Open Access Article
Open Access ArticleSecondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes
Xiaofeng Zhang ,
Qiongbo Hu
,
Qiongbo Hu * and
Qunfang Weng
* and
Qunfang Weng *
*
College of Agriculture, South China Agricultural University, Guangzhou 510642, China. E-mail: hqbscau@scau.edu.cn; wengweng@scau.edu.cn
First published on 21st December 2018
Abstract
Both Isaria cicadae and Isaria tenuipes are important entomopathogenic fungi used in health foods and traditional herbal medicines in East Asia. However, the safety concerns for both fungal species have been attracting significant attention. Thus, surveying their secondary metabolites (SMs) will be beneficial to improving the safety of their fungal products. In the case of I. cicadae, its SMs mainly include nucleosides, amino acids, beauvericins, myriocin, and oosporein. In contrast, trichothecene derivatives, isariotins, cyclopenta benzopyrans and PKs, are found in the case of I. tenuipes. Among them, beauvericins, myriocin, oosporein and many trichothecene derivatives are toxic compounds. The toxicity and side effects of the fungal products may be related to these SMs. Thus, to ensure the safety of fungal products, the residues standards of SMs need to be reported. Furthermore, methods for the detection of their SMs and biological identification of their strains must be considered. This review gives new insight into the secondary metabolites of medical and edible fungi.
1 Introduction
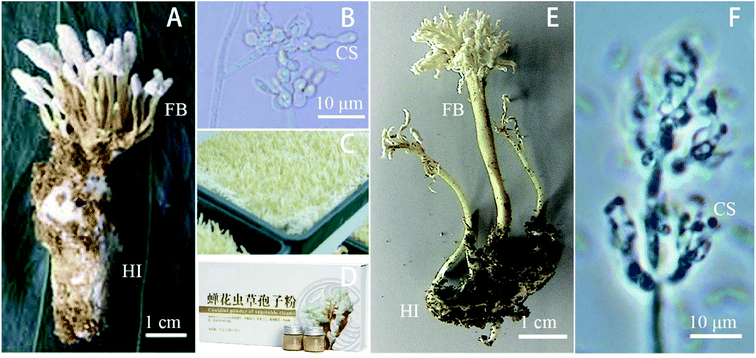
Isaria is an entomopathogenic fungal genus with more than 100 species (http://www.mycobank.org/), which plays important roles in biodiversity conservation and is utilized in medicines and agriculture.1,2 Both I. cicadae and I. tenuipes (Fig. 1) belonging to the anamorph of Cordyceps genus, are popular mushrooms mostly infecting insects (in nature, often the underground nymphs of cicadas), leading to their death, and then produces white fruit body from the dead insect, which releases white powdery spores. The fruit body is used in expensive traditional medicines and health products in East Asia.2–4I. cicadae, the asexual type of C. cicadae, is a famous mushroom. In nature, the fruiting bodies form uniquely on pupated cicadae (Platylomia spp.) and are referred to as “cicada flower” or the Chinese name “Jingchanhua” in China. In the laboratory, this fungus can be inoculated in the pupae of Chinese tussah silkworm (Antheraea pernyi). Interestingly, in contrast to other Cordyceps species that produce sexual fruiting bodies in nature, C. cicadae forms synnema-like asexual structures.5 I. sinclairii was once known as C. cicadae, C. kobayasii, Paecilomyces cicadae, P. sinclairii and C. sinclairii.6–8 However, it is considered as the synonym of I. cicadae.9 This species is found in Asia (particularly China, Japan, and Korea) and New Zealand. It infects cicadas of the genera Amphipsalta and Melampsalta in nature, and in the lab it can be cultured on the bodies of silkworms,10 which also shows potential as a biological control agent against the agricultural pest Plutella xylostella.11 Its fruit body is used to treatment cancers and improve the eyesight and renal function and immunity of patients.12 It also has anti-hypertensive effect in spontaneously hypertensive rats.13 However, it was reported that it induced kidney toxicity in rats exposed to its fungus fruit bodies.14,15
I. tenuipes is a common entomopathogenic fungus that usually infects Lepidopteran insects.16 It has the synonymies of C. tenuipes and P. tenuipes, and it was also called Spicaria heliothis, C. concurrens, C. polyarthra, and C. subpolyarthra (http://www.mycobank.org/). Moreover, it is considered as the anamorph of C. takaomontana17 and synonymy I. japonica.18 Its fruiting body is used in the production of health foods and traditional medicine with the functions of lowering blood-glucose, antitumor, antibacterial, anti-depression, anti-oxidation, and anti-aging activity, lowering blood fat and immunoregulation.7,18,19
However, safety concerns of consuming these entomopathogenic fungi have been frequently raised due to the uncertainty of the fungal production of human-toxic mycotoxins.20 In fact, Isaria fungi have rigorous secondary metabolisms through different pathways, including non-ribosomal peptide synthetase (NRPS), polyketide synthase (PKS) and terpenoid synthetase (TS), which produce various secondary metabolites (SMs), such as ribosomal peptides (NRPs), polyketides (PKs), terpenoid and miscellaneous types.5,21,22 According to the research (patents not included) published in the past forty years, it is estimated that more than 200 SMs have been isolated and identified from both Isaria fungi. Many SMs are the functional components with anti-virus, anti-bacteria, and anti-tumor activity and immunity regulation.23,24 Interestingly, SMs are considered as important drug resources. For example, fingolimod, a novel type of immunosuppressive agent, is a new medicine derived from I. sinclairii, which is used for treating multiple sclerosis, renal cancer, and asthma.25,26 However, many SMs belong to mycotoxins, which are toxic to humans and animals. Nevertheless, there are few documents reviewing the risks of Isaria fungi mycotoxins. Thus, the current review focuses on the mycotoxins of both Isaria fungi, including their structures, bioactivities and toxicities, as well as their risk evaluation.
2 SMs from I. cicadae
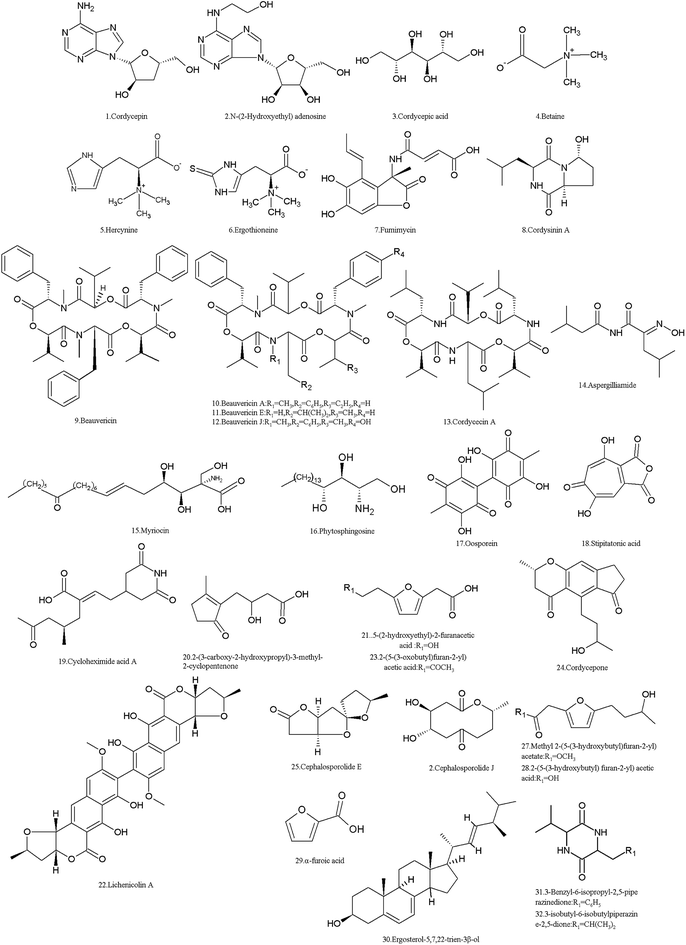
I. cicadae produces numerous SMs (Table 1 and Fig. 2). Using metabolism omics analysis, Lu et al. reported that more than 110 compounds were detected in the mycelia, primordia and stroma stages of the fungus.5 While eight compounds were found in C. cicadae sporoderm-broken spore powders (CCBSP) using UPLC-ESI-Q-TOF-MS, and forty-nine volatile molecules representing 99.56% of CCBSP were clearly identified by GC-MS.27 Moreover, another experiment reported that 60 metabolites including amino acids and their derivatives, saccharides, organic acids and fatty acids were identified in the methanol extract of the complex of the cicadae body and I. cicadae stroma, while inositol, gamma-aminobutyric acid, ornithine and threonine were the most abundant compounds.28| Metabolite | CAS no. | Material source | Biological activity |
|---|---|---|---|
| Cordycepin (1) | 73-03-0 | Sporoderm-broken spore powders of wild C. cicadae | Immunomodulatory, antibacterial, antiviral, and anti-tumor effects on cells |
| N-(2-Hydroxyethyl) adenosine (2) | 4338-48-1 | Cultivated complex of fruit body of I. sinclairii and silkworm | |
| Cordycepic acid (3) | 69-65-8 | Sporoderm-broken spore powders of wild C. cicadae | Bacteriostatic activity |
| Betaine (4) | 107-43-7 | Mycelia of C. cicadae CCAD02 | Regulate internal osmotic pressure, relieve stress, and promote fat metabolism and protein synthesis |
| Hercynine (5) | 534-30-5 | Mycelia of C. cicadae CCAD02 | Antioxidant |
| Ergothioneine (6) | 497-30-3 | Mycelia of C. cicadae CCAD02 | Antioxidant |
| Fumimycin (7) | 942472-95-9 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | |
| Cordysinin A (8) | 1330197-09-5 | Mycelia of C. cicadae CCAD02 | Inhibiting the proliferation of human glioma U87 mg and U251 cells. Anti-inflammatory activity in human neutrophils |
| Beauvericin (9) | 26048-05-5 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | |
| Beauvericin A (10) | 165467-50-5 | Wild complex of fruit body of C. cicadae and insect-body (specimen no. 2012081827) | Significant inhibitory effect on hepg2 and hepg2/ADM cells with IC50 values ranging from 2.40–14.48 μM. Cytotoxicity against hepg2/ADM cell line with IC50 value 25-fold more sensitive to doxorubicin |
| Beauvericin E (11) | 728912-27-4 | Wild complex of fruit body of C. cicadae and insect-body (specimen no. 2012081827) | Significant inhibitory effect on hepg2 and hepg2/ADM cells with IC50 values ranging from 2.40–14.48 μM |
| Beauvericin J (12) | 1342821-26-4 | Wild complex of fruit body of C. cicadae and insect-body (specimen no. 2012081827) | Significant inhibitory effect on hepg2 and hepg2/ADM cells with IC50 values ranging from 2.40–14.48 μM |
| Cordycecin A (13) | 1799406-02-2 | Wild complex of fruit body of C. cicadae and insect-body (specimen no. 2012081827) | |
| Aspergilliamide (14) | 1691204-76-8 | Mycelia of C. cicadae CCAD02 | Medium brine shrimp toxicity |
| Myriocin (15) | 35891-70-4 | Sporoderm-broken spore powders of wild C. cicadae. Cultivated mycelia of C. cicadae | Serine palmitoyltransferase inhibitor |
| Phytosphingosine (16) | 554-62-1 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | |
| Oosporein (17) | 475-54-7 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | Food refusal effect on crop pests Prodenia litura and Spodoptera exigua. Insecticide to Lepidopteran pests |
| Stipitatonic acid (18) | 606-39-3 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | Bactericidal and insecticidal activities, as well as growth-inhibitory effects on some pathogenic fungi |
| Cycloheximide acid A (19) | 1610848-67-3 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | |
| 2-(3-Carboxy-2-hydroxypropyl)-3-methyl-2-cyclopentenone (20) | 1019196-97-4 | Cultivated mycelia of C. cicadae | |
| 5-(2-Hydroxyethyl)-2-furanacetic acid (21) | 1018901-08-0 | Cultivated mycelia of C. cicadae | |
| Lichenicolin A (22) | 883977-62-6 | Mycelia, cultivated complex of fruit body of C. cicadae CCAD02 and Antheraea pernyi | |
| 2-(5-(3-Oxobutyl)furan-2-yl)acetic acid (23) | A strain of cell fusion from C. cicadae and C. militaris | Weak inhibitory activity against AChE | |
| Cordycepone (24) | A strain of cell fusion from C. cicadae and C. militaris | ||
| Cephalosporolide E (25) | 97373-15-4 | A strain of cell fusion from C. cicadae and C. militaris | |
| Cephalosporolide J (26) | 97344-02-0 | A strain of cell fusion from C. cicadae and C. militaris | |
| Methyl-2-(5-(3-hydroxybutyl)furan-2-yl)acetate (27) | 851868-04-7 | A strain of cell fusion from C. cicadae and C. militaris | Weak inhibitory activity against AChE |
| 2-(5-(3′-Hydroxybutyl) furan-2-yl) acetic acid (28) | 170445-59-7 | A strain of cell fusion from C. cicadae and C. militaris | Weak inhibitory activity against AChE |
| α-furoic acid (29) | 88-14-2 | A strain of cell fusion from C. cicadae and C. militaris | Weak inhibitory activity against AChE. Moderate inhibitory activity against the nematode Panagrellus redivivus |
| (22E,24R)-Ergosta-5,7,trien-3β-ol (ergosterol) (30) | 57-87-4 | A strain of cell fusion from C. cicadae and C. militaris | |
| 3-Benzyl-6-isopropyl-2,5-piperazinedione (31) | 14474-71-6 | A strain of cell fusion from C. cicadae and C. militaris | Moderate inhibitory activity against the nematode Panagrellus redivivus |
| 3-Isobutyl-6-isobutylpiperazine-2,5-dione (32) | 5625-50-3 | A strain of cell fusion from C. cicadae and C. militaris |
Based on their molecular structures, I. cicadae SMs are mainly categorized as organic acids, amino acids, lipids and phospholipids, nucleosides, carbohydrates and their derivatives. Among them, some compounds are functional or bioactive components, for example, cordycepin (1) and cordycepic acid (3) are considered as the bioactive components of I. cicadae, similar with Cordyceps spp.29 Furthermore, many other compounds such as adenosine, guanosine, uridine, inosine and thymidine and other nucleosides, ergosterol and its peroxide, and D-mannitol beneficial for pharmacological functions.30,31 However, some of the SMs are toxic to humans and animals, while probably most SMs are unknown, and their molecular structures and biological functions have not been elucidated.5 The main nucleoside of this fungus is cordycepin (1), 3′-deoxyadenosine, which was isolated from the sporoderm-broken spore powders and fruit bodies of C. cicadae.27,32 As an adenosine derivative, it is an important parameter for quality control in medicines, referring to Dongchongxiacao CORDYCEPS, Cordyceps sinensis (BerK.) Sacc. in the Chinese Pharmacopoeia, 2015. Since cordycepin (1) is similar to adenosine, it can participate in certain biochemical reactions (for example, be incorporated into an RNA molecule, thus causing the premature termination of its synthesis)33 and has multiple bioactivities such as anti-cancer, antimetastatic, antioxidant, antidepressant and immunoregulation properties.34–37 It also enhances male reproduction by stimulating in vitro and in vivo steroidogenesis in mouse Leydig cells by activating the PKA pathway.38 In 2007, the US Food and Drug Administration (FDA) granted cordycepin orphan drug designation for the treatment of TdT-positive acute lymphocytic leukemia (https://www.fda.gov/). Cordycepin plus pentostatin was subjected to a clinic trail of phase 1/phase 2 in patients with refractory TdT-positive leukemia in 2008–2010 (https://www.clinicaltrials.gov/). In China, it was reported that cordycepin was used for clinic adjuvant treatment of non-small cell lung cancer39 and nephropathy of type 2 diabetes mellitus.40 However, the main fungal species used to produce cordycepin in industry is C. militaris.41
Another adenosine derivative, N-(2-hydroxyethyl) adenosine (2) was isolated and proven to exhibit antioxidant activity by measuring its radical scavenging effect on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals.42
Cordycepic acid (3) (D-mannitol), an isomer of quinic acid with a polyol structure, was isolated from sporoderm-broken spore powders.27 It is believed to be another active ingredient and is used as an important index of quality control for C. sinensis products. It exhibits diuretic action and prophylaxis against postoperative acute renal failure, relieving cough and asthma, and anti-free radical activities. Specifically, it can be used to provide effective protection or treatment for patients after cerebral ischemia and trauma, such as improving cerebral microcirculation and cerebral blood flow.43
The amino derivative betaine (4) (N,N,N-trimethylglycine), which was first found in sugar beet in the nineteenth century, was detected in the mycelia, primordia and stroma of C. cicadae.5 Betaine (4) is a methyl donor of increasingly recognized significance in biology, and serves as an organic osmolyte, which is a substance synthesized or taken up from the environment by cells for protection against osmotic stress, drought, high salinity, and high temperature.44 Hercynine (5), a derivative of histidine, was detected in the mycelia, primordia and stroma of C. cicadae.5 However, ergothioneine (6) was identified in its fruit body and mycelia.45 Hercynine (5) is considered as the primer for ergothioneine biosynthesis, which is a secreted antioxidant that protects cells from oxidative stress.46 Fumimycin (7), an unusual metabolite incorporating an unusual alanine unit linked to a phenyl group at the alpha-carbon with both lactone and amide moieties, was first isolated from Aspergillus fumisynnematus, and it was detected in the mycelia, primordia and stroma of C. cicadae.5 It showed antibacterial activity against Staphylococcus aureus with an IC50 value of 4.1 μM, inhibiting peptide deformylase.47
Cordysinin A (8), a diketopiperazine first identified from C. sinensis,48,49 was detected in the mycelia of C. cicadae.5 It shows activity in inhibiting the proliferation of human glioma U87-MG and U251 cells.50 It has anti-inflammatory activity in human neutrophils, which was assessed by its inhibition of FMLP/CB-induced superoxide anion generation (https://pubchem.ncbi.nlm.nih.gov).
Some of the SMs in I. cicadae are toxic to animals and humans. Among them, five NRPs are common mycotoxins. Beauvericin (9) and its analogues A, E, J (10–12), and cordycecin A (13), were isolated from its fruit body,51 and beauvericin was also detected in other stages (mycelia, primordia and stroma).5 The four beauvericins (9–12) exhibited a significant inhibitory effect on HepG2 and HepG2/ADM cells with IC50 values ranging from 2.40–14.48 μM. However, cordycecin A (13) had no such activity.51 Beauvericins have insecticidal effects at a microgram level to several insects.52 The cytotoxicity of beauvericins on human cells and cancer cells was also discovered.53–55 Thus, the risks of beauvericins to contaminate foods and influence human health are attracting the attention of researchers.56,57 Aspergilliamide (14) is also an amino derivative, which was detected in the primordia of I. cicadae.5 It was toxic to brine shrimp with an LC50 value of 71.09 nM.58
Myriocin (15) (ISP-1, thermozymocidin), an atypical amino acid, was isolated from the culture broth, mycelia and sporoderm-broken spore powders of C. cicadae27,59–61 and from the culture broth of the I. sinclairii ATCC24400 strain.62 In fact, it was named myriocin because it was first identified from the thermophilic ascomycete Myriococcum albomyces. It exhibited good anti-fungal activity against Candida albicans. However, the acute toxicity of myriocin is too high for therapeutic purposes. It had intraperitoneal toxicity with an LD50 of 5–10 and 2–5 mg kg−1 for mice and rats, respectively, and resulted in the death of dogs 48–72 hours after they were treated by subcutaneous injection of 0.25 mg kg−1.63 Therefore, myriocin did not attract more attention until it was found in the culture broth of the strain CTCC 24400 of I. sinclairii and exhibited 10- to 100-fold more potency than cyclosporin A as an immunosuppressive agent of the immune response in vitro and in vivo.64 In 1995, a novel immunosuppressant, FTY720 (fingolimod), was first synthesized and screened based on the knowledge that 2-substituted 2-aminoethanol is the minimum essential structure for the immunosuppression activity of myriocin.65,66 Different from the action mechanism of myriocin, which inhibits serine palmitoyl transferase, FTY720 is a sphingosine 1-phosphate (S1P) receptor modulator, which is mainly phosphorylated by sphingosine kinase 2 in vivo. The phosphorylated drug (-P) acts as a potent agonist of four of the five G protein-coupled receptors for S1P: S1P(1), S1P(3), S1P(4), and S1P(5).67 FTY720 is now used worldwide for the treatment of multiple sclerosis.68
Phytosphingosine (16), a myriocin-like long-chain compound, was also found in the mycelia, primordia and stroma of C. cicadae.5 In the budding yeast Saccharomyces cerevisiae, the endoplasmic reticulum stress surveillance pathway is activated by phytosphingosine, which ensures that daughter cells inherit a functional ER.69
Oosporein (17), an important dibenzoquinone pigment identified early,70 was also detected in the mycelia, primordia and stroma of C. cicadae.5 It exhibited median oral toxicity to 1 day-old cockerels.71 Oosporein inhibits total erythrocyte membrane ATPase activity in a dose-dependent manner due to alterations in erythrocyte morphology and promotes varying degrees of cell lysis.72 Oosporein also exhibits broad spectrum in vitro antimicrobial, antioxidant and cytotoxic activities.73 Oosporein is a rather strong organic acid and can hardly be adsorbed by organisms; thus, oosporein is unlikely to enter the food chain and influence human health.74 Also, this fungus produces other pigments and is considered as a resource of natural color materials for the food industry.75,76 However, the exact components in its pigments are not clear.
Stipitatonic acid (18), a tropolone compound isolated in early 1959 from Penicillium stipitatum, was also detected in the mycelia, primordia and stroma of C. cicadae.5 Stipitatonic acid shows bactericidal and insecticidal activities, as well as growth-inhibitory effects on some pathogenic fungi, especially Pythium aphanidermatum.77
Many SMs are unknown, and their bioactivity, toxicity, and even molecular structure are unclear. For example, twelve unknown compounds were detected in C. cicadae by Lu et al.5 Meanwhile, cycloheximide acid A (19), 2-(3-carboxy-2-hydroxypropyl)-3-methyl-2-cyclopentenone (20), 5-(2-hydroxyethyl)-2-furanacetic acid (21) and lichenicolin A (22) were identified in different stages of C. cicadae,78 but their bioactivities are not yet known.
Moreover, some new compounds were found in fungal hybrids. A strain from the cell fusion of C. cicadae and C. militaris produces different SMs including two new compounds, 2-(5-(3-oxobutyl) furan-2-yl) acetic acid (23) and cordycepone (24), as well as eleven known compounds, cephalosporolide E (25), cephalosporolide J (26), methyl 2-(5-(3-hydroxybutyl)furan-2-yl)acetate (27), 2-(5-(3-hydroxybutyl) furan-2-yl) acetic acid (28), α-furoic acid (29), (22E,24R)-ergosta-5,7,22-trien-3β-ol (ergosterol) (30), 3-benzyl-6-isopropyl-2,5-piperazinedione (31), and 3-isobutyl-6-isobutylpiperazine-2,5-dione (32).79 The AChE inhibitory activity of all the compounds was tested, and the results showed that compounds 23 and 27–29 at a concentration of 50 μg mL−1 showed weak inhibitory activity against AChE with inhibition rates of 15.45%, 17.89%, 15.07%, and 16.41%, respectively. Moreover, the bioassay for the anti-nematode activity of all the isolates showed that compounds 29 and 31 exhibited moderate inhibitory activity against the nematode Panagrellus redivivus with mortality ratios of 71.66% and 72.29% at 2.5 mg mL−1, respectively.79
3 SMs from I. tenuipes
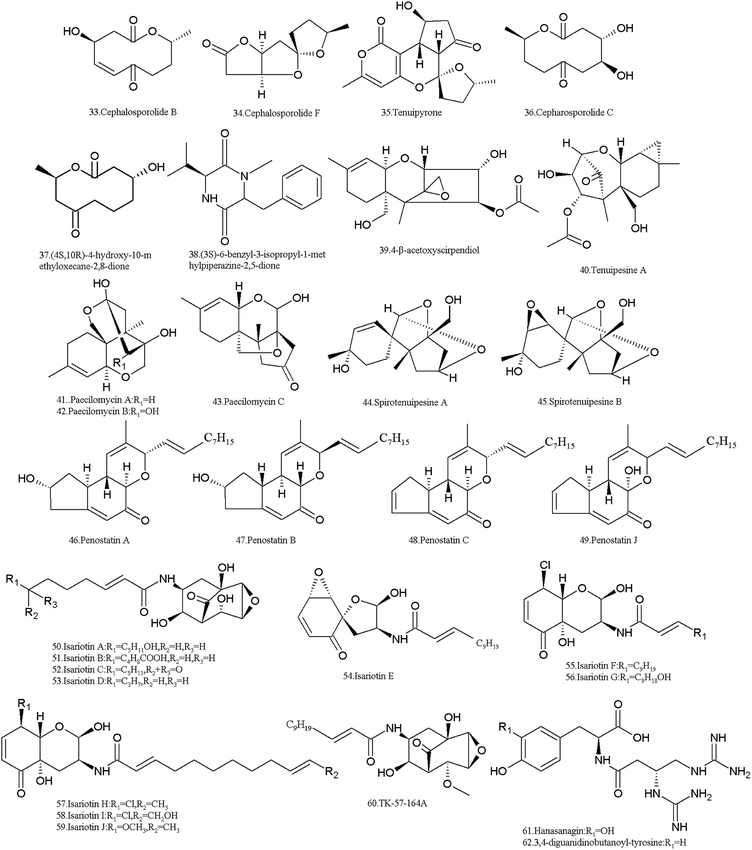
More than twenty SMs were isolated from I. tenuipes, and most of them belong to compounds from the PKS pathway (Table 2 and Fig. 3). Cephalosporolides B (33), F (34) and tenuipyrone (35) were isolated in 2012 by Asai et al.80 Cephalosporolide B is thought to be a true biosynthetic precursor of tenuipyrone,80 which can be used as a precursor for the chemical synthesis of cephalosporin C (36), G and (4-OMe-) G.81 Cephalosporolide F (34) showed moderate inhibitory activity against the nematode Panagrellus redivivus with a mortality ratio of 79.0% at 2.5 mg mL−1.79| Metabolites | CAS no. | Material source | Biological activity |
|---|---|---|---|
| Cephalosporolide B (33) | 97344-03-1 | Culture broth | |
| Cephalosporolide F (34) | 97344-04-2 | Culture broth of I. tenuipes | Moderate inhibitory activity against the nematode Panagrellus redivivus |
| Tenuipyrone (35) | 1354559-27-5 | Culture broth of I. tenuipes | |
| Cephalosporolide C (36) | 97344-02-0 | Culture broth of I. tenuipes | |
| (4R,10R)-4-Hydroxy-10-methyloxecane-2,8-dione (37) | 2098945-28-7 | Culture broth of I. tenuipes | Inhibit prostate cancer cells 22RV1 and DU-145 at the rates of 32.5% and 40.6% at 5 μmol L−1, respectively |
| (3S)-6-Benzyl-3-isopropyl-1-methylpiperazine-2,5-dione (38) | 2101845-87-6 | Culture broth of I. tenuipes | Inhibit prostate cancer cells 22RV1 and DU-145 at the rates of 37.8% and 38.6% at 5 μmol L−1, respectively |
| 4-β-Acetoxyscirpendiol (39) | 2531-11-5 | Cultivated fruiting bodies of I. japonica (voucher no. CHO-6133) | Induces apoptosis of human leukemia cells (HL-60). Lower blood sugar levels in the circulatory system as effective SGLT-1 inhibitors |
| Tenuipesine A (40) | 816448-01-8 | Cultivated fruiting bodies of P. tenuipes MH19912 | |
| Paecilomycine A (41) | 594840-03-6 | Cultivated fruiting bodies of P. tenuipes MH19912 | Activity in neurotrophic factor biosynthesis in glial cells |
| Paecilomycine B (42) | 594840-04-7 | Cultivated fruiting bodies of P. tenuipes MH19912 | |
| Paecilomycine C (43) | 765942-39-0 | Cultivated fruiting bodies of P. tenuipes MH19912 | |
| Spirotenuipesine A (44) | 594840-05-8 | Cultivated fruiting bodies of P. tenuipes MH19912 | Potent activity in neurotrophic factor biosynthesis in glial cells |
| Spirotenuipesine B (45) | 594840-06-9 | Cultivated fruiting bodies of P. tenuipes MH19912 | Potent activity in neurotrophic factor biosynthesis in glial cells |
| Penostatin A (46) | 173485-70-6 | Mixture of cultivated mycelium and medium of P. tenuipes RCEF 37776 | Protein phosphatase 1B inhibitors |
| Penostatin B (47) | 173655-56-6 | Mixture of cultivated mycelium and medium of P. tenuipes RCEF 37776 | Protein phosphatase 1B inhibitors |
| Penostatin C (48) | 173485-71-7 | Mixture of cultivated mycelium and medium of P. tenuipes RCEF 37776 | Protein phosphatase 1B inhibitors |
| Penostatin J (49) | 1695557-12-0 | Mixture of cultivated mycelium and medium of P. tenuipes RCEF 37776 | Protein phosphatase 1B inhibitors |
| Isariotin A (50) | 952703-96-7 | Culture broth of I. tenuipes BCC7831 | Marginal activity against the Mycobacterium tuberculosis H37Ra with the MIC value of 486 μM |
| Isariotin B (51) | 952703-97-8 | Culture broth of I. tenuipes BCC7831 | |
| Isariotin C (52) | 952703-98-9 | Culture broth of I. tenuipes BCC7831 | Marginal activity against the Mycobacterium tuberculosis H37Ra with the MIC value of 488 μM |
| Isariotin D (53) | 952703-99-0 | Culture broth of I. tenuipes BCC7831 | Marginal activity against the Mycobacterium tuberculosis H37Ra with the MIC value of 544 μM |
| Isariotin E (54) | 1137665-60-1 | Culture broth of I. tenuipes BCC12625 | |
| Isariotin F (55) | 1137665-61-2 | Culture broth of I. tenuipes BCC12625 | Antimalarial activity against Plasmodium falciparum K1; Antitubercular activity against Mycobacterium tuberculosis H37Ra; Antifungal activity against Candida albicans; cytotoxic activities against three cancer cell lines (KB, BC, and NCI-H187) |
| TK-57-164A (60) | 745050-50-4 | Culture broth of I. tenuipes BCC12625, BCC21283 | Antimalarial activity; cytotoxic activities against KB, MCF-7, NCI-H187 and Vero cells |
| Isariotin G (56) | 1383930-91-3 | Culture broth of I. tenuipes BCC21283 | Antimalarial activity; cytotoxic activities against KB, MCF-7, NCI-H187 and Vero cells |
| Isariotin H (57) | 1383930-93-5 | Culture broth of I. tenuipes BCC15621 | Antimalarial activity; cytotoxic activities against KB, MCF-7, NCI-H187 and Vero cells |
| Isariotin I (58) | 1383930-95-7 | Culture broth of I. tenuipes BCC15621 | Antimalarial activity; cytotoxic activities against KB, MCF-7, NCI-H187 and Vero cells |
| Isariotin J (59) | 1383930-97-9 | Culture broth of I. tenuipes BCC15621 | Antimalarial activity; cytotoxic activities against KB, MCF-7, NCI-H187 and Vero cells |
| Hanasanagin (61) | 1187336-16-8 | Fruiting body of I. japonica cultivated on silkworm pupae | Antioxidant activity |
| 3′-Deoxyhanasanagin(3,4-diguanidinobutanoyl-tyrosine) (62) | 1187059-33-1 | Fruiting body of I. japonica cultivated on silkworm pupae |
The ten-membered macrolide, (4R,10R)-4-hydroxy-10-methyloxecane-2,8-dione (37) and a novel diketopiperazine, (3S)-6-benzyl-3-isopropyl-1-methylpiperazine-2,5-dione (38), were obtained from the culture broth.82 They showed moderate cytotoxicity in the MTT assay against prostate cancer cells 22RV1 and DU-145, and their inhibition rates were 37.8% and 38.6% and 32.5% and 40.6%, respectively.
The trichothecene derivative, 4-β-acetoxyscirpendiol (4-ASD) (39) was isolated from the fruit body in 2001, which induced apoptosis in human leukemia cells (HL-60).83 It lowered the blood sugar levels in the circulatory system by inhibiting Na+/glucose transporter-1 (SGLT-1)84 and also showed significant apoptosis-inducing activity in various human cancer cell lines.85
Tenuipesine A (40) and paecilomycines A–C (41–43) were isolated from the cultivated fruiting bodies also, but only paecilomycine A (41) exhibited activity in neurotrophic factor biosynthesis in glial cells.86,87 The spirocyclic trichothecanes, spirotenuipesines A (44) and B (45), were isolated from fruiting body grown in barley grain. The two compounds had the potent activity in neurotrophic factor biosynthesis in glial cells.88 As is known, trichothecenes are very important mycotoxins with multiple toxicities including carcinogenic, teratogenic and mutagenic effects.89
Cyclopenta benzopyran and penostatins A–C (46–48) and J (49) were isolated in 2014 by Chen et al. All of them are protein tyrosine phosphatase 1B (PTP1B) inhibitors.90 In fact, penostatins A–C were identified in a strain of Penicillium sp. originally separated from the marine alga Enteromorpha intestinalis. They exhibited significant cytotoxicity against cultured P388 cells.91
I. tenuipes produces isariotins, which are alkaloids probably biosynthesized through the hybrid NRPS-PKS pathway. Isariotins A–D (50–53) were isolated from the culture broth of I. tenuipes BCC7831 strain in 2007 by Haritakun et al. These compounds showed no bioactivity against malaria parasites and fungi, and no cytotoxicity against three cancer cell lines and Vero cells.92 Isariotins E (54), F (55) and TK-57-164A (56) were isolated from the strain BCC12625 in 2009 by Bunyapaiboonsri et al.93,94 Isariotin F (55) exhibited activity against the malaria parasite Plasmodium falciparum K1 with an IC50 value of 5.1 nM and cytotoxic activities against cancer cell lines (KB, BC, and NCI-H187) and nonmalignant (Vero) cells with IC50 values of 15.8, 2.4, 1.6, and 2.9 nM, respectively. However, TK-57-164A (56) exhibit no such activity.93 Both isariotin G (57) and TK-57-164A (56) were isolated from the strain BCC21283, while isariotins H–J (58–60) were isolated from the strain BCC1562195. Isariotins G (57), I (59) and J (60) showed antimalarial activity with IC50 values of 2.10–5.51 μg mL−1, while isariotin H (58) had IC50 values of >10 μg mL−1. Isariotins G–J (57–60) all exhibited cytotoxic activities against KB, MCF-7, NCI-H187 and Vero cells.95
Two pseudo-di-peptides, hanasanagin (61) (3,4-diguanidinobutanoyl-DOPA) and 3,4-diguanidinobutanoyl-tyrosine (62), were isolated from the fruiting bodies of I. japonica cultivated on silkworm pupae.96,97 Hanasanagin (61) exhibited antioxidant activity, but 3,4-diguanidinobutanoyl-tyrosine (62) showed no antioxidant activity.96,97
In addition, I. tenuipes produces the depsipeptide beauvericin (9). It was confirmed that beauvericin is one of the active principles of three strains of I. tenuipes, which strongly inhibited mycelial growth of the two phytopathogens Phytophthora sojae and Aphanomyces cochlioides.98
4 Problems and perspectives
In recent years, the concerns about the safety risks of medical and edible Isaria fungi have been attracting the attention of researchers and consumers. In fact, several research reports about the biosafety analysis of both fungi were published.For I. cicadae, there were four reports indicating its safety to humans, but one report demonstrated its toxicity to the kidneys (Table 3). The possible toxicity arising from repeated exposure to freeze-dried submerged mycelial culture of C. cicadae for 90 days was evaluated. The results indicated that there were no adverse effects to male and female Sprague Dawley rats by gavage >2000 mg kg−1 C. cicadae whole broth.99 Also, toxicological effects were not recorded in the adult Sprague Dawley rats administered orally with a complex powder suspension of P. sinclairii and larvae of Bombyx mori at doses ranging from 0.008 to 5 g kg−1 body weight for 2 weeks, except for a decline in the weight of the thymus in males.100 The extract of wild C. cicadae fruit bodies was not toxic to mice even at a dosage of 80 g kg−1, which was 444 times the clinical daily dosage.101 Moreover, the genotoxicity of the fruiting bodies of I. sinclairii together with its parasitic host larva was evaluated by using short-term genotoxicity tests, namely, the Ames, chromosome aberration (CA), and micronuclei (MN) tests. The results indicated that this complex has no mutagenic potential in the in vitro and in vivo systems.102 However, concerns about the nephrotoxicity of the complex of P. sinclairii and its host insect (Bombyx mori) have been raised. Kidney toxicity was investigated after 13 weeks of administering the complex orally to rats. Dose-dependent kidney cell karyomegaly and tubular hypertrophy were observed. There was a dose-dependent increase in kidney injury molecule 1 (KIM-1) and matrix metalloproteinase 1 (TIMP-1) levels in the kidney and urinary KIM-1, cystatin C, beta-2-microglobulin, and osteopontin levels. KIM-1 and TIMP-1 increased in the male kidneys, and they did not recover 2 weeks after stopping exposure. Cystatin C in the kidney was significantly lowered in all treatment groups at 13 weeks of administration. All the changes were more noticeable in males.14 In addition, some clinical cases of poisoning by eating wild the complex of C. cicadae and the host insect (Cicadae flammata) were reported in China.103
| Material source | Toxicity descript |
|---|---|
| Freeze-dried powder of C. cicadae (MU30106, Bioresource Collection and Research Center at the Food Industry Research and Development Institute, Taiwan, China) submerged mycelial culture | A total of eighty 8 week-old Sprague Dawley rats were divided into 4 groups (10 males and 10 females in each group). C. cicadae was administered daily to the animals by gavage at doses of 0, 500, 1000, and 2000 mg kg−1 body weight for 90 days. No observed adverse effect level to both sexes of Sprague Dawley rats exposed dietary >2000 mg kg−1 for 90 days |
| Water extract from the powder of wild C. cicadae fruit bodies and hosts (Hangzhou, China) | No acute toxicity to mice even at a dosage 80 g kg−1 for 7 days, which was 444 times the clinical daily dosage |
| Water extract from the powder of P. sinclairii from the National Institute of Agricultural Science and Technology (Suwon, Korea) | No toxicological effects to the adult Sprague Dawley rats orally treated at doses of 0.008–5 g kg−1 body weight for 2 weeks, except for a decline in the weight of the thymus in the males |
| Extract of phosphate buffered saline (PBS) from the complex of the fruiting bodies of I. sinclairii and its parasitic host larva (Rural Development Administration, Korea) | In the Ames test, no mutagenic response in the absence or presence of 59 mix with TA98, TA100, TA1535, and TA1537. In the chromosome aberration (CA) test, no significant effect on Chinese hamster ovary (CHO) cells. In the micronuclei (MN) test, no significant change in the occurrence of micronucleated polychromatic erythrocytes in male ICR mice intraperitoneally administered at doses of 15, 150, or 1500 mg kg−1 |
| Powder of the cultured fruiting bodies of P. sinclairii (isolated from parasitizing dead or living Cicadae subspecies) and its silk worm larvae hosts (Rural Development Administration, Korea) | Kidney toxicity was investigated after 13 weeks of administering the complex orally to Sprague Dawley rats at doses of 0, 5000, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, and 50 000, and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm. Dose-dependent kidney cell karyomegaly and tubular hypertrophy were observed, with higher severity in males. There was a dose-dependent increase in kidney injury molecule 1 (KIM-1) and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1) levels in the kidney and urinary KIM-1, cystatin C, b2m, and osteopontin levels. KIM-1 and TIMP-1 increased in the male kidneys, and they did not recover 2 weeks after stopping exposure. Cystatin C in the kidney was significantly lowered in all treatment groups at 13 weeks of administration. All the changes were more noticeable in males. These data indicate that the complex damages renal tubule cells with histopathological lesions and changes in biomarker levels. Kidney and urinary KIM-1 and cystatin C were the most markedly affected and early increased indicators among the biomarkers tested; whereas, blood urea nitrogen (BUN) and creatinine were not affected 000 ppm. Dose-dependent kidney cell karyomegaly and tubular hypertrophy were observed, with higher severity in males. There was a dose-dependent increase in kidney injury molecule 1 (KIM-1) and tissue inhibitor of matrix metalloproteinase 1 (TIMP-1) levels in the kidney and urinary KIM-1, cystatin C, b2m, and osteopontin levels. KIM-1 and TIMP-1 increased in the male kidneys, and they did not recover 2 weeks after stopping exposure. Cystatin C in the kidney was significantly lowered in all treatment groups at 13 weeks of administration. All the changes were more noticeable in males. These data indicate that the complex damages renal tubule cells with histopathological lesions and changes in biomarker levels. Kidney and urinary KIM-1 and cystatin C were the most markedly affected and early increased indicators among the biomarkers tested; whereas, blood urea nitrogen (BUN) and creatinine were not affected |
| Aqueous and ethanol extracts of I. tenuipes N45 (CCTCC M2011145) (P. tenuipes RCEF 4339, Anhui Agricultural University, China) | In acute toxicity, neither mortality nor toxicological signs were found in mice and rats with the maximum tolerance dose of 15 g kg−1. No mortality or adverse effects was observed in the subchronic toxicity studies, in which no significant difference in bodyweight, relative organ weight or hematological parameters, and no abnormality of internal organs were found between the treatment and control groups |
| Water extract from the powder of fruiting bodies of P. tenuipes (Korea Food Research Institute, Seongnam, Korea) | The acute oral LD50 to Sprague Dawley rats was estimated to be greater than 2000 mg kg−1 of body weight. In the subchronic study, the oral treatment of rats with 500, 1000 or 2000 mg kg−1 daily for 13 weeks did not induce any dose-related changes (body weight, food consumption, clinical observation, urinalysis, hematology, clinical chemistry and organ weight). In contrast, histopathological observation revealed that the extract induced karyomegaly in the outer medulla of the kidney in all the treated rats. In the Ames tests, the extract significantly produced His+ mutants at the highest concentration of 5000 lg per plate in S. typhimurium TA102 and 1535 without S9 mix. In the presence of S9 mix, it also significantly produced His+ mutants in TA98, 100 and 102 at 5000 lg per plate of the extract. The increase in His+ mutants was dose-dependent although it was less than 2-fold in comparison to that of the control group |
For I. tenuipes, there were two different research reports about its biosafety (Table 3). In the first report, the genotoxicity, acute and subchronic toxicity of the water-macerated extracts of its fungus fruiting bodies were evaluated in Korea.104 The acute oral LD50 to rats was >2000 mg kg−1 of body weight. In the subchronic test, the oral treatment of rats with 500, 1000 or 2000 mg kg−1 extract daily for 13 weeks did not induce any dose-related changes (body weight, food consumption, clinical observation, urinalysis, hematology, clinical chemistry and organ weight). However, histopathological observation revealed that the I. tenuipes extract induced karyomegaly in the outer medulla of the kidneys of all the treated rats. Importantly, the I. tenuipes extract exerted mutagenic potential in the Ames assay. Since karyomegalic alterations are known to be associated with carcinogenicity, I. tenuipes probably has the risk of carcinogenicity.104 In the second experiment, the aqueous and ethanol extracts of I. tenuipes N45 caused neither mortality nor toxicological signs in mice and rats with the maximum tolerance dose of 15 g kg−1. No mortality or adverse effects was observed in the subchronic toxicity tests, in which no significant difference in bodyweight, relative organ weight or hematological parameters, and no abnormality of internal organs were found between the treatment and control groups. This suggests that the fungus extract is safe.105
Obviously, there are contradictions in these research reports, but some safety risks indeed exist in the both fungi. Of course, the toxicity is based on the substances in the fungus or the complex of the fungus and its insect hosts. Thus, from rational deduction, the toxicities of both Isaria fungi to humans may be related to the toxic MSs described above; however, this needs to be further validated because other toxic substances including mycotoxins and macro biological molecules in both fungi may not have been discovered yet. Also, their toxicity is related to the genetic backgrounds of the fungal strains and the hosts, and their growing environment and culture conditions.106 In fact, different fungal strains under the same culture conditions or the same strain under different culture conditions, usually produce different toxic substances.107,108 Therefore, controlling the culturing conditions and growth environments of Isaria fungi is very important for the quality and safety management of their fungal products.
To date, the products of I. cicadae and I. tenuipes have no standards for quality and safety control. Referring to C. sinensis in the Chinese Pharmacopoeia, 2015, the adenosine marker is the key parameter for quality control. Cordycepin and cordycepic acid are considered as the bioactive components.29 Their other functional compounds such as nucleosides, amino acids, fatty acids and carbohydrates are attracting researchers attention and certain detection methods have been developed.31,109 However, to absolutely ensure the safety of the products of both fungi, only detecting their functional components is not sufficient. Two other aspects must be emphasized. First, identification standards for both fungi and hosts must be developed. As it is known, distinguishing fungal species based on their morphological features is very difficult, especially in commercially processed products. A good choice is may be methods based on DNA. In fact, DNA barcode-based species-specific sequence characterized amplified region (SCAR) markers to discriminate authentic herbal Cordyceps medicines and Cordyceps-derived dietary supplements from related but inauthentic species were recently reported. The ITS-based SCAR markers and the real-time PCR assay constitute a useful genetic tool for preventing the adulteration of Cordyceps and Cordyceps-related dietary supplements.110 Second, the accurate detection and differentiation of toxic components (mycotoxins) in the products of both fungi must be established. Furthermore, residue standards of their toxic components should be developed. In recent years, metabonomics based on GC/HPLC-MS has been used for the analysis of Cordyceps compounds5,28,111 and other effective methods such as HPLC fingerprint for functional substances have also been developed.31,109,112 These technologies will support the detection and identification of SMs in the both Isaria fungi and their products.
In conclusion, both I. cicadae and I. tenuipes are important edible and medicinal fungal species with multiple pharmacological functions. They produce various SMs that affect the quality and safety of their fungal products. For I. cicadae, its SMs include nucleosides, amino acids, beauvericins, and myriocin, oosporein. For I. tenuipes, trichothecene derivatives, isariotins, cyclopenta benzopyrans and PKs were found. Among them, beauvericins, myriocin, oosporein and many trichothecene derivatives are toxic compounds. Thus, the toxicity and side-effects of the fungal products of I. cicadae and I. tenuipes may be related to these SMs. To ensure the quality and safety of their fungal products, residues standards for their SMs must be developed. Furthermore, methods for SM detection and strain biological identification must be give attention.
Conflicts of interest
The authors declare no conflict of interests.Abbreviations
| SMs | Secondary metabolites |
| NRPS | Non-ribosomal peptide synthetase |
| PKS | Polyketide synthase |
| TS | Terpenoid synthetase |
| PK | Polyketides |
| AChE | Acetylcholine esterase |
| PTP1B | Protein tyrosine phosphatase 1B |
| MN | Micronuclei |
| PCR | Polymerase chain reaction |
Acknowledgements
This research is supported by The National Key Research and Development Program of China (2016YFD0200506) and National Natural Science Foundation of China (31572053). We cordially thank Dr Sengodan Karthi for his carefully linguistic modification to this article.References
- J. J. Luangsa-Ard, N. L. Hywel-Jones, L. Manoch and R. A. Samson, Mycol. Res., 2005, 109, 581–589 CrossRef CAS PubMed.
- C. Dong, S. Guo, W. Wang and X. Liu, Mycology, 2015, 6, 121–129 CrossRef PubMed.
- J. Holliday and M. Cleaver, Int. J. Med. Mushrooms, 2008, 10, 219–234 CrossRef CAS.
- K. Yue, M. Ye, Z. J. Zhou, W. Sun and X. Lin, J. Pharm. Pharmacol., 2013, 65, 474–493 CrossRef CAS PubMed.
- Y. Lu, F. Luo, K. Cen, Y. Yin, S. Zhan, C. Wang, H. Zhang, G. Xiao, C. Li and Z. Li, BMC Genomics, 2017, 18 DOI:10.1186/s12864-017-4060-4.
- E. Yokoyama, M. Arakawa, K. Yamagishi and A. Hara, FEMS Microbiol. Lett., 2006, 264, 182–191 CrossRef CAS PubMed.
- F. Takano, N. Yahagi, R. Yahagi, S. Takada, M. Yamaguchi, S. Shoda, T. Murase, S. Fushiya and T. Ohta, Int. Immunopharmacol., 2005, 5, 903–916 CrossRef CAS PubMed.
- B. Song, Q. Liu, T. Lin, Y. Shen, J. Li and D. Luo, J. Fungal Res., 2006, 4, 10–25 Search PubMed.
- C. Dong, W. Li, Z. Li, W. Yan, T. Li and X. Liu, Mycosystema, 2016, 35, 1–15 CrossRef PubMed.
- G. H. Cunningham, Trans. R. Soc. N. Z., 1921, 53, 372–382 Search PubMed.
- R. T. Duarte, K. C. Goncalves, D. J. Espinosa, L. F. Moreira, S. A. De Bortoli, R. A. Humber and R. A. Polanczyk, J. Econ. Entomol., 2016, 109, 594–601 CrossRef CAS PubMed.
- Z. Li, Z. Chen and Y. Chen, Guobao Chongcao Jingchanhua, Hefei University of Technology Publishing House, China, 1st edn, 2014 Search PubMed.
- M. Y. Ahn, Y. S. Jung, S. D. Jee, C. S. Kim, S. H. Lee, C. H. Moon, S. I. Cho, B. M. Lee and K. S. Ryu, Arch. Pharmacal Res., 2007, 30, 493–501 CrossRef CAS.
- M. Jeong, Y. W. Kim, J. R. Min, M. Kwon, B. S. Han, J. G. Kim and S. H. Jeong, Food Chem. Toxicol., 2013, 59, 177–186 CrossRef CAS PubMed.
- M. Jeong, Y.-W. Kim, J.-R. Min, M. Kwon, B.-S. Han, J.-G. Kim and S.-H. Jeong, Toxicol. Res., 2012, 28, 179–185 CrossRef CAS PubMed.
- P. Vega-Aquino, S. Sanchez-Pena and C. A. Blanco, J. Invertebr. Pathol., 2010, 103, 145–149 CrossRef PubMed.
- Y. Han, J. Liang, X. Zou, X. Dong and Z. Liang, Acta Edulis Fungi, 2011, 18, 89–94 Search PubMed.
- K. H. Shin, S. S. Lim, S. Lee, Y. S. Lee, S. H. Jung and S. Y. Cho, Phytother. Res., 2003, 17, 830–833 CrossRef PubMed.
- J. Liang, Y. Han, W. Tian, Z. Liang and Z. Li, Acta Edulis Fungi, 2018, 25, 113–119 Search PubMed.
- S. S. Sum and J. Ziegler, Nutr. Clin. Pract., 2016, 31, 695–697 CrossRef PubMed.
- X. Wang, X. Gong, P. Li, D. Lai and L. Zhou, Molecules, 2018, 23, 169, DOI:10.3390/molecules23010169.
- K. Zhou, X. Zhang, F. Zhang and Z. Li, Microb. Ecol., 2011, 62, 644–654 CrossRef PubMed.
- J.-H. Hsu, B.-Y. Jhou, S.-H. Yeh, Y.-L. Chen and C.-C. Chen, J. Nutr. Food Sci., 2015, 5, 432 Search PubMed.
- S.-C. Weng, C.-J. Chou, L.-C. Lin, W.-J. Tsai and Y.-C. Kuo, J. Ethnopharmacol., 2002, 83, 79–85 CrossRef PubMed.
- Y. Shen, M. Cai, W. Xia, J. Liu, Q. Zhang, H. Xie, C. Wang, X. Wang and S. Zheng, Cancer Lett., 2007, 254, 288–297 CrossRef CAS PubMed.
- K. Su, P. Zeng, W. Liang, Z. Luo, Y. Wang, X. Lv, Q. Han, M. Yan and C. Chen, Mediators Inflammation, 2017, 3701385, DOI:10.1155/2017/3701385.
- Y. Sun, M. Wink, P. Wang, H. Lu, H. Zhao, H. Liu, S. Wang, Y. Sun and Z. Liang, Phytomedicine, 2017, 36, 217–228 CrossRef CAS PubMed.
- J. Zhang, H. Yu, X. Zhong, G. Zhang and X. Liu, Chin. J. Exp. Tradit. Med. Formulae, 2018, 24, 23–29 Search PubMed.
- X. Zhou, Z. Gong, Y. Su, J. Lin and K. Tang, J. Pharm. Pharmacol., 2009, 61, 279–291 CrossRef CAS PubMed.
- L. He, S. Ma, J. Cheng, W. Li and X. Wu, Shipin Yu Shengwu Jishu Xuebao, 2012, 31, 8–16 CAS.
- L. Li, H. Liang, Z. Wu, Z. Zhang, L. Zhang, Y. Wang, T. Zhang and B. Wang, Lishizhen Med. Mater. Med. Res., 2017, 28, 1537–1542 Search PubMed.
- Y. Wang, Y. Guo, L. Zhang and J. Wu, Braz. J. Microbiol., 2012, 43, 449–455 CrossRef CAS PubMed.
- M. Siev, R. Weinberg and S. Penman, J. Cell Biol., 1969, 41, 510–520 CrossRef CAS PubMed.
- O. J. Olatunji, Y. Feng, J. Tang, O. O. Olatunji, Z. Ouyang and Z. Su, Biomed. Pharmacother., 2016, 81, 7–14 CrossRef CAS PubMed.
- K. Nakamura, K. Shinozuka and N. Yoshikawa, J. Pharmacol. Sci., 2015, 127, 53–56 CrossRef CAS PubMed.
- H. S. Tuli, A. K. Sharma, S. S. Sandhu and D. Kashyap, Life Sci., 2013, 93, 863–869 CrossRef CAS PubMed.
- B. Li, Y. Hou, M. Zhu, H. Bao, J. Nie, G. Y. Zhang, L. Shan, Y. Yao, K. Du, H. Yang, M. Li, B. Zheng, X. Xu, C. Xiao and J. Du, Int. J. Neuropsychopharmacol., 2016, 19 DOI:10.1093/ijnp/pyv112.
- Y. C. Chen, Y. H. Chen, B. S. Pan, M. M. Chang and B. M. Huang, J. Food Drug Anal., 2017, 25, 197–205 CrossRef CAS PubMed.
- Z. Wang, X. Zhao, F. Gao, Z. Zhao and K. Wang, Zhong Xi Yi Jiehe Yanjiu, 2017, 9, 169–171 Search PubMed.
- Z. Wang, X. Zhao, L. Song, F. Gao, Z. Zhao and K. Wang, World Journal of Integrated Traditional and Western Medicine, 2018, 13, 1306–1309 Search PubMed.
- H. C. Kuo, I. C. Huang and T. Y. Chen, Int. J. Med. Mushrooms, 2015, 17, 1077–1085 CrossRef PubMed.
- M. Y. Ahn, J. E. Heo, J.-H. Ryu, S. D. Ji, H. Jeong, H. C. Park and H. S. Sim, Int. J. Indust. Entomol., 2008, 17, 197–200 Search PubMed.
- S. Lin, Z. Q. Liu, Y. P. Xue, P. J. Baker, H. Wu, F. Xu, Y. Teng, M. E. Brathwaite and Y. G. Zheng, Appl. Biochem. Biotechnol., 2016, 179, 633–649 CrossRef CAS PubMed.
- M. Saeed, D. Babazadeh, M. Naveed, M. A. Arain, F. U. Hassan and S. Chao, Trop. Anim. Health Prod., 2017, 49, 1329–1338 CrossRef PubMed.
- S.-Y. Chen, K.-J. Ho, Y.-J. Hsieh, L.-T. Wang and J.-L. Mau, LWT–Food Sci. Technol., 2012, 47, 274–278 CrossRef CAS.
- J. H. Jeong, H. J. Cha, S. C. Ha, C. Rojviriya and Y. G. Kim, Biochem. Biophys. Res. Commun., 2014, 452, 1098–1103 CrossRef CAS PubMed.
- Y. J. Kwon, M. J. Sohn, C. J. Zheng and W. G. Kim, Org. Lett., 2007, 9, 2449–2451 CrossRef CAS PubMed.
- P. X. Chen, S. A. Wang, S. P. Nie and M. Marcone, J. Funct. Foods, 2013, 5, 550–569 CrossRef CAS.
- M. L. Yang, P. C. Kuo, T. L. Hwang and T. S. Wu, J. Nat. Prod., 2011, 74, 1996–2000 CrossRef CAS PubMed.
- X. W. Ye, W. Y. Chai, X. Y. Lian and Z. Z. Zhang, Nat. Prod. Res., 2017, 31, 1390–1396 CrossRef CAS PubMed.
- J. Wang, D.-M. Zhang, J.-F. Jia, Q.-L. Peng, H.-Y. Tian, L. Wang and W.-C. Ye, Fitoterapia, 2014, 97, 23–27 CrossRef CAS PubMed.
- Q. G. Wang and L. J. Xu, Molecules, 2012, 17, 2367–2377 CrossRef CAS PubMed.
- Y. W. Tao, Y. C. Lin, Z. G. She, M. T. Lin, P. X. Chen and J. Y. Zhang, Anti-Cancer Agents Med. Chem., 2015, 15, 258–266 CrossRef CAS PubMed.
- W. Watjen, A. Debbab, A. Hohlfeld, Y. Chovolou and P. Proksch, Toxicol. Lett., 2014, 231, 9–16 CrossRef PubMed.
- X. F. Wu, R. Xu, Z. J. Ouyang, C. Qian, Y. Shen, X. D. Wu, Y. H. Gu, Q. Xu and Y. Sun, PLoS One, 2013, 8, e83013, DOI:10.1371/journal.pone.0083013.
- M. J. Ruiz, P. Franzova, A. Juan-Garcia and G. Font, Toxicon, 2011, 58, 315–326 CrossRef CAS PubMed.
- C. Luz, F. Saladino, F. B. Luciano, J. Manes and G. Meca, Food Chem. Toxicol., 2017, 107, 430–439 CrossRef CAS PubMed.
- X. L. Xu, L. Y. Yin, S. Y. S. Wang, H. H. Liu, J. H. Gao and S. J. Zhao, Rec. Nat. Prod., 2013, 7, 292–295 CAS.
- J. Yu, Z. Mo, X. Mao, H. Xu and H. Zhu, Yaowu Fenxi Zazhi, 2010, 30, 664–667 CAS.
- J. Yu, H. Xu, Z. Mo, H. Zhu and X. Mao, Anal. Sci., 2009, 25, 855–859 CrossRef CAS PubMed.
- H. Xu, Z. Mo, J. Yu, X. Mao and H. Zhu, Nat. Prod. Res. Dev., 2010, 22(5), 794–797 CAS.
- T. Fujita, K. Inoue, S. Yamamoto, T. Ikumoto, S. Sasaki, R. Toyama, K. Chiba, Y. Hoshino and T. Okumoto, J. Antibiot., 1994, 47, 208–215 CrossRef CAS.
- D. Kluepfel, J. Bagli, H. Baker, M. P. Charest and A. Kudelski, J. Antibiot., 1972, 25, 109–115 CrossRef CAS PubMed.
- T. Fujita, K. Inoue, S. Yamamoto, T. Ikumoto, S. Sasaki, R. Toyama, K. Chiba, Y. Hoshino and T. Okumoto, J. Antibiot., 1994, 47, 208–215 CrossRef CAS PubMed.
- T. Fujita, R. Hirose, N. Hamamichi, Y. Kitao, S. Sasaki, M. Yoneta and K. Chiba, Bioorg. Med. Chem. Lett., 1995, 5, 1857–1860 CrossRef CAS.
- K. Adachi, T. Kohara, N. Nakao, M. Arita, K. Chiba, T. Mishina, S. Sasaki and T. Fujita, Bioorg. Med. Chem. Lett., 1995, 5, 853–856 CrossRef CAS.
- K. Adachi, J. Synth. Org. Chem., Jpn., 2011, 69, 904–912 CrossRef CAS.
- M. Sanford, Drugs, 2014, 74, 1411–1433 CrossRef CAS PubMed.
- F. Pina, F. Yagisawa, K. Obara, J. D. Gregerson, A. Kihara and M. Niwa, J. Cell Biol., 2018, 217, 495–505 CrossRef CAS.
- H. Takeshita and M. Anchel, Science, 1965, 147, 152–153 CrossRef CAS.
- R. J. Cole, J. W. Kirksey, H. G. Cutler and E. E. Davis, J. Agric. Food Chem., 1974, 22, 517–520 CrossRef CAS PubMed.
- L. B. Jeffs and G. G. Khachatourians, Toxicon, 1997, 35, 1351–1356 CrossRef CAS.
- R. Alurappa, M. R. M. Bojegowda, V. Kumar, N. K. Mallesh and S. Chowdappa, Nat. Prod. Res., 2014, 28, 2217–2220 CrossRef CAS.
- C. Seger, D. Erlebach, H. Stuppner, U. J. Griesser and H. Strasser, Helv. Chim. Acta, 2005, 88, 802–810 CrossRef CAS.
- J. W. Choi and J. P. Park, Biotechnol. Bioprocess Eng., 2018, 23, 246–249 CrossRef CAS.
- F. A. E. Torres, B. R. Zaccarim, L. C. D. Novaes, A. F. Jozala, C. A. dos Santos, M. F. S. Teixeira and V. C. Santos-Ebinuma, Appl. Microbiol. Biotechnol., 2016, 100, 2511–2521 CrossRef CAS.
- R. Bentley, Nat. Prod. Rep., 2008, 25, 118–138 RSC.
- S.-W. Zhang and L.-J. Xuan, J. Antibiot., 2008, 61, 43–45 CrossRef CAS PubMed.
- N. N. Yang, N. Jiang, Q. Y. Ma, F. D. Kong, Q. Y. Xie, L. M. Zhou, Z. F. Yu and Y. X. Zhao, J. Asian Nat. Prod. Res., 2018 DOI:10.1080/10286020.2018.1451518.
- T. Asai, Y. M. Chung, H. Sakurai, T. Ozeki, F. R. Chang, K. Yamashita and Y. Oshima, Org. Lett., 2012, 14, 513–515 CrossRef CAS PubMed.
- L. Y. Song, Y. Liu and R. B. Tong, Org. Lett., 2013, 15, 5850–5853 CrossRef CAS PubMed.
- Y. Zheng, H. Pang, J. Wang, D. Chen, G. Shi and J. Huang, Chem. J. Chin. Univ., 2012, 17, 1665–1669 Search PubMed.
- G.-S. Oh, K.-H. Hong, H. Oh, H.-O. Pae, I.-K. Kim, N.-Y. Kim, T.-O. Kwon, M.-K. Shin and H.-T. Chung, Biol. Pharm. Bull., 2001, 24, 785–789 CrossRef CAS.
- O. Yoo, J.-H. Son and D.-H. Lee, J. Biochem. Mol. Biol., 2005, 38, 211–217 CAS.
- K. S. Nam, Y. S. Jo, Y. H. Kim, J. W. Hyun and H. W. Kim, Life Sci., 2001, 69, 229–237 CrossRef CAS PubMed.
- H. Kikuchi, Y. Miyagawa, Y. Sahashi, S. Inatomi, A. Haganuma, N. Nakahata and Y. Oshima, Tetrahedron Lett., 2004, 45, 6225–6228 CrossRef CAS.
- H. Kikuchi, Y. Miyagawa, K. Nakamura, Y. Sahashi, S. Inatomi and Y. Oshima, Org. Lett., 2004, 6, 4531–4533 CrossRef CAS PubMed.
- H. Kikuchi, Y. Miyagawa, Y. Sahashi, S. Inatomi, A. Haganuma, N. Nakahata and Y. Oshima, J. Org. Chem., 2004, 69, 352–356 CrossRef CAS PubMed.
- S. Aupanun, S. Poapolathep, M. Giorgi, K. Imsilp and A. Poapolathep, J. Vet. Med. Sci., 2017, 79, 6–13 CrossRef CAS PubMed.
- Y.-P. Chen, C.-G. Yang, P.-Y. Wei, L. Li, D.-Q. Luo, Z.-H. Zheng and X.-H. Lu, Molecules, 2014, 19, 1663–1671 CrossRef PubMed.
- C. Takahashi, A. Numata, T. Yamada, K. Minoura, S. Enomoto, K. Konishi, M. Nakai, C. Matsuda and K. Nomoto, Tetrahedron Lett., 1996, 37, 655–658 CrossRef CAS.
- R. Haritakun, P. Srikitikulchai, P. Khoyaiklang and M. Isaka, J. Nat. Prod., 2007, 70, 1478–1480 CrossRef CAS PubMed.
- T. Bunyapaiboonsri, S. Yoiprommarat, K. Intereya, P. Rachtawee, N. L. Hywel-Jones and M. Isaka, J. Nat. Prod., 2009, 72, 756–759 CrossRef CAS PubMed.
- J. Y. Cha, Y. Huang and T. R. R. Pettus, Angew. Chem., Int. Ed., 2009, 48, 9519–9521 CrossRef CAS PubMed.
- T. Bunyapaiboonsri, S. Yoiprommarat, U. Srisanoh, W. Choowong, K. Tasanathai, N. L. Hywel-Jones, J. J. Luangsa-ard and M. Isaka, Phytochem. Lett., 2011, 4, 283–286 CrossRef CAS.
- A. Sakakura, K. Shioya, H. Katsuzaki, T. Komiya, T. Imamura, Y. Aizono and K. Imai, Tetrahedron, 2009, 65, 6822–6827 CrossRef CAS.
- A. Sakakura, K. Suzuki, H. Katsuzaki, T. Komiya, T. Imamura, Y. Aizono and K. Imai, Tetrahedron Lett., 2005, 46, 9057–9059 CrossRef CAS.
- S. P. Putri, K.-i. Ishido, H. Kinoshita, S. Kitani, F. Ihara, Y. Sakihama, Y. Igarashi and T. Nihira, J. Biosci. Bioeng., 2014, 117, 557–562 CrossRef CAS PubMed.
- Y.-L. Chen, C.-S. Chen, S.-H. Yeh, T.-W. Lin, C.-C. Chen and C.-F. Kuo, Int. J. Med. Mushrooms, 2015, 17, 771–781 CrossRef PubMed.
- S. J. Kwack and B. M. Lee, Toxicol. Res., 2009, 25, 101–106 CrossRef CAS.
- J. Song, J. Xin, Y. Zhu and X. Zhang, Chin JMAP, 2004, 21, 12–13 Search PubMed.
- M. Y. Ahn, K. S. Ryu, S. D. Jee, I. Kim, J. W. Kim, Y. S. Kim, H. S. Kim, I. S. Kim, S. C. Kang, H. J. Koo, Y. A. Park, S. M. Choi, E. J. Yoo, S. J. Kwack, S. D. Yoo and B. M. Lee, J. Toxicol. Environ. Health, Part A, 2004, 67, 2037–2044 CrossRef CAS PubMed.
- P. Song, X. Liu and Q. Zhuo, World Chin. J. Digestol., 1997, 5, 22 Search PubMed.
- J.-H. Che, J.-W. Yun, E.-Y. Cho, S.-H. Kim, Y.-S. Kim, W. H. Kim, J.-H. Park, W.-C. Son, M. K. Kim and B.-C. Kang, Regul. Toxicol. Pharmacol., 2014, 70, 527–534 CrossRef CAS.
- L. Du, Y. Liu, C. Liu, Q. Meng, J. Song, D. Wang, J. Lu, L. Teng, Y. Zhou and L. Teng, Comb. Chem. High Throughput Screening, 2015, 18, 809–818 CrossRef CAS.
- H. Zhang, X. Shin, T. Liu, W. Ma, Y. Zhang and X. Shi, Global Tradit. Chin. Med., 2017, 10, 297–301 Search PubMed.
- Z. Zhang, T. Chen, B. YIin, Q. Cui, S. Zhang and Y. Wang, Drug Eval. Res., 2016, 39, 797–805 Search PubMed.
- H. Zhang, X. Gao, W. Chen and K. Huang, Acta Agric. Shanghai, 2016, 32, 101–104 Search PubMed.
- H. Lu, A. Yang and H. Zhang, Pract. Pharm. Clin. Rem., 2015, 18, 1466–1469 Search PubMed.
- B. C. Moon, W. J. Kim, I. Park, G. H. Sung and P. Noh, Molecules, 2018, 23, 1932 CrossRef PubMed.
- W. Zhang, L. Chen, S. Gao, F. Xu, J. Liu, R. Lu and F. Hu, Junwu Xuebao, 2015, 34, 252–268 CAS.
- W. Lu, J. Xu, S. Xu and Q. Yuan, Zhejiang Zhongyiyao Daxue Xuebao, 2013, 37, 601–605 CAS.
| This journal is © The Royal Society of Chemistry 2019 |