Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrochemical sensor based on a three dimensional nanostructured MoS2 nanosphere-PANI/reduced graphene oxide composite for simultaneous detection of ascorbic acid, dopamine, and uric acid†
Shuaihui Lia,
Yashen Mab,
Yongkang Liub,
Gu Xin*c,
Minghua Wangb,
Zhihong Zhang *b and
Zhongyi Liu
*b and
Zhongyi Liu *a
*a
aSchool of Chemical Engineering and Energy, Zhengzhou University, No. 100 Science Avenue, Zhengzhou 450001, Henan, China. E-mail: liuzhongyi@zzu.edu.cn; 2006025@zzuli.edu.cn; Tel: +86-37186609676
bHenan Provincial Key Laboratory of Surface & Interface Science, Zhengzhou University of Light Industry, Zhengzhou 450002, China
cCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou, 450001, China. E-mail: guxin@zzu.edu.cn
First published on 23rd January 2019
Abstract
A three dimensional (3D) nanostructured composite based on the self-assembly of MoS2 nanospheres and polyaniline (PANI) loaded on reduced graphene oxide (denoted by 3D MoS2-PANI/rGO) was prepared via a feasible one-pot hydrothermal process. The 3D MoS2-PANI/rGO nanocomposite not only exhibits good functionality and bioaffinity but also displays high electrochemical catalytic activity. As such, the developed 3D MoS2-PANI/rGO nanocomposite can be employed as the sensing platform for simultaneously detecting small biomolecules, i.e., ascorbic acid (AA), dopamine (DA), and uric acid (UA). The peak currents obtained from the differential pulse voltammetry (DPV) measurements depended linearly on the concentrations in the wide range from 50 μM to 8.0 mM, 5.0 to 500 μM, and 1.0 to 500 μM, giving low detection limits of 22.20, 0.70, and 0.36 μM for AA, DA, and UA, respectively. Furthermore, the 3D MoS2-PANI/rGO-based electrochemical sensor also exhibited high selectivity, good reproducibility and stability toward small molecule detection. The present sensing strategy based on 3D MoS2-PANI/rGO suggests a good reliability in the trace determination of electroactive biomolecules.
Introduction
Ascorbic acid (AA), dopamine (DA), and uric acid (UA) are crucial biomolecules that usually coexist in human physiological fluids.1–3 Abnormal DA level in the central nervous system may lead to several neurological diseases, e.g., schizophrenia and Parkinson's disease.4,5 Similarly, AA associates to atherosclerosis,6 and abnormality of UA level in the human metabolism causes diseases like hyperuricemia and gout.7 Therefore, sensitive and rapid detection of AA, DA, and UA in biological systems is vital for healthcare, routine analysis and clinical diagnostics. Several analytical techniques including chromatography,8 surface plasmon resonance spectroscopy,9 chemiluminescence,10 fluorescence,11 and electrochemical analysis,12 have been conducted for detecting biomolecules. Among them, electrochemical techniques have been receiving considerable interest because of the low cost, fast response, easy operation, and inexpensive instrumentation required.13,14 Notably, as electroactive molecules, AA, DA, and UA can be detected simultaneously by using electrochemical technique.15 However, the electro-catalytic oxidation of these biomolecules at bare electrodes occur at very similar potentials, leading to poor selectivity.16Many efforts have been made to develop effective electrochemically active and large surface area sensors with highly selective and sensitive electrochemical current responses. To address these issues, various sensing materials, e.g., metal nanoparticles, semiconductors, polymers and carbon materials, have been employed.17–19 With high electron transfer rate, electrochemical active surface area, and chemical functionality, reduce graphene oxide (rGO) has been investigated widely in electrochemical sensing.20,21 Chen et al. reported that cobalt manganese oxides increased the amount of graphene captured in the composites and improved sensing activities.22,23 Moreover, as a two-dimensional (2D) layered nanomaterial, molybdenum disulfide (MoS2) has been focused on the fields like energy conversion and storage, electronic devices, catalysts, and sensors,24–28 due to its highly optical transmittance, unique electrochemical properties, and large electrochemical active surface area. MoS2-based nanocomposites have been used as matrix for simultaneously detecting of AA, DA, and UA. For instance, the gold nanoparticle-decorated MoS2 nanocomposite modified electrode exhibited good electro-catalytic oxidation of AA, DA, and UA, giving detection limits of 100, 0.05, and 10 μM, respectively.29 Interestingly, MoS2/graphene hierarchical frameworks have been reported where Mo6+ cations were found to induce the self-assembly of graphene oxide, and the aerogel structures depend strongly on different MoS2 loadings.30 What's more, most of the reported MoS2 used as the sensing materials for biomolecules detection were still based on 2D planar structures. Considering that the effective electrochemically active surface areas of MoS2 for adsorption and electro-catalytic oxidation of biomolecules may be closely associated with their morphology, the structure modulation needs to be further studied.31,32 Diverse shapes of nanoparticles distributed and anchored on graphene sheets can be achieved by varying synthesis conditions.33,34 Additionally, conductive polymers such as polyaniline (PANI) have also been used to functionalize MoS2 to improve the electro conductivity of the materials for the detection of dopamine.35 MoS2/PANI/rGO aerogel nanocomposite exhibited excellent long-term cycling stability and a high capacity.36 RGO/MoS2/PANI@AuNPs nanocomposite-based electrochemical aptasensor was also reported for detection of aflatoxin B1.37 However, the nanocomposites were synthesized via multiple steps.
This work aims to enhance the electrochemical activity of the MoS2/graphene and improve the bioaffinity between the small biomolecules and the sensing layer. 3D nanostructured composite based on the self-assembly of MoS2 nanospheres and PANI loaded on rGO (3D MoS2-PANI/rGO) was synthesized by a one-pot hydrothermal reaction, following by the application as electrochemical catalyst toward the trace determination of biomolecules in blood and urine samples (Scheme 1). As expected, the synergistic effects of the effective electro-catalytic performance of MoS2, electrochemically activity of PANI, and the excellent charge transfer of graphene impart superior electro-catalytic activity of 3D nanostructured MoS2-PANI/rGO composite for the oxidation reactions of AA, DA, and UA.
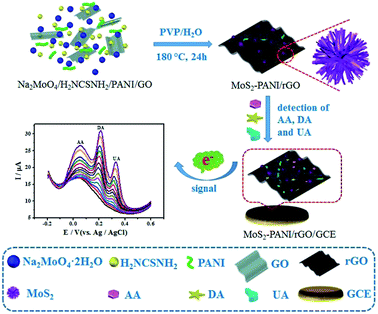 | ||
| Scheme 1 Schematic diagram of MoS2-PANI/rGO-based electrochemical biosensor for simultaneous detection of AA, DA, and UA. | ||
Experimental
Materials and chemicals
Graphite powders, potassium permanganate (KMnO4), sulfuric acid (H2SO4), 30% H2O2 solution, sodium molybdate (Na2MoO4·2H2O), thiourea (H2NCSNH2), polyvinylpyrrolidone (PVP), aniline, ammonium persulfate, HClO4, AA, DA, and UA were supplied by Aladdin reagent (Shanghai, China) and used as received. Milli-Q water (≥18.2 MΩ cm) was used throughout the experiments. The 0.1 M phosphate buffer solution (PBS, pH 7.0) was prepared by mixing stock solutions of Na2HPO4 and KH2PO4.Preparation of 3D MoS2-PANI/rGO nanocomposite
The preparation of GO and PANI are described in detail in the ESI (S1).† MoS2-PANI/rGO nanocomposite was synthesized by a one-pot hydrothermal approach. Briefly, 0.40 g Na2MoO4·2H2O, 0.66 g thiourea, 0.20 g PANI and 0.25 g PVP were added in 40 mL of GO aqueous dispersion (1.25 mg mL−1) to form a homogeneous solution under stirring. Then the solution was transferred into a 50 mL Teflon-lined stainless steel autoclave and heated at 180 °C for 24 h. After cooling to ambient temperature, the precipitate was collected by centrifugation and washed thoroughly using ultrapure water and ethanol. Finally, the solid was dried in a vacuum oven at 60 °C for 12 h and the 3D MoS2-PANI/rGO nanocomposite was then obtained. MoS2/rGO composite was prepared in a similar manner in the absence of PANI.Apparatus
X-ray diffraction (XRD) spectra were performed using a D8 advance X-ray diffraction instrument (Germany) in the 2θ range of 5–80°. Fourier-transform infrared (FT-IR) spectra were recorded on a Bruker TENSOR27 instrument from 400 to 4000 cm−1. The X-ray photoelectron spectroscopy (XPS) analysis was measured by a Thermo Fisher ESCALAB 250Xi spectrometer equipped with an Al anode (Al-Kα 1486.6 eV). Surface morphologies of the samples were investigated by field-emission scanning electron microscopy (FE-SEM, JSM-6490LV, Japan) and transmission electron microscopy (TEM, JEM-2100F, Japan), respectively.Fabrication of biosensor and electrochemical measurements
The glassy carbon electrodes (GCE, 3.0 mm in diameter) were polished using 0.3 μm and 0.05 μm alumina slurry, followed by sonicating in nitric acid, ethanol, and ultrapure water sequentially, and drying at room temperature. Afterward, 10 μL of the MoS2-PANI/rGO suspension (1.0 mg mL−1) was dropped onto GCE electrode surface, then dried in the ambient air to develop the sensor (MoS2-PANI/rGO/GCE). For comparison, the MoS2/rGO/GCE and PANI/GCE were made in the same way.Electrochemical measurements were performed on a CHI660D electrochemical workstation (Chenhua, Shanghai, China) with a conventional three-electrode system. The GCE or modified GCEs, platinum slide, and Ag/AgCl (saturated KCl) electrode were used as working electrode, counter electrode, and reference electrode, respectively. Cyclic voltammetry (CV) was recorded in 0.1 M PBS (pH 7.0; containing 0.1 M KCl). The potential range was from −0.2 to 0.8 V and the scan rate was 50 mV s−1. Differential pulse voltammetry (DPV) was collected at from −0.2 V to 0.8 V with pulse amplitude of 50 mV.
Results and discussion
Characterization
The chemical structure and components of nanomaterials were determined by FT-IR (S2†) and XPS. The FT-IR spectra (Fig. S1†) suggested that PANI was present in MOS2-PANI/rGO nanocomposite. The chemical composition and valence states of C, N, Mo and S containing in MoS2/rGO and MoS2-PANI/rGO (Fig. 1, S2 and Table S1†) nanocomposites were investigated by XPS in detail. From the XPS survey spectrum (Fig. S2a†), C, N, O, Mo and S can be clearly identified in MoS2-PANI/rGO with atomic% of 74.5, 11.8, 10.8, 0.9, and 2.0, respectively. Fig. 1a shows the C 1s characteristic peak which is caused by the component of rGO and PANI. Three main peaks are centered at 284.6, 285.6, and 287.7 eV, which are assigned with the groups of C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–H, C–OH/C–N, and C
C/C–H, C–OH/C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.38 The graphene possess numerous edge planes and defects, which are conductive to the fast electron transfer as well as electro-catalytic activity.39,40 Apparently, the C–N was originated from PANI. The N 1s core-level XPS spectrum (Fig. 1b) was fitted into three components, 398.8, 399.8 and 400.8 eV, which are corresponded to imine (
O, respectively.38 The graphene possess numerous edge planes and defects, which are conductive to the fast electron transfer as well as electro-catalytic activity.39,40 Apparently, the C–N was originated from PANI. The N 1s core-level XPS spectrum (Fig. 1b) was fitted into three components, 398.8, 399.8 and 400.8 eV, which are corresponded to imine (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–), amine (–NH), and protonated amine units (–NH+/
N–), amine (–NH), and protonated amine units (–NH+/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+–) containing in PANI, respectively.41 The amine and imine groups can form hydrogen bonds with hydroxyl groups present in the small biomolecules and eventually results strong bioaffinity.42 Fig. 1c and d depict the high-resolution scan XPS spectra of the Mo 3d, S 2s and S 2p electrons. The binding energies of Mo 3d3/2, Mo 3d5/2 and S 2s peaks are located at 232.4, 229.2 and 226.5 eV, whereas S 2p1/2 and S 2p3/2 peaks are observed at 163.3, and 162.1 eV, respectively,30,43 suggesting that Mo4+ chemical state existed and the formation of crystalline MoS2. The combination of the MoS2, rGO and PANI may effectively increase the conductivity, bioaffinity and catalytic activity of the nanocomposite.
N+–) containing in PANI, respectively.41 The amine and imine groups can form hydrogen bonds with hydroxyl groups present in the small biomolecules and eventually results strong bioaffinity.42 Fig. 1c and d depict the high-resolution scan XPS spectra of the Mo 3d, S 2s and S 2p electrons. The binding energies of Mo 3d3/2, Mo 3d5/2 and S 2s peaks are located at 232.4, 229.2 and 226.5 eV, whereas S 2p1/2 and S 2p3/2 peaks are observed at 163.3, and 162.1 eV, respectively,30,43 suggesting that Mo4+ chemical state existed and the formation of crystalline MoS2. The combination of the MoS2, rGO and PANI may effectively increase the conductivity, bioaffinity and catalytic activity of the nanocomposite.
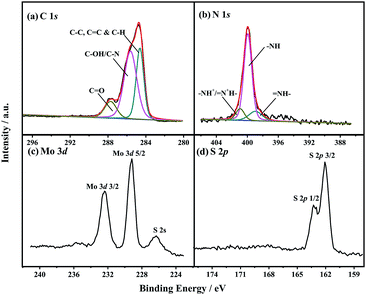 | ||
| Fig. 1 High-resolution XPS spectra of (a) C 1s, (b) N 1s, (c) Mo 3d, and (d) S 2p of MoS2-PANI/rGO nanocomposite. | ||
Morphological studies
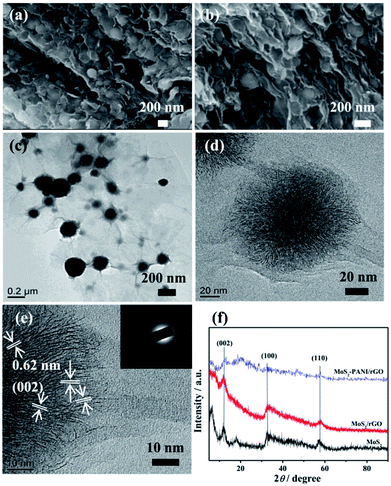
The surface morphology of as-prepared MoS2/rGO, PANI, and MoS2-PANI/rGO were investigated by FE-SEM, as shown in Fig. 2 and S3.† The results show that the MoS2 nanospheres were formed with a diameter of 300–400 nm (Fig. S3a†), approximately, whereas the PANI exhibits a rod-like shape (Fig. S3b†). The FE-SEM images of MoS2/rGO reveal spherical-shaped MoS2 nanoparticles wrapped by crumpled structure of graphene sheets (Fig. S3c and d†). As for the MoS2-PANI/rGO, the honeycomb-like structure was formed with MoS2 nanospheres interpenetrating in 3D graphene sheets (Fig. 2a and b), which could increase the electrochemical active surface area of the nanocomposite. Further structural insights were obtained in TEM analysis. The graphene appears a curved and crinkled texture (Fig. 2c, d and S4a–c†), which is well anchored by MoS2 nanospheres. For MoS2-PANI/rGO, dendritic hierarchical structure with several layers can be found in high-resolution TEM (HR-TEM) image. In Fig. 2e, the layered structures with an interlayer distance of ∼0.62 nm corresponds to the (002) crystal planes of MoS2. The corresponding selected area electron diffraction (SAED) patterns of MoS2/rGO (Fig. S4d†) and MoS2-PANI/rGO (inset of Fig. 2e) confirm the hexagonal structure of MoS2 with different crystallographic orientations and present separated diffraction rings that can be indexed to the XRD patterns (Fig. 2f). The peaks that appeared in the XRD patterns of all the three samples are indexed based on MoS2.The major diffraction peaks at 2θ = 14.4°, 32.7°, and 58.3° are in accordance with the (002), (100), and (110) crystal planes of hexagonal MoS2 (JCPDS 37-1492).44,45 Nevertheless, the intensity of diffraction peak for MoS2-PANI/rGO decreased, indicating that PANI slightly affected the crystallization performance of MoS2.
Electrochemical performance of the as-synthesized nanomaterials
To evaluate the electrochemical performance of the different materials, CV curves were tested on the bare GCE, MoS2/rGO, PANI, and 3D MoS2-PANI/rGO-modified glassy carbon electrodes. The MoS2-PANI/rGO/GCE (0.125 cm2) exhibited larger electrochemical active surface area than MoS2/rGO/GCE (0.101 cm2) and PANI/GCE (0.102 cm2), suggesting it can provide quick mass transport of molecules to the electrocatalyst (Fig. S5†). When the AA, DA and UA biomolecules were added in 0.1 M PBS solution (Fig. 3a), the GCE and PANI/GCE showed broad peak from −0.2 to 0.8 V, while MoS2/rGO/GCE had two obvious peak responses to DA and UA. In comparison, the MoS2-PANI/rGO/GCE exhibited the largest anodic peaks in the CV measurements. Three peaks were observed on MoS2-PANI/rGO/GCE in DPV measurements, indicating a simultaneous electrochemical detection of the three bio-molecules (Fig. 3b). The MoS2-PANI/rGO/GCE exhibits the highest peak current intensity and three well-resolved peaks at 0.020, 0.196, and 0.320 V (ΔEAA–DA = 176 mV, ΔEDA–UA = 124 mV, ΔEAA–UA = 300 mV), implying that synergistic effect existed among the MoS2, rGO and PANI toward the electrochemical catalysis of three small biomolecules. The observed anodic peaks are ascribed to the oxidation of hydroxyl groups to carbonyl groups in AA, catechol to o-quinone in DA, and bridging double bond to hydroxyl groups in UA.16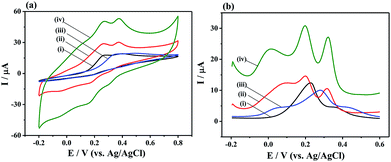 | ||
| Fig. 3 (a) CV and (b) DPV curves of (i) bare GCE, (ii) MoS2/rGO/GCE, (iii) PANI/GCE, and (iv) MoS2-PANI/rGO/GCE in 0.1 M PBS (pH 7.0, 0.1 M KCl) containing 2.0 mM AA, 150 μM DA, and 200 μM UA. | ||
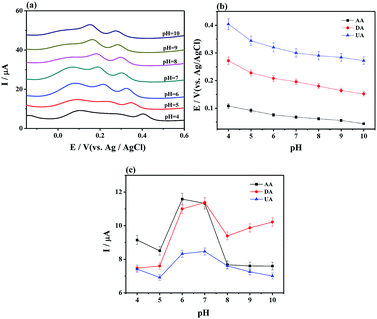
Moreover, the effect of pH on electrochemical oxidation of AA, DA, and UA with MoS2-PANI/rGO/GCE was investigated (Fig. 4). The results show that the anodic peak potential of AA, DA, and UA shifted negatively with increasing of their pH value (pH 4.0–10.0) (Fig. 4a), and oxidation peak potential of AA, DA, and UA were linearly proportional to the pH value (Fig. 4b), demonstrating that the proton took part in the electrochemical oxidation reaction process.6,29 The peak current of AA, DA, and UA was also changed with the pH value. The AA, DA, and UA achieved the maximum value at pH 6.0, 7.0, and 7.0, respectively (Fig. 4c). Considering sensitivity and selectivity, the electrolyte solution with pH 7.0 was selected for further measurements of the analytes.
Simultaneous detection of AA, DA and UA
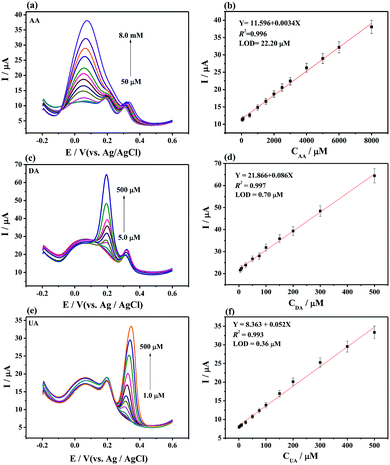
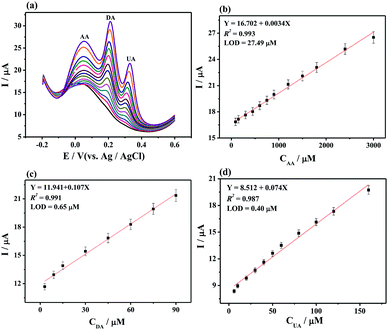
Individually or simultaneously analyze AA, DA, and UA on MoS2-PANI/rGO/GCE was studied by DPV. It is found that the response current corresponding to the analyte increases with increasing concentration of the ternary mixture. In Fig. 5a–b, the peak currents increased linear with the AA concentrations in the range of 50 μM to 8.0 mM. The regression equation of Ip,AA (μA) = 11.596 + 0.0034CAA (μM) (R2 = 0.996) was obtained. The limit of detection (LOD) was determined to be 22.20 μM for AA at a signal-to-noise ratio of 3. Similarly, the peak currents of DA (Fig. 5c and d) and UA (Fig. 5e and f) were proportional to the concentration in the range of 5.0–500 μM (DA) and 1.0–500 μM (UA). The calibration equations were Ip,DA (μA) = 21.866 + 0.086CDA (μM) (R2 = 0.997) and Ip,UA (μA) = 8.363 + 0.052CUA (μM) (R2 = 0.993) with LOD of 0.70 μM for DA and 0.36 μM for UA, respectively.In the simultaneous detection of AA, DA, and UA experiments, three well- resolved anodic peaks at potentials of 0.052, 0.196, and 0.304 mV were presented at MoS2-PANI/rGO/GCE from DPV plots (Fig. 6). The three peak currents were linearly dependent on the concentrations ranging from 30 μM to 3.0 mM for AA, 3.0 to 300 μM for DA, and 2.0 to 200μM for UA while the LOD of AA, DA, and UA were 27.49, 0.65, and 0.40 μM, respectively. The results demonstrate that the 3D MoS2-PANI/rGO-based sensor exhibited wide linear ranges and low detection limits for detection of biomolecules comparative to those of other sensors (Table 1).
| Electrode | Method | Linear range (μM) | LOD (μM) | Reference | ||||
|---|---|---|---|---|---|---|---|---|
| AA | DA | UA | AA | DA | UA | |||
| a LSV is linear sweep voltammetry. | ||||||||
| RGO-ZnO/GCE | DPV | 50–2350 | 1–70 | 3–330 | 3.71 | 0.33 | 1.08 | 6 |
| AuNPs@MoS2/GCE | DPV | 1000–70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.05–4000 | 10–7000 | 100 | 0.05 | 10 | 29 |
| Fe-Meso-PANI/GCE | LSVa | 10–300 | 10–300 | 10–300 | 6.5 | 9.8 | 5.3 | 16 |
| GO-PANI/GCE | DPV | 150–1050 | 1–14 | 3–26 | 50 | 0.5 | 1 | 46 |
| Au/RGO/GCE | DPV | 240–1500 | 6.8–41 | 8.8–53 | 51 | 1.4 | 1.8 | 47 |
| Graphene/SnO2/GCE | DPV | 100–1000 | 1–20 | 3–21 | 100 | 1 | 3 | 48 |
| MoS2PANI/rGO/GCE | DPV | 50–8000 | 5.0–500 | 1.0–500 | 22.20 | 0.70 | 0.36 | This work |
Selectivity, reproducibility, and stability of the fabricated biosensor
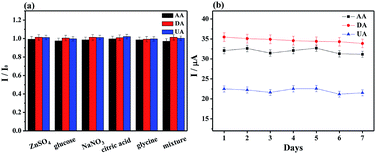
Several potential interferential species were used to examine the selectivity at MoS2-PANI/rGO/GCE for simultaneously detecting of AA (1.0 μM), DA (75 μM) and UA (75 μM) (Fig. 7a).There were negligible interferences detected in the presence of NaNO3, ZnSO4, glucose, glycine, and citric acid, whose concentrations are 1.0 mM. The peak response changes were less than ±3%, revealing a good selectivity of the developed electrochemical sensor.
Five MoS2-PANI/rGO modified GCEs were prepared independently and their peak currents for AA, DA and UA mixture were investigated (Fig. S6†). The relative standard deviation (RSD) were 4.0% for AA, 4.56% for DA, and 4.24% for UA, indicating the good reproducibility of the developed sensor. In successive 15 times DPV measurements, the RSD values of 1.13%, 1.70%, and 2.49% for AA, DA and UA were observed. Furthermore, the stability of the electrode was also determined by DPV measurements for 7 days. The oxidation currents decreased by less than 5.0% of the initial currents (Fig. 7b), confirming a good stability of the biosensor.
Real sample analysis
To evaluate the applicability of the MoS2-PANI/rGO-based sensor, the simultaneous detection of AA, DA, and UA in human serum and urine samples was investigated by using standard addition method. Human serum (obtained from Beijing Solarbio Science & Technology Co., Ltd.) and human urine (supplied by an adult male student in the laboratory) were filtered through 0.22 μm filter, and diluted 100 times with 0.1 M PBS (pH 7.0) to prepare the real samples. Subsequently, DPV curves were collected before and after spiking with different concentrations of UA, DA and AA in the real samples (Fig. S7†). As shown in Table 2, the recoveries of the three kinds of small biomolecules varied from 99.0% to 103.5% in human serum, whereas they changed from 96.2 to 103.6% in human urine samples. The RSD values were also accurate and precise, indicating the applicability of the developed sensor for detection of these electroactive molecules in real biological system.| Sample no. | Species | Spiking (μM) | Detected (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| a The number “1” and “2” samples were prepared separately with different concentrations of AA, DA, and UA. | |||||
| Human serum1 | AA | 1200 | 1241.8 | 103.5 | 1.71 |
| DA | 120 | 119.5 | 99.6 | 3.38 | |
| UA | 120 | 121.5 | 101.2 | 1.20 | |
| Human serum2 | AA | 1800 | 1810.4 | 100.6 | 1.03 |
| DA | 180 | 183.0 | 101.7 | 1.89 | |
| UA | 180 | 178.3 | 99.0 | 2.21 | |
| Human urine1 | AA | 1200 | 1199.0 | 99.9 | 1.34 |
| DA | 120 | 121.6 | 101.3 | 2.30 | |
| UA | 120 | 124.3 | 103.6 | 2.45 | |
| Human urine2 | AA | 1800 | 1783.7 | 99.1 | 1.35 |
| DA | 180 | 180.1 | 100.0 | 0.76 | |
| UA | 180 | 173.2 | 96.2 | 3.12 | |
Conclusion
A 3D nanostructured MoS2-PANI/rGO nanocomposite based on the self-assembly of MoS2 nanospheres and PANI loaded on reduced graphene oxide was synthesized by a one-pot hydrothermal reaction, and employed as the platform for electrochemical oxidation of biomolecules. The result showed that synergistic effect existed among MoS2, rGO and PANI. The ternary composite exhibited good electrochemical activity, high bioaffinity and strong catalytic effect on the oxidation of biomolecules. The 3D MoS2-PANI/rGO-based sensing platform exhibited high sensitivity for simultaneous detection of AA, DA, and UA with three distinguished oxidation peaks (ΔEAA–DA = 176 mV, ΔEDA–UA = 124 mV, ΔEAA–UA = 300 mV) in DPV measurements. The MoS2-PANI/rGO/GCE showed excellent responses toward AA, DA, and UA in the linear range of 50 μM to 8.0 mM, 5.0–500 μM, and 1.0–500 μM with low detection limit of 22.20, 0.70, and 0.36 μM (S/N = 3), respectively. The sensor also exhibited good selectivity, reproducibility and stability for simultaneous detection of these biomolecules in human serum and urine samples.Ethical conduct of research
The authors state that for investigations involving human subjects, informed consent was obtained from all human subjects.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Programs for the National Natural Science Foundation of China (NSFC: Account No. U1604127).Notes and references
- T. D. Thanh, J. Balamurugan, S. H. Lee, N. H. Kim and J. H. Lee, Biosens. Bioelectron., 2016, 81, 259–267 CrossRef CAS PubMed.
- S. Pakapongpan, J. P. Mensing, D. Phokharatkul, T. Lomas and A. Tuantranont, Electrochim. Acta, 2014, 133, 294–301 CrossRef CAS.
- K. Q. Deng, X. F. Li and H. W. Huang, Microchim. Acta, 2016, 183, 2139–2145 CrossRef CAS.
- J. S. Lee, J. Oh, S. G. Kim and J. Jang, Small, 2015, 11, 2399–2406 CrossRef CAS PubMed.
- X. M. Feng, Y. Zhang, Z. Z. Yan, N. N. Chen, Y. W. Ma, X. F. Liu, X. Y. Yang and W. H. Hou, J. Mater. Chem. A, 2013, 1, 9775–9780 RSC.
- X. Zhang, Y. C. Zhang and L. X. Ma, Sens. Actuators, B, 2016, 227, 488–496 CrossRef CAS.
- L. P. Mei, J. J. Feng, L. Wu, J. R. Chen, L. G. Shen, Y. L. Xie and A. J. Wang, Microchim. Acta, 2016, 183, 2039–2046 CrossRef CAS.
- P. Uutela, R. Reinila, K. Harju, P. Piepponen, R. A. Ketola and R. Kostiainen, Anal. Chem., 2009, 81, 8417–8425 CrossRef CAS PubMed.
- P. Dutta, R. B. Pernites, C. Danda and R. C. Advincula, Macromol. Chem. Phys., 2011, 212, 2439–2451 CrossRef CAS.
- J. H. Yu, S. M. Wang, L. Ge and S. G. Ge, Biosens. Bioelectron., 2011, 26, 3284–3289 CrossRef CAS PubMed.
- C. G. Qian, S. Zhu, P. J. Feng, Y. L. Chen, J. C. Yu, X. Tang, Y. Liu and Q. D. Shen, ACS Appl. Mater. Interfaces, 2015, 7, 18581–18589 CrossRef CAS PubMed.
- D. Y. Zhao, G. L. Yu, K. L. Tian and C. X. Xu, Biosens. Bioelectron., 2016, 82, 119–126 CrossRef CAS PubMed.
- N. J. Ronkainen, H. B. Halsall and W. R. Heineman, Chem. Soc. Rev., 2010, 39, 1747–1763 RSC.
- X. L. Chai, X. G. Zhou, A. W. Zhu, L. M. Zhang, Y. Qin, G. Y. Shi and Y. Tian, Angew. Chem., Int. Ed., 2013, 52, 8129–8133 CrossRef CAS PubMed.
- L. W. Zhao, H. J. Li, S. M. Gao, M. J. Li, S. Xu, C. P. Li, W. L. Guo, C. Q. Qu and B. H. Yang, Electrochim. Acta, 2015, 168, 191–198 CrossRef CAS.
- M. U. A. Prathap and R. Srivastava, Sens. Actuators, B, 2013, 177, 239–250 CrossRef.
- N. G. Tsierkezos, S. H. Othman, U. Ritter, L. Hafermann, A. Knauer, J. M. Kohler, C. Downing and E. K. McCarthy, Sens. Actuators, B, 2016, 231, 218–229 CrossRef CAS.
- H. F. Hu, Y. P. Song, M. Feng and H. B. Zhan, Electrochim. Acta, 2016, 190, 40–48 CrossRef CAS.
- A. K. Yang, Y. Xue, Y. Zhang, X. F. Zhang, H. Zhao, X. J. Li, Y. J. He and Z. B. Yuan, J. Mater. Chem. B, 2013, 1, 1804–1811 RSC.
- A. Ambrosi, C. K. Chua, N. M. Latiff, A. H. Loo, C. H. A. Wong, A. Y. S. Eng, A. Bonanni and M. Pumera, Chem. Soc. Rev., 2016, 45, 2458–2493 RSC.
- T. Kuila, S. Bose, P. Khanra, A. K. Mishra, N. H. Kim and J. H. Lee, Biosens. Bioelectron., 2011, 26, 4637–4648 CrossRef CAS PubMed.
- C.-C. Kuo, W.-J. Lan and C.-H. Chen, Nanoscale, 2014, 6, 334–341 RSC.
- W.-J. Lan and C.-H. Chen, Electrochim. Acta, 2015, 180, 1014–1022 CrossRef CAS.
- C. N. R. Rao, K. Gopalakrishnan and U. Maitra, ACS Appl. Mater. Interfaces, 2015, 7, 7809–7832 CrossRef CAS PubMed.
- M. Park, Y. J. Park, X. Chen, Y. K. Park, M. S. Kim and J. H. Ahn, Adv. Mater., 2016, 28, 2556–2562 CrossRef CAS PubMed.
- W. Y. Gao, M. Q. Wang, C. X. Ran and L. Li, Chem. Commun., 2015, 51, 1709–1712 RSC.
- W. S. Zhang, P. P. Zhang, Z. Q. Su and G. Wei, Nanoscale, 2015, 7, 18364–18378 RSC.
- B. J. Guo, K. Yu, H. L. Li, H. L. Song, Y. Y. Zhang, X. Lei, H. Fu, Y. H. Tan and Z. G. Zhu, ACS Appl. Mater. Interfaces, 2016, 8, 5517–5525 CrossRef CAS PubMed.
- H. F. Sun, J. Chao, X. L. Zuo, S. Su, X. F. Liu, L. H. Yuwen, C. H. Fan and L. H. Wang, RSC Adv., 2014, 4, 27625–27629 RSC.
- W. J. Zhou, K. Zhou, D. M. Hou, X. J. Liu, G. Q. Li, Y. H. Sang, H. Liu, L. G. Li and S. W. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 21534–21540 CrossRef CAS PubMed.
- Y. H. Tan, K. Yu, T. Yang, Q. F. Zhang, W. T. Cong, H. H. Yin, Z. L. Zhang, Y. W. Chen and Z. Q. Zhu, J. Mater. Chem. C, 2014, 2, 5422–5430 RSC.
- Y. Zhao, Y. C. Liu, H. Q. Liu, H. Y. Kang, K. Z. Cao, Q. H. Wang, C. L. Zhang, Y. J. Wang, H. T. Yuan and L. F. Jiao, J. Power Sources, 2015, 300, 358–364 CrossRef CAS.
- W.-Q. Chen, M.-C. Chung, J. A. A. Valinton, D. P. Penaloza, S.-H. Chuang and C.-H. Chen, Chem. Commun., 2018, 54, 7900–7903 RSC.
- W.-Y. Kao, W.-Q. Chen, Y.-H. Chiu, Y.-H. Ho and C.-H. Chen, Sci. Rep., 2016, 6, 37174 CrossRef CAS PubMed.
- K.-J. Huang, J.-Z. Zhang, Y.-J. Liu and L.-L. Wang, Sens. Actuators, B, 2014, 194, 303–310 CrossRef CAS.
- C. Sha, B. Lu, H. Mao, J. Cheng, X. Pan, J. Lu and Z. Ye, Carbon, 2016, 99, 26–34 CrossRef CAS.
- G. S. Geleta, Z. Zhao and Z. Wang, Analyst, 2018, 143, 1644–1649 RSC.
- O. Akhavan and E. Ghaderi, J. Phys. Chem. C, 2009, 113, 20214–20220 CrossRef CAS.
- X. Huang, Z. Yin, S. Wu, X. Qi, Q. He, Q. Zhang, Q. Yan, F. Boey and H. Zhang, Small, 2011, 7, 1876–1902 CrossRef CAS PubMed.
- J. T. Robinson, F. K. Perkins, E. S. Snow, Z. Wei and P. E. Sheehan, Nano Lett., 2008, 8, 3137–3140 CrossRef CAS PubMed.
- T. Zhou, C. Li, H. Jin, Y. Lian and W. Han, ACS Appl. Mater. Interfaces, 2017, 9, 6030–6043 CrossRef CAS PubMed.
- B. Mu and A. Wang, J. Mater. Chem. A, 2015, 3, 281–289 RSC.
- W. J. Zhou, Z. Y. Yin, Y. P. Du, X. Huang, Z. Y. Zeng, Z. X. Fan, H. Liu, J. Y. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- H. L. Li, K. Yu, X. Lei, B. J. Guo, C. Li, H. Fu and Z. Q. Zhu, Dalton Trans., 2015, 44, 10438–10447 RSC.
- Y. Bao, J. X. Song, Y. Mao, D. X. Han, F. Yang, L. Niu and A. Ivaska, Electroanalysis, 2011, 23, 878–884 CrossRef CAS.
- C. Q. Wang, J. Du, H. W. Wang, C. E. Zou, F. X. Jiang, P. Yang and Y. K. Du, Sens. Actuators, B, 2014, 204, 302–309 CrossRef CAS.
- Y. L. Xie, J. Yuan, H. L. Ye, P. Song and S. Q. Hu, J. Electroanal. Chem., 2015, 749, 26–30 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra09511f |
| This journal is © The Royal Society of Chemistry 2019 |