Open Access Article
Open Access ArticlePreparation of triazine containing porous organic polymer for high performance supercapacitor applications†
Lirong Xu *a,
Ruiying Liua,
Fang Wanga,
Shina Yana,
Xinxin Shia and
Jiaqin Yang*ab
*a,
Ruiying Liua,
Fang Wanga,
Shina Yana,
Xinxin Shia and
Jiaqin Yang*ab
aSchool of Chemistry and Chemical Engineering, Qufu Normal University, Qufu, 273165, Shandong, China. E-mail: xulr2015@qfnu.edu.cn; yjq8681@163.com
bKey Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), College of Chemistry, Nankai University, Tianjin, 300071, China
First published on 11th January 2019
Abstract
By condensing M and TFP under solvothermal conditions, a new porous organic polymer POPM–TFP was obtained. The electrode modified with triazine containing POPM–TFP exhibits well-defined rapid redox processes and showed a high specific capacitance of 130.5 F g−1 at 2 A g−1, suggesting well electrochemical performance.
Due to the overconsumption of oil and coal over the past few decades, it is highly desirable to develop safe sources of energy, as well as the eco-friendly use of conventional energy through energy storage devices.1,2 Supercapacitors have attracted much attention because of their high power density, long recycle life and a wide temperature range.3 Generally, supercapacitors are classified into two types: electrochemical double layer capacitor and pseudocapacitor.4–6 Electrode materials are the key ingredient for this electrochemically occurring process.7 The applied voltage depended ion adsorption on the electrode surface depending on the structure of the material used for the electrodes. To obtain a device with good performance, significant study has been paid to the exploration of redox-active materials for supercapacitors.8,9 In order to improve the electronic performance of electrode materials, the functionalized porous structure having a high surface area and hierarchical porosity is important.10
Nanoporous organic polymers with high nitrogen content have got great interest because of their applications in semiconductors, gas storage, and electrochemical supercapacitors etc.11–13 Recently, nitrogen-doped carbon materials exhibit good electrical properties because the pseudocapacitance effect provided by N atoms.14 Nanoporous organic polymers, such as covalent organic frameworks (COFs), covalent triazine-based frameworks (CTFs), porous organic polymers (POPs) which have controllable pore structure and heteroatom doping are promising electrode materials in supercapacitors.15–19 Among them, POPs which are built by the polymerization of rigid organic molecules showing good supercapacitor applications are TaPa-Py COF, HCPANIs, TpDAB and Aza-CMPs etc.20–23 POPs have micropores with high specific surface area and heteroatom doping, which should be quite conductive to the electrochemical performance when applied as electrode materials. Consequently, polytriazines with high surface area stand out due to their high nitrogen content.24
Nowadays, nitrogen-enriched polymers attract increasing attention for application in supercapacitors.25 It is a challenge to synthesize nitrogen-enriched nanoporous polymers by using cheap reagents.26 POPs or COFs are prepared by using different synthetic method such as ultrahigh vacuum surface synthesis,27 microwave assisted solvothermal synthesis,28 ionthermal synthesis29 and vapor-assisted conversion etc.30 Solvothermal synthesis often used to produce nanoporous materials through exploring reaction conditions appropriately.31–33 CTFs represent a class of functionalized polymers which are obtained by the trimerization of aromatic nitriles under ionthermal conditions in the presence of a catalyst. This method involves harsh experimental conditions such as very high temperature.29 Melamine is a trimer of aromatic nitriles, with a 1,3,5-triazine skeleton considered as Lewis base.34 The lone pair of electrons in N plays an important role in electrochemical applications. Introducing triazine skeleton into POP materials by using precursor bearing triazine moieties is a straightforward approach in POP synthesis.
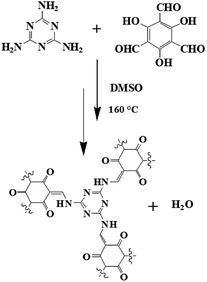
Herein, we choose a trigonal-symmetrical nitrogen-containing melamine (M) as a monomer, which can be used to construct conjugated microporous covalent triazine-based organic polymer through the coupling with triformylphloroglucinol (TFP) monomer under solvothermal conditions without any catalysts. The doping of nitrogen atoms in POPM–TFP can affect the electron distribution of the materials and further enhance the wettability and the electro-active surface area of the electrode. Otherwise, the nitrogen atom can also introduce pseudocapacitance to the supercapacitors, which are beneficial for enhancing the supercapacitor performance. Scheme 1 illustrates the synthesis of POPM–TFP at 160 °C. The synthesized POPM–TFP was employed as an electrode material for supercapacitor application. The electrodes which are modified with POPM–TFP exhibit well-defined rapid redox processes and high capacitance.
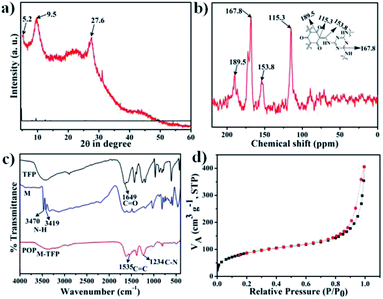
The crystalline structure of POPM–TFP was first characterized by powder X-ray diffraction (XRD). The XRD pattern of POPM–TFP material has been shown in Fig. 1a which reveals the crystalline nature of the material. As seen from Fig. 1a, the peaks at 5.2°, 9.5° and 27.6° are attributed to the corresponding (100), (200) and (201) planes diffractions of POPM–TFP respectively. Successful condensation of M and TFP under a simple solvothermal conditions without any catalysts yields product, which has been confirmed by 13C CP-MAS NMR and The Fourier transform infrared (FT-IR) spectroscopic investigations. In order to further understand the chemical environment of various types of carbon atoms in POPM–TFP, 13C solid state NMR spectroscopy was performed. POPM–TFP exhibits a clear signal at 167.8 ppm confirms the presence of the triazine skeletons (Fig. 1b).35–37 The additional characteristic 13C resonance in the C–N was observed to be located at 153.8 ppm. The characteristic peak of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appearing at 189.5 ppm. In summary, it can be confirmed that the M has successfully reacted with TFP to form POPM–TFP material.
O appearing at 189.5 ppm. In summary, it can be confirmed that the M has successfully reacted with TFP to form POPM–TFP material.
The FT-IR spectra of M, TFP and POPM–TFP between 4000 to 400 cm−1 are shown in Fig. 1c. The two separated peaks at 3470 and 3419 cm−1 originate from stretching vibrations of amine (–NH2) groups in M. The peak at 1649 cm−1 belongs to stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in TFP. The FT-IR spectra of POPM–TFP shows the disappearance of the –NH2 stretching bands of the M. The appearance of a broad –NH peak at 3379 cm−1 further indicates the formation of POPM–TFP. Additionally, the emergence of a new band at 1234 cm−1 is characteristic of C–N stretch moieties. The peak at 1535 cm−1 belongs to stretching vibrations of C
O in TFP. The FT-IR spectra of POPM–TFP shows the disappearance of the –NH2 stretching bands of the M. The appearance of a broad –NH peak at 3379 cm−1 further indicates the formation of POPM–TFP. Additionally, the emergence of a new band at 1234 cm−1 is characteristic of C–N stretch moieties. The peak at 1535 cm−1 belongs to stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in POPM–TFP.16,38 Further, the chemical environment of the elements present in the specimen was studied using X-ray photoelectron spectroscopy (XPS). Full scan XPS spectra of POPM–TFP showed in Fig. S1† reveals the peaks of C, N and O. To analyze the detailed chemical states of N elements, the high-resolution XPS scan was also recorded (Fig. S2†). The N 1s spectra of POPM–TFP split into two peaks located at 398.8 eV and 399.8 eV, respectively. The peak at 398.8 eV can be assigned to triazine N, while the peak at 399.8 eV can be assigned to the N in the –C
C in POPM–TFP.16,38 Further, the chemical environment of the elements present in the specimen was studied using X-ray photoelectron spectroscopy (XPS). Full scan XPS spectra of POPM–TFP showed in Fig. S1† reveals the peaks of C, N and O. To analyze the detailed chemical states of N elements, the high-resolution XPS scan was also recorded (Fig. S2†). The N 1s spectra of POPM–TFP split into two peaks located at 398.8 eV and 399.8 eV, respectively. The peak at 398.8 eV can be assigned to triazine N, while the peak at 399.8 eV can be assigned to the N in the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH–. These peak positions are in agreement with previous reports.20,35 The N2 adsorption/desorption was carried out for POPM–TFP in order to evaluate the permanent porosity. The N2 sorption isotherm measured at 77 K as showed in Fig. 1d. POPM–TFP exhibits characteristics of type-I adsorption isotherms with sharp uptake at a low pressure region indicating the microporous nature of this material. The Brunauer–Emmett–Teller (BET) surface area of POPM–TFP was found to be 317 m2 g−1. The pore size distribution plot has been demonstrated in Fig. S3.† The two sharp peaks at 1.5 and 2.5 nm are observed from the pore size distribution plot. Micropores of dimension 1.5 nm could be attributed to the porous network of the POPM–TFP, whereas mesopores of 2.5 nm could be originated due to void spaces associated to the grain boundary.
C–NH–. These peak positions are in agreement with previous reports.20,35 The N2 adsorption/desorption was carried out for POPM–TFP in order to evaluate the permanent porosity. The N2 sorption isotherm measured at 77 K as showed in Fig. 1d. POPM–TFP exhibits characteristics of type-I adsorption isotherms with sharp uptake at a low pressure region indicating the microporous nature of this material. The Brunauer–Emmett–Teller (BET) surface area of POPM–TFP was found to be 317 m2 g−1. The pore size distribution plot has been demonstrated in Fig. S3.† The two sharp peaks at 1.5 and 2.5 nm are observed from the pore size distribution plot. Micropores of dimension 1.5 nm could be attributed to the porous network of the POPM–TFP, whereas mesopores of 2.5 nm could be originated due to void spaces associated to the grain boundary.
The morphology of the synthesized POPM–TFP can be well investigated by field emission scanning electron microscopy (FE-SEM) and transmission electron microscope (TEM) analysis respectively. FE-SEM images of POPM–TFP at two different magnifications are shown in Fig. 2a and b. SEM images show that POPM–TFP reveals irregular nanowires morphologies. The TEM characterization further confirms the nanowire structures morphology with porous architecture (Fig. 2c and d.). Fig. 2d represents the high-resolution TEM image of POPM–TFP where the micropores of about 1.5 nm (white spot) are spread throughout the specimen.
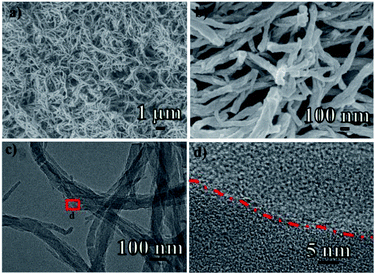 | ||
| Fig. 2 (a and b) FE-SEM images of POPM–TFP at two different magnifications. (c and d) TEM images of POPM–TFP at two different magnifications. | ||
Considering the synthesized POPM–TFP with 1,3,5-triazine skeleton distributed in nanopores, this may hold promising applications in electrode materials for supercapacitors. The pore channels and triazine units in POPM–TFP could render this material able to store electric energy. The electrochemical performance of POPM–TFP was investigated by using a typical three-electrode system in 2 M KOH aqueous electrolyte. The working electrodes were fabricated by mixing POPM–TFP, acetylene black and binder (polyvinylidene fluoride) in a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
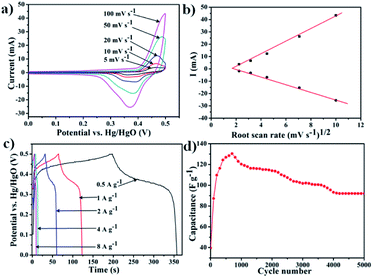
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and loaded to a piece of nickel foam. A Pt plate and Hg/HgO electrode were used as the counter electrode and reference electrode, respectively. The electrochemical charge storage activity of the POPM–TFP was analysed by cyclic voltammetry (CV), galvanostatic charge discharge (GCD) and electrochemical impedance spectroscopy (EIS) in 2 M KOH electrolyte. The CV curves of POPM–TFP at 5 mV s−1, 10 mV s−1, 20 mV s−1, 50 mV s−1, and 100 mV s−1 are presented in Fig. 3a. The CV recorded with the as-prepared electrode over different scan rates shows redox peaks at ∼0.34–0.45 V during forward and backward sweeps, suggesting its pseudo capacitive nature. The redox signature is attributed to the oxidation and reduction of the triazine skeleton in the polymer. The small separation between cathodic and anodic peaks indicated that the fast electron transfer kinetics between POPM–TFP and the electrode interface. The current plateau of CV over the increasing scan rate increased, suggesting fast accessible ions electrolyte through the pore channel of POPM–TFP.
10 and loaded to a piece of nickel foam. A Pt plate and Hg/HgO electrode were used as the counter electrode and reference electrode, respectively. The electrochemical charge storage activity of the POPM–TFP was analysed by cyclic voltammetry (CV), galvanostatic charge discharge (GCD) and electrochemical impedance spectroscopy (EIS) in 2 M KOH electrolyte. The CV curves of POPM–TFP at 5 mV s−1, 10 mV s−1, 20 mV s−1, 50 mV s−1, and 100 mV s−1 are presented in Fig. 3a. The CV recorded with the as-prepared electrode over different scan rates shows redox peaks at ∼0.34–0.45 V during forward and backward sweeps, suggesting its pseudo capacitive nature. The redox signature is attributed to the oxidation and reduction of the triazine skeleton in the polymer. The small separation between cathodic and anodic peaks indicated that the fast electron transfer kinetics between POPM–TFP and the electrode interface. The current plateau of CV over the increasing scan rate increased, suggesting fast accessible ions electrolyte through the pore channel of POPM–TFP.
The specific capacitance of the electrode materials is estimated from the follow equation,39
| Csp = (∫IdV)/vmV | (1) |
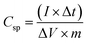 | (2) |
Furthermore, the interfacial charge transfer activity and electrocapacitive characteristics of POPM–TFP are studied by EIS analysis. The impedance characteristic of the electrode is represented by a Nyquist plot (Fig. S6†). From the analysis of the Nyquist plots, POPM–TFP displays a near vertical line within the low-frequency region, indicative of a well capacitive behavior. The slope of the line at low frequency region is related to the diffusive resistivity of the electrolyte ions within the nanopores. The small semicircular shapes in the EIS plots in the high frequency could be neglected indicates a low charge transfer resistance at the electrode/electrolyte interface (inset in Fig. S3†).43 We also estimated the accessible electro-active sites of POPM–TFP system by the method already discussed in the previous literature.16,20 These calculations suggest the about 1.99% of the triazine moieties are accessible.
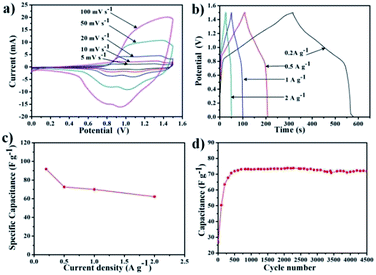
To evaluate the energy and power density, the supercapacitor performance of POPM–TFP was tested in two electrode system using 2 M KOH as electrolyte. Activated carbon and as-prepared POPM–TFP were used as negative and positive electrodes in the asymmetric supercapacitor fabrication, respectively. The asymmetric supercapacitor was investigated in the potential window of 0 to 1.5 V at different scan rates (Fig. 4a). The current increases with increasing scan rate suggesting a good rate capability for energy storage. The CV curves and GCD curves (Fig. 4b) indicated their typical pseudo capacitive behaviour. The calculated specific capacitances of the supercapacitor at different current densities are summarized in Fig. 4c. The specific capacitance at current density of 0.2 A g−1 was 91.7 F g−1. The highest energy density and power density were calculated to be 25.8 W h kg−1 and 727 W kg−1, respectively (Fig. S7†). The cycle life of the asymmetric supercapacitor was further investigated at 0.5 A g−1 (Fig. 4d). The specific capacitance retained 72.5 after 4500 GCD cycles at current density of 0.5 A g−1, demonstrating the outstanding stability of the device.
In summary, by a simple solvothermal synthesis method, we have successfully synthesized a crystalline triazine containing porous organic polymer POPM–TFP through simple condensation reaction between M and TFP using dimethyl sulfoxide solvent without any catalysts. Further, we have used POPM–TFP as an electrode material for supercapacitor application. The electrochemical measurement of POPM–TFP material showed well electro-chemical performance in supercapacitors. The wonderful electrochemical properties of the POPM–TFP can be ascribed to the synergistic effect of the π-conjugated triazine contained POPM–TFP and nanopores structure. Active sites triazine moieties which put up redox behavior distributed in the pore channels. These findings will meet the requirements like energy storage ability and help more energy efficient electrode materials in the near future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21505085).Notes and references
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928 CrossRef CAS PubMed
.
- X. Liu, N. Wen, X. Wang and Y. Zheng, ACS Sustainable Chem. Eng., 2015, 3, 475 CrossRef CAS
.
- M. Beidaghi and Y. Gogotsi, Energy Environ. Sci., 2014, 7, 867 RSC
.
- A. M. Khattak, H. Yin, Z. A. Ghazi, B. Liang, A. Iqbal, N. A. Khan, Y. Gao, L. Li and Z. Tang, RSC Adv., 2016, 6, 58994 RSC
.
- J. P. Paraknowitsch and A. Thomas, Energy Environ. Sci., 2013, 6, 2839 RSC
.
- C. Merlet, B. Rotenberg, P. A. Madden, P.-L. Taberna, P. Simon, Y. Gogotsi and M. Salanne, Nat. Mater., 2012, 11, 306 CrossRef CAS PubMed
.
- Y. Kou, Y. Xu, Z. Guo and D. Jiang, Angew. Chem., Int. Ed., 2011, 50, 8753 CrossRef CAS PubMed
.
- C. Wang, Y. Xi, M. Wang, C. Zhang, X. Wang, Q. Yang, W. Li, C. Hu and D. Zhang, Nano Energy, 2016, 28, 115 CrossRef CAS
.
- C. R. Mulzer, L. Shen, R. P. Bisbey, J. R. McKone, N. Zhang, H. D. Abruna and W. R. Dichtel, ACS Cent. Sci., 2016, 2, 667 CrossRef CAS PubMed
.
- S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC
.
- K. Sakaushi, G. Nickerl, H. C. Kandpal, L. Cano-Cortes, T. Gemming, J. Eckert, S. Kaskel and J. van den Brink, J. Phys. Chem. Lett., 2013, 4, 2977 CrossRef CAS
.
- S. Hug, L. Stegbauer, H. Oh, M. Hirscher and B. V. Lotsch, Chem. Mater., 2015, 27, 8001 CrossRef CAS
.
- L. Hao, B. Luo, X. Li, M. Jin, Y. Fang, Z. Tang, Y. Jia, M. Liang, A. Thomas, J. Yang and L. Zhi, Energy Environ. Sci., 2012, 5, 9747 RSC
.
- T. Lin, I. W. Chen, F. Liu, C. Yang, H. Bi, F. Xu and F. Huang, Science, 2015, 350, 1508 CrossRef CAS PubMed
.
- L. Xu, Y. Yu, J. Lin, X. Zhou, W. Q. Tian, D. Nieckarz, P. Szabelski and S. Lei, Nanoscale, 2016, 8, 8568 RSC
.
- C. R. DeBlase, K. E. Silberstein, T. Thanh-Tam, H. D. Abruna and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821 CrossRef CAS PubMed
.
- C. R. DeBlase, K. Hernandez-Burgos, K. E. Silberstein, G. G. Rodriguez-Calero, R. P. Bisbey, H. D. Abruna and W. R. Dichtel, ACS Nano, 2015, 9, 3178 CrossRef CAS PubMed
.
- L. Hao, J. Ning, B. Luo, B. Wang, Y. Zhang, Z. Tang, J. Yang, A. Thomas and L. Zhi, J. Am. Chem. Soc., 2015, 137, 219 CrossRef CAS PubMed
.
- Y. Su, Y. Liu, P. Liu, D. Wu, X. Zhuang, F. Zhang and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 1812 CrossRef CAS PubMed
.
- A. M. Khattak, Z. A. Ghazi, B. Liang, N. A. Khan, A. Iqbal, L. Li and Z. Tang, J. Mater. Chem. A, 2016, 4, 16312 RSC
.
- V. Sharma, S. Khilari, D. Pradhan and P. Mohanty, RSC Adv., 2016, 6, 56421 RSC
.
- B. C. Patra, S. Khilari, L. Satyanarayana, D. Pradhan and A. Bhaumik, Chem. Commun., 2016, 52, 7592 RSC
.
- X. Zhan, Z. Chen and Q. Zhang, J. Mater. Chem. A, 2017, 5, 14463 RSC
.
- J. Zhang, Z. Wang, L. Li, J. Zhao, J. Zheng, H. Cui and Z. Zhu, J. Mater. Chem. A, 2014, 2, 8179 RSC
.
- Y. Li, S. Zheng, X. Liu, P. Li, L. Sun, R. Yang, S. Wang, Z.-S. Wu, X. Bao and W.-Q. Deng, Angew. Chem., Int. Ed., 2018, 57, 7992 CrossRef CAS PubMed
.
- W. Wang, Y. Yuan, F.-X. Sun and G.-S. Zhu, Chin. Chem. Lett., 2014, 25, 1407 CrossRef CAS
.
- B. Yang, J. Bjork, H. Lin, X. Zhang, H. Zhang, Y. Li, J. Fan, Q. Li and L. Chi, J. Am. Chem. Soc., 2015, 137, 4904 CrossRef CAS PubMed
.
- H. Wei, S. Chai, N. Hu, Z. Yang, L. Wei and L. Wang, Chem. Commun., 2015, 51, 12178 RSC
.
- P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450 CrossRef CAS PubMed
.
- D. D. Medina, J. M. Rotter, Y. Hu, M. Dogru, V. Werner, F. Auras, J. T. Markiewicz, P. Knochel and T. Bein, J. Am. Chem. Soc., 2015, 137, 1016 CrossRef CAS PubMed
.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, 923 CrossRef CAS PubMed
.
- F. J. Uribe-Romo, J. R. Hunt, H. Furukawa, C. Klock, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 4570 CrossRef CAS PubMed
.
- M. G. Schwab, B. Fassbender, H. W. Spiess, A. Thomas, X. Feng and K. Muellen, J. Am. Chem. Soc., 2009, 131, 7216 CrossRef CAS PubMed
.
- N. Sahiner, S. Demirci and K. Sel, J. Porous Mater., 2016, 23, 1025 CrossRef CAS
.
- X. Huang, Z. Wu, H. Zheng, W. Dong and G. Wang, Green Chem., 2018, 20, 664 RSC
.
- Y. Cui, Z. Ding, X. Fu and X. Wang, Angew. Chem., Int. Ed., 2012, 51, 11814 CrossRef CAS PubMed
.
- B. Jurgens, E. Irran, J. Senker, P. Kroll, H. Muller and W. Schnick, J. Am. Chem. Soc., 2003, 125, 10288 CrossRef PubMed
.
- S. Chandra, T. Kundu, K. Dey, M. Addicoat, T. Heine and R. Banerjee, Chem. Mater., 2016, 28, 1489 CrossRef CAS
.
- Y. Ma, W. Chen, P. Zhang, F. Teng, J. Zhou, X. Pan and E. Xie, RSC Adv., 2014, 4, 47609 RSC
.
- J.-W. Jeon, R. Sharma, P. Meduri, B. W. Arey, H. T. Schaef, J. L. Lutkenhaus, J. P. Lemmon, P. K. Thallapally, M. I. Nandasiri, B. P. McGrail and S. K. Nune, ACS Appl. Mater. Interfaces, 2014, 6, 7214 CrossRef CAS
.
- W. Lv, M. Guo, M.-H. Liang, F.-M. Jin, L. Cui, L. Zhi and Q.-H. Yang, J. Mater. Chem., 2010, 20, 6668 RSC
.
- F. Zhao, Y. Wang, X. Xu, Y. Liu, R. Song, G. Lu and Y. Li, ACS Appl. Mater. Interfaces, 2014, 6, 11007 CrossRef CAS PubMed
.
- J. Romero, D. Rodriguez-San-Miguel, A. Ribera, R. Mas-Balleste, T. F. Otero, I. Manet, F. Liscio, G. Abellan, F. Zamora and E. Coronado, J. Mater. Chem. A, 2017, 5, 4343 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, instrumentation and characterization of POPM–TFP, other figures and electrochemical measurements. See DOI: 10.1039/c8ra09099h |
| This journal is © The Royal Society of Chemistry 2019 |