the synthesis of 2-aminobenzothiazoles. Such a transformation can be achieved through metal-catalyzed C–H activation3 or via radical reactions.6 For example, Doi and coworkers reported a Pd-catalyzed intramolecular C–H sulfurization of arylthiourea using O2 as an oxidant.3c Metal-free iodine-catalyzed intramolecular C–H sulfurization also provides an effective pathway to 2-aminobenzothiazoles.6e Zhu et al. developed an efficient oxidative radical strategy for the synthesis of 2-aminobenzothiazoles via an aminyl radical addition to aryl isothiocyanates followed by C–H sulfurization in the presence of n-Bu4NI and TBHP.6c Interestingly, Lei et al. reported a “green” synthesis of 2-aminobenzothiazoles by employing electro-catalysis technology for an external oxidant-free intramolecular C–H sulfurization.6d In terms of metal-catalyzed C–H sulfurization,8 palladium-catalyzed C–H sulfurization has not been widely reported because thioureas are often strong metal-chelating species that may lead to poisoning of palladium catalysts. Thus, only a few examples of Pd-catalyzed protocols have been reported (Scheme 1b).3b,c,e Therefore, the development of novel palladium-catalyzed C–H sulfurization reaction is of great interest. As a continuation of our interest in sulfur chemistry and C–H sulfurization,9 we attempted to transform isothiocyanates into sulfur-containing compounds.10 Unexpectedly, a newly formed 2-aminobenzothiazole was observed for the reaction of a phenylisothiocyanate. Encouraged by this discovery, we developed a new, facile and efficient DMSO-mediated palladium-catalyzed C–H sulfurization/cyclization of two isothiocyanates for the synthesis of 2-aminobenzothiazoles (Scheme 1c).
Our study began with the reaction of phenyl isothiocyanate (1a) with PdCl2 in different solvents (Table 1). Solvents including toluene, chlorobenzene, DCE, CH3CN, DMF, NMP (N-methyl-2-pyrrolidinone), and DMSO, were examined (Table 1, entries 1–7). Only highly polar solvents, such as DMF, NMP, and DMSO were suitable for this reaction; however, DMF and NMP gave low yields of the desired product (entries 5 and 6). DMSO was found to be the preferable solvent for the reaction giving the desired product 2 in 72% yield in the presence of 10 mol% PdCl2 at 80 °C over 24 hours (entry 7). Increasing or decreasing the reaction temperature led to lower yields (e.g. 32% at 100 °C and 58% at 60 °C) (entries 8 and 9). Next, other palladium catalysts, such as PdBr2, Pd(OAc)2, and Pd(PPh3)2Cl2, were evaluated (entries 10–12). Compared with PdCl2, PdBr2 was more effective affording product 2 in 82% yield (entry 10). However, Pd(OAc)2 and Pd(PPh3)2Cl2 were not suitable catalysts for this reaction (entries 11 and 12). The yield of the desired product decreased to 70% when the reaction was conducted in the presence of 5 mol% PdBr2 (entry 13). The reaction did not proceed in the absence of PdBr2 (entry 14). When the reaction was carried out in a mixed DMSO/CH3CN solvent system, lower yields of product were obtained (entries 15 and 16). The influence of water was also examined. The addition of water facilitated the formation of thiourea, but reduced the yield of target product (entries 17 and 18).
| Entry | Catalyst | Solvent | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1a (0.4 mmol), [Pd] (10 mol%), stirred at 80 °C in solvent (2 mL) for 24 h.b Isolated yield.c At 100 °C.d At 60 °C.e PdBr2 (5 mol%).f DMSO/CH3CN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.g DMSO/CH3CN = 1 1.g DMSO/CH3CN = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.h DMSO/H2O = 10 4.h DMSO/H2O = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.i DMSO/H2O = 20 1.i DMSO/H2O = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. |
|||
| 1 | PdCl2 | Toluene | 0 |
| 2 | PdCl2 | Chlorobenzene | 0 |
| 3 | PdCl2 | DCE | 0 |
| 4 | PdCl2 | CH3CN | 0 |
| 5 | PdCl2 | DMF | 12 |
| 6 | PdCl2 | NMP | 16 |
| 7 | PdCl2 | DMSO | 72 |
| 8c | PdCl2 | DMSO | 32 |
| 9d | PdCl2 | DMSO | 58 |
| 10 | PdBr2 | DMSO | 82 |
| 11 | Pd(OAc)2 | DMSO | Trace |
| 12 | Pd(PPh3)2Cl2 | DMSO | Trace |
| 13e | PdBr2 | DMSO | 70 |
| 14 | — | DMSO | 0 |
| 15f | PdBr2 | DMSO/CH3CN | 55 |
| 16g | PdBr2 | DMSO/CH3CN | 23 |
| 17h | PdBr2 | DMSO/H2O | 61 |
| 18i | PdBr2 | DMSO/H2O | 75 |
With the optimized reaction conditions in hand, the reaction scope was investigated (Table 2). Initially, 4-substituted arylisothiocyanates were subjected to the reaction conditions. Substituents, including methyl, ethyl, butyl, t-butyl, methoxy, ethoxyl, bromo, and iodo groups, were all tolerated. Electron-donating substituents promoted the reaction, affording their corresponding products 2–8 in moderate to good yields. Although both the C–Br and C–I bonds are reactive units for cross-coupling reactions, the C–H sulfurization was selectively carried out to afford products 9 and 10 in 38% and 34% yields, respectively. However, the electron-withdrawing cyano group was not amenable to the reaction conditions (products 11). This suggests that the reaction may involve an electrophilic cyclopalladation process and the electron-withdrawing group would thus decrease the reactivity.
| a Reaction conditions: 1 (0.4 mmol), PdBr2 (10 mol%), stirred at 80 °C in DMSO (2 mL) for 24 h. |
|---|
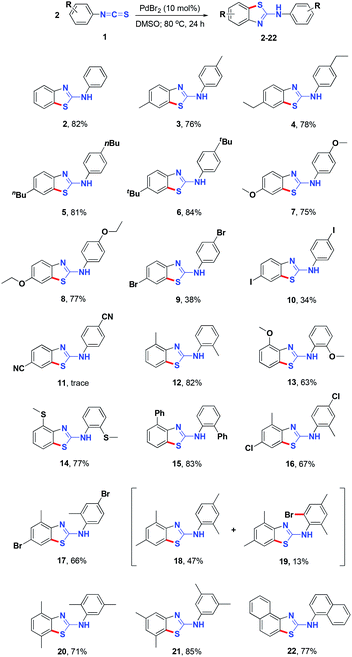 |
Ortho-substituents, such as methyl, methoxy, methylthio, and phenyl, which increase steric hindrance, still performed well to give their corresponding products in moderate to good yields (products 12–20). For reactions involving ortho-substituted substrates, a small amount of brominated by-product similar to compound 19 was observed; however, the majority of these by-products were obtained in far lower yield than 19. To our delight, a naphthyl isothiocyanate also underwent reaction smoothly to give product 22 in 77% yield. 3-Isothiocyanatopyridine was also subjected to the reaction conditions. However, no cyclization/C–H sulfurization occurred; the electron-deficient pyridine ring is not reactive enough for C–H sulfurization. The reaction of meta-substituted methyl phenylisothiocyanates provided two isomers which could not be easily purified.
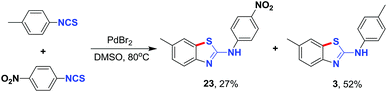
To prove the feasibility of the cyclization of two different aryl isothiocyanates, a cross-reaction between 4-methyl-phenylisothiocyanate and 4-nitro-phenyliso-thiocyanate was conducted. The cross-reaction product 23, was obtained in 27% yield with compound 3 being formed as the main product in 52% yield (Scheme 2). The strong electron-withdrawing nitro group greatly reduces the electron density of the benzene ring, thus C–H sulfurization is disfavored; C–H sulfurization only occurred on the methyl-substituted benzene ring.
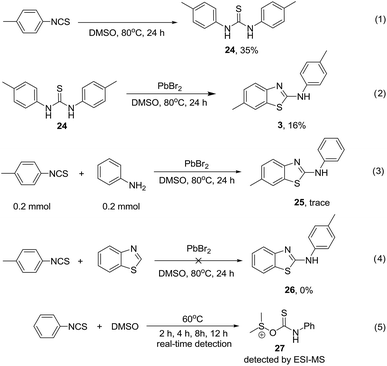
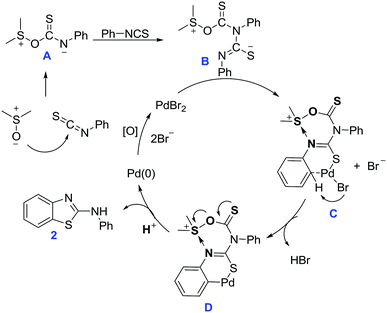
To gain insight into the reaction mechanism, control experiments were conducted, as shown in Scheme 3. In the absence of PdBr2, thiourea 24 was obtained in 35% yield for the reaction of 4-methyl phenylisothiocyanate in DMSO at 80 °C over 24 hours (Scheme 3, eqn (1)). This suggested that the thiourea may be the reaction intermediate; however, when thiourea 24 was subjected to reaction with PdBr2, product 3 was obtained in only 16% yield (Scheme 3, eqn (2)). Aryl isothiocyanate may decompose to aniline, and react with isothiocyanate for C–H sulfurization. However, only a trace amount of product 25 was observed in the reaction between 4-methylphenyl isothiocyanate and aniline (Scheme 3, eqn (3)). None of the target product 26 was observed for the reaction of benzothiazole with 4-methyl phenylisothiocyanate, suggesting that benzothiazole is not a reaction intermediate (Scheme 3, eqn (4)). To our delight, a mass peak corresponding to intermediate 27 was observed with real-time ESI-MS when phenylisothiocyanate was stirred in DMSO at 60 °C (Scheme 3, eqn (5)) (see the ESI, Fig. S1†). Based on theoretical calculations,11 the dipole moment of the DMSO molecule is 3.9274 debye (gas) or 5.1281 debye in solvent form. Natural bond orbital analysis shows the differences in the charge distribution on the O and S atoms. Obviously, the polarity of the S–O bond increases and a greater distribution of the negative charge on the O atom leads to an improvement in its nucleophilic character. The oxygen atom attacks the carbon atom with the most positive NBO charge in PhNCS (see the ESI, Fig. S2 and S3†). These results indicate that DMSO acts as a nucleophilic agent for the activation of arylisothiocyanates for C–H sulfurization.
Based on the above results and previous work,3 a possible reaction mechanism has been proposed and is shown in Scheme 4. Firstly, the polarized DMSO undergoes a nucleophilic addition with phenylisothiocyanate to afford the active intermediate A, which then reacts with another equivalent of phenylisothiocyanate to produce an intermediate B. Intermediate B chelates with PdBr2 to form an intermediate C. Subsequent elimination of HBr leads to the formation of intermediate D, which undergoes reductive elimination to afford the desired product 2. The Pd(0) is oxidized by DMSO to PdBr2 for the next catalytic cycle.
Conclusions
In summary, a DMSO-mediated PdBr2-catalyzed synthesis of 2-aminobenzothiozoles via the cyclization/C–H sulfurization of two aryl isothiocyanates has been developed for the first time. In the presence of DMSO and PdBr2, a variety of aryl isothiocyanates underwent reaction to afford the corresponding 2-aminobenzothiozoles in moderate to good yields. In this tandem reaction, DMSO acts as a key initiator for the activation of aryl isothiocyanates, although the exact role of DMSO is unclear.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Science Technology Program Project of Guangdong Province (No. 2016B020204005), Natural Science Foundation of Guangdong Province (Grant 2016A030313387), and China Postdoctoral Science Foundation (Grant 2018M643105).Notes and references
- (a) P. C. Sharma, K. K. Bansal, A. Deep and M. Pathak, Curr. Top. Med. Chem., 2017, 17, 208–237 CrossRef CAS PubMed; (b) J. B. Sweeney, M. Rattray, V. Pugh and L. A. Powell, ACS Med. Chem. Lett., 2018, 9, 552–556 CrossRef CAS PubMed; (c) L. Hroch, P. Guest, O. Benek, O. Soukup, J. Janockova, R. Dolezal, K. Kuca, L. Aitken, T. K. Smith, F. Gunn-Moore, D. Zala, R. R. Ramsay and K. Musilek, Bioorg. Med. Chem., 2017, 25, 1143–1152 CrossRef CAS PubMed; (d) Z. Ji, F. Zhou and S. Wei, Bioorg. Med. Chem. Lett., 2015, 25, 4065–4068 CrossRef CAS PubMed.
- For reviews, see: (a) T. L. Dadmal, S. D. Katre, M. C. Mandewale and R. M. Kumbhare, New J. Chem., 2018, 42, 776–797 RSC; (b) N. P. Prajapati, R. H. Vekariya, M. A. Borad and H. D. Patel, RSC Adv., 2014, 4, 60176–60208 RSC.
- Synthesis of benzothiazoles via C–H sulfurization, see: (a) A. D. Jordan, C. Luo and A. B. Reitz, J. Org. Chem., 2003, 68, 8693–8696 CrossRef CAS PubMed; (b) L. L. Joyce and R. A. Batey, Org. Lett., 2009, 11, 2792–2795 CrossRef CAS PubMed; (c) K. Inamoto, C. Hasegawa, J. Kawasaki, K. Hiroya and T. Doi, Adv. Synth. Catal., 2010, 352, 2643–2655 CrossRef CAS; (d) H. Wang, L. Wang, J. Shang, X. Li, H. Wang, J. Gui and A. Lei, Chem. Commun., 2012, 48, 76–78 RSC; (e) S. K. Sahoo, A. Banerjee, S. Chakraborty and B. K. Patel, ACS Catal., 2012, 2, 544–551 CrossRef CAS; (f) J. Wang, Y. Zong, X. Zhang, Y. Gao, Z. Li, G. Yue, Z. Quan and X. Wang, Synlett, 2014, 25, 2143–2148 CrossRef CAS; (g) S. Sharma, R. S. Pathare, A. K. Maurya, K. Gopal, T. K. Roy, D. M. Sawant and R. T. Pardasani, Org. Lett., 2016, 18, 356–359 CrossRef CAS PubMed.
- Synthesis of benzothiazoles via cross-coupling reaction of ortho-halothiourea, see: (a) C. Benedi, F. Bravo, P. Uriz, E. Fernández, C. Claver and S. Castillón, Tetrahedron Lett., 2003, 44, 6073–6077 CrossRef CAS; (b) L. L. Joyce, G. Evindar and R. A. Batey, Chem. Commun., 2004, 446–447 RSC; (c) R. Wang, W.-J. Yang, L. Yue, W. Pan and H.-Y. Zeng, Synlett, 2012, 23, 1643–1648 CrossRef CAS; (d) S. K. Rout, S. Guin, J. Nath and B. K. Patel, Green Chem., 2012, 14, 2491–2498 RSC; (e) H. Min, G. Xiao, W. Liu and Y. Liang, Org. Biomol. Chem., 2016, 14, 11088–11091 RSC; (f) W. Zeng, P. Dang, X. Zhang, Y. Liang and C. Peng, RSC Adv., 2014, 4, 31003–31006 RSC.
- Metal-catalyzed tandem reaction of isothiocyanates with amines, see: (a) Q. Ding, X. He and J. Wu, J. Comb. Chem., 2009, 11, 587–591 CrossRef CAS PubMed; (b) Y.-J. Guo, R.-Y. Tang, P. Zhong and J.-H. Li, Tetrahedron Lett., 2010, 51, 649–652 CrossRef CAS; (c) N. Khatun, L. Jamir, M. Ganesh and B. K. Patel, RSC Adv., 2012, 2, 11557–11565 RSC; (d) L. Chen, B. Huang, Q. Nie and M. Cai, Appl. Organomet. Chem., 2016, 30, 446–450 CrossRef CAS; (e) N. Zhao, L. Liu, F. Wang, J. Li and W. Zhang, Adv. Synth. Catal., 2014, 356, 2575–2579 CrossRef CAS; (f) Q. Ding, B. Cao, X. Liu, Z. Zong and Y.-Y. Peng, Green Chem., 2010, 12, 1607–1610 RSC.
- Metal-free conditions: (a) Z.-G. Le, J.-P. Xu, H.-Y. Rao and M. Ying, J. Heterocycl. Chem., 2006, 43, 1123–1124 CrossRef CAS; (b) W. Zhang, Y. Yue, D. Yu, L. Song, Y.-Y. Xu, Y.-J. Tian and Y.-J. Guo, Adv. Synth. Catal., 2012, 354, 2283–2287 CrossRef CAS; (c) Y. He, J. Li, S. Luo, J. Huang and Q. Zhu, Chem. Commun., 2016, 52, 8444–8447 RSC; (d) P. Wang, S. Tang and A. Lei, Green Chem., 2017, 19, 2092–2095 RSC; (e) Y. Xu, B. Li, X. Zhang and X. Fan, J. Org. Chem., 2017, 82, 9637–9646 CrossRef CAS PubMed.
- (a) S. Toulot, T. Heinrich and F. R. Leroux, Adv. Synth. Catal., 2013, 355, 3263–3272 CrossRef CAS; (b) A. Armstrong and J. C. Collins, Angew. Chem., Int. Ed., 2010, 49, 2282–2285 CrossRef CAS PubMed; (c) S. Mitsuda, T. Fujiwara, K. Kimigafukuro, D. Monguchi and A. Mori, Tetrahedron, 2012, 68, 3585–3590 CrossRef CAS; (d) D. Monguchi, T. Fujiwara, H. Furukawa and A. Mori, Org. Lett., 2009, 11, 1607–1610 CrossRef CAS PubMed.
- (a) C. Shen, P. Zhang, Q. Sun, S. Bai, T. S. A. Hor and X. Liu, Chem. Soc. Rev., 2015, 44, 291–314 RSC; (b) F.-J. Chen, G. Liao, X. Li, J. Wu and B.-F. Shi, Org. Lett., 2014, 16, 5644–5647 CrossRef CAS PubMed.
- (a) J.-R. Zhang, L.-Z. Zhan, L. Wei, Y.-Y. Ning, X.-L. Zhong, J.-X. Lai, L. Xu and R.-Y. Tang, Adv. Synth. Catal., 2018, 360, 533–543 CrossRef CAS; (b) J.-C. Deng, J.-H. Chen, J.-R. Zhang, T.-T. Lu and R.-Y. Tang, Adv. Synth. Catal., 2018, 360, 4795–4806 CrossRef CAS; (c) J.-R. Zhang, Y.-Y. Liao, J.-C. Deng, K.-Y. Feng, M. Zhang, Y.-Y. Ning, Z.-W. Lin and R.-Y. Tang, Chem. Commun., 2017, 53, 7784–7787 RSC; (d) J.-C. Deng, S.-B. Zhuang, Q.-Z. Liu, Z.-W. Lin, Y.-L. Su, J.-H. Chen and R.-Y. Tang, RSC Adv., 2017, 7, 54013–54016 RSC; (e) J. Jiao, L. Wei, X.-M. Ji, M.-L. Hu and R.-Y. Tang, Adv. Synth. Catal., 2016, 358, 268–275 CrossRef CAS; (f) X.-M. Ji, S.-J. Zhou, F. Chen, X.-G. Zhang and R.-Y. Tang, Synthesis, 2015, 47, 659–671 CrossRef CAS.
- Y.-Y. Liao, J.-C. Deng, Y.-P. Ke, X.-L. Zhong, L. Xu, R.-Y. Tang and W. Zheng, Chem. Commun., 2017, 53, 6073–6076 RSC.
- M. J. Frisch, G. W. Trucks and H. B. Schlegel, et al., Gaussian 09, rev. D. 01, Gaussian, Inc., Wallingford, CT 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09784d |
| This journal is © The Royal Society of Chemistry 2019 |