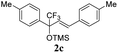
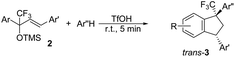
TfOH-promoted transformation of TMS-ethers of diarylsubstituted CF3-allyl alcohols with arenes into CF3-indanes†
Aleksey V.
Zerov
a,
Anastasia A.
Bulova
a,
Olesya V.
Khoroshilova
b and
Aleksander V.
Vasilyev
 *ac
*ac
aDepartment of Organic Chemistry, Institute of Chemistry, Saint Petersburg State University, Universitetskaya nab., 7/9, Saint Petersburg, 199034, Russia. E-mail: aleksvasil@mail.ru; a.vasilyev@spbu.ru
bResearch Center for X-ray Diffraction Studies, Research Park, St. Petersburg State University, Universitetskiy pr. 26, Saint Petersburg, Petrodvoretz 198504, Russia
cDepartment of Chemistry, Saint Petersburg State Forest Technical University, Institutsky per., 5, Saint Petersburg, 194021, Russia
First published on 5th August 2019
Abstract
The reaction of TMS-ethers of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-ols with arenes in TfOH at room temperature for 5 min results in the formation of trans-/cis-1,3-diaryl-1-trifluoromethyl indanes in good yields (up to 99%). The predominant or exclusive formation of indanes with a trans-configuration of 1,3-diaryl groups has been observed. The reaction proceeds through an intermediate formation of the corresponding mesomeric CF3-allyl cations. A plausible reaction mechanism is discussed.
Introduction
Allyl alcohols and their derivatives are widely used in organic synthesis. There are many various pathways for transformations of allyl alcohols leading to the formation of new carbon–carbon and carbon–heteroatom bonds. The most important reactions are C–H activation at the double bond,1 addition to the double bond without breaking the C–O bond,2–6 and transformations associated with the cleavage of the C–O bond,7–15 including the classical Friedel–Crafts process.16–19 Under the action of Brønsted superacids, some allyl alcohols gave rise to relatively stable intermediate allyl cations, which were studied by means of NMR.20 The introduction of the trifluoromethyl group CF3 into the allylic position of allyl alcohols significantly increases the electrophilicity of the corresponding intermediate allyl cations.In general, including fluorinated moieties in organic molecules often changes important pharmacokinetic parameters such as lipophilicity, bioavailability and metabolic activity. Due to this, fluorinated organic compounds have a great theoretical and practical importance in chemistry, biology, medicine, and materials science.21–26
We have previously shown that various CF3-substituted allyl alcohols and their derivatives under the action of Brønsted and Lewis acids undergo the Friedel–Crafts intra- and inter-molecular reactions, yielding a number of important compounds, among them CF3-substituted indanes and indenes.27–31 Moreover, it was found that various 1-CF3-indanes are effective ligands for cannabinoid receptors CB1 and CB2 types,32 and ligands for endocannabinoid degrading enzymes (FAAH and MAGL), and AEA uptake.33
Taking into account the importance of fluorinated indanes and based on our recent study on acid-promoted intramolecular cyclization of diarylsubstituted CF3-allyl alcohols into CF3-indenes,28 we have decided to develop a novel method for the synthesis of CF3-indanes based on transformations of such CF3-allyl alcohols under superelectrophilic activation conditions.
The main goal of this work was a study of reactions of TMS-ethers of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-ols with arenes in Brønsted superacid, and trifluoromethanesulfonic acid CF3SO3H (TfOH).
Results and discussion
The initial TMS-ethers of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-ols 2a–u were obtained by the reaction of the chalcones 1a–u with Rupert–Prakash reagent CF3SiMe3 in the presence of CsF in 1,2-dimethoxyethane (DME) at room temperature for 4 h (Scheme 1, see the ESI† for details). | ||
| Scheme 1 Synthesis of TMS-esters of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-ols 2a–u, used in this study. | ||
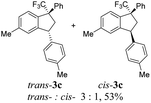
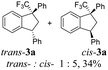
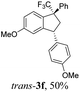
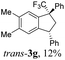
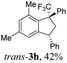
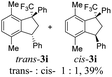
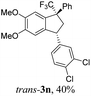
Then, reactions of TMS-ethers 2 with arenes under the action of various Brønsted and Lewis acids were conducted. It was found that reactions of compound 2a with benzene in H2SO4 or with strong Lewis acids AlX3 (X = Cl, Br) afforded complex mixtures of oligomeric compounds. In contrast to that, the use of TfOH for electrophilic activation of 2a in this reaction led to the formation of trans-/cis-indanes 3a (entry 1, Table 1). Analogously, other TMS-ethers 2 in the reaction with benzene in TfOH at room temperature for just 5 min (!!!) gave mainly indanes 3 (see Table 1). With rare exceptions, the major or even sole reaction products were trans-indanes 3, bearing aryl groups in the trans-position in the indane core. The stereochemical trans-/cis-configuration of compounds 3 was determined by H–F NOESY correlations between the 1-CF3 group and 3-H proton in the indane ring (Fig. 1). Exact structures of indanes 3b–h,k–n, and q–r were additionally confirmed by X-ray analysis (see the ESI†).
 | ||
| Fig. 1 H–F NOESY correlations in indanes 3, clarifying their trans-/cis-stereochemical configuration. | ||
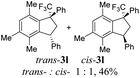
However, in some cases the formation of indanes 3 was accompanied by aryl group exchange under the superacidic conditions. Thus, TMS-ethers 2e and 2k bearing electron donating aryl rings gave indene 3a (entries 5 and 11) as a result of exchange of these aryl groups into phenyl one (see the below discussion on the reaction mechanism). Apart from indanes 3, in some reactions, other compounds were obtained, such as indenes 4a–d1 (entries 14, 16, 19) as products of intramolecular cyclization of initial TMS-ethers without any interaction with benzene. Also, E-/Z-alkene 5 was obtained from compound 2s (entry 19). All initial TMS-ethers 2 were successfully involved in the transformation with benzene (Table 1), except compound 2t having two electron poor 3,4-dichlorophenyl rings; the reaction of it with benzene finally led to a complex mixture of products.
Having the data obtained (Table 1) and based on our previous work,28 one may propose a plausible mechanism of the reaction of TMS-ethers 2 with benzene in TfOH (Scheme 2). In the beginning, protonation of oxygen in ether 2 followed by elimination of TMSOH gives rise to mesomeric CF3-allyl cation A ↔ A′, which is cyclized into indene C through resonance form A′. Under superacidic reaction conditions, indene C is further protonated leading to CF3-benzyl cation D. The latter reacts with benzene forming finally trans-/cis-indenes 3. The predominant formation of trans-indenes 3 (see Table 1) may be explained by less sterical hindrance in the corresponding intermediates of electrophilic substitution between species D and benzene. An exchange of donating aryl moiety Ar′ into the phenyl group at the benzylic position in indanes 3 may occur in the superacid TfOH, as it was previously observed for various substrates.34,35 This is why the formation of indene 3a from ethers 2e and k took place (entries 5 and 11, Table 1). Compounds 4 were obtained after chromatographic isolation as a result of isomerization of indene C. This isomerization has been already described by us.28 The formation of alkene 5 is caused by alternative alkylation of benzene by species A ↔ A′, rather than its cyclization into an electron poor 3,4-dichlorophenyl ring leading to the corresponding indene C that seems to be less favorable.
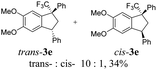
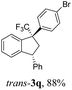
We also ran reactions of TMS-ethers 2a,b, ande with selected arenes (Table 2). Thus, reactions with bromobenzene, meta-xylene, and veratrol gave stereoselectively only trans-indanes 3q–s (entries 1–3). However, transformations with mesitylene resulted in the formation of indenes 4e,f (entries 4 and 5). Probably, in this case, an interaction of the corresponding CF3-benzyl cations D with bulky mesitylene molecules does not take place.
Conclusions
We have developed a novel synthesis of 1,3-diaryl-1-trifluoromethylindanes on the basis of the reaction of TMS-ethers of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-ols with arenes under superelectrophilic activation in TfOH. The reaction proceeds rather highly stereoselectively leading mainly or solely to such 1-CF3-indanes having a trans-configuration of 1,3-diaryl substituents. These CF3-indanes have great importance for medicinal chemistry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Russian Scientific Foundation (grant no. 18-13-00008). Spectral studies were performed at the Center for Magnetic Resonance, Center for Chemical Analysis and Materials Research, and Research Center for X-ray Diffraction Studies of Saint Petersburg State University, Saint Petersburg, Russia.References
- E. Alacida and C. Najera, Adv. Synth. Catal., 2007, 349, 2572–2584 CrossRef.
- S. Albert, S. Robin and G. Rousseau, Tetrahedron Lett., 2001, 42, 2477–2479 CrossRef CAS.
- S. Amirat and A. Djellal, Arab. J. Chem., 2014, 7, 518–521 CrossRef CAS.
- O. Hamed, P. M. Henry and D. P. Becker, Tetrahedron Lett., 2010, 51, 3514–3517 CrossRef CAS.
- Y. Ishizuka, H. Fujimori, T. Noguchi, M. Kawasaki, M. Kishida, T. Nagai, N. Imai and M. Kirihara, Chem. Lett., 2013, 42, 1311–1313 CrossRef CAS.
- J. B. Shi, S. Xu, Y. P. Wang, J. J. Li and Q. Z. Yao, Chin. Chem. Lett., 2011, 22, 899–902 CrossRef CAS.
- S. Arimori, M. Takada and N. Shibata, Dalton Trans., 2015, 44, 19456–19459 RSC.
- Y. Bunno, N. Murakami, Y. Suzuki, M. Kanai, T. Yoshino and S. Matsunaga, Org. Lett., 2016, 18, 2216–2219 CrossRef CAS PubMed.
- L. Calmus, A. Corbu and J. Cossy, Adv. Synth. Catal., 2015, 357, 1381–1386 CrossRef CAS.
- X.-Q. Hu, Z. Hu, A. S. Trita, G. Zhanga and L. J. Gooßen, Chem. Sci., 2018, 9, 5289–5294 RSC.
- J. Wang, Y. Masui and M. Onaka, ACS Catal., 2011, 1, 446–454 CrossRef CAS.
- X. Wu and H. Ji, Org. Biomol. Chem., 2018, 16, 5691–5698 RSC.
- B. Yang and Z.-X. Wang, J. Org. Chem., 2017, 82, 4542–4549 CrossRef CAS PubMed.
- M. Yoshida, J. Org. Chem., 2017, 82, 12821–12826 CrossRef CAS PubMed.
- A. B. Zaitsev, S. Gruber, P. A. Pluess, P. S. Pregosin, L. F. Veiros and M. Woerle, J. Am. Chem. Soc., 2008, 130, 11604–11605 CrossRef CAS PubMed.
- S. Guo and Y. Liu, Org. Biomol. Chem., 2008, 6, 2064–2070 RSC.
- J. Wang, L. Zhang, Y. Jing, W. Huang and X. Zhou, Tetrahedron Lett., 2009, 50, 4978–4982 CrossRef CAS.
- J. Zhang, J. Zhang, K. Fang, G. Shu and M. Xie, Chin. J. Org. Chem., 2012, 32, 1533–1538 CrossRef CAS.
- P. Shanmugam and P. Rajasingh, Chem. Lett., 2005, 34, 1494–1495 CrossRef CAS.
- G. K. S. Prakash, V. V. Krishnamurthy, G. A. Olah and D. G. Farnum, J. Am. Chem. Soc., 1985, 107, 3928–3935 CrossRef CAS.
- M. Shimizu, Y. Takeda, M. Higashi and T. Hiyama, Angew. Chem., Int. Ed., 2009, 48, 3653–3656 CrossRef CAS PubMed.
- J. P. Begue and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, 2008 Search PubMed.
- A. Tressaud and G. Haufe, Fluorine and Health: Molecular Imaging, Biomedical Materials and Pharmaceuticals, Elsevier, Amsterdam, 2008 Search PubMed.
- V. A. Petrov, Fluorinated Heterocyalic Compounds: Synthesis, Chemistry, and Applications, Wiley, Hoboken, 2009 Search PubMed.
- V. G. Nenajdenko, Fluorine in Heterocyclic Chemistry, Springer, Berlin, 2014 Search PubMed.
- R. V. Prakash., Organofluorine Compounds in Biology and Medicine, Elsevier, Amsterdam, 2015 Search PubMed.
- A. N. Kazakova, R. O. Iakovenko, I. A. Boyarskaya, A. Y. Ivanov, M. S. Avdontceva, A. A. Zolotarev, T. L. Panikorovsky, G. L. Starova, V. G. Nenajdenko and A. V. Vasilyev, Org. Chem. Front., 2017, 2, 255–265 RSC.
- M. Yu. Martynov, R. O. Iakovenko, A. N. Kazakova, I. A. Boyarskaya and A. V. Vasilyev, Org. Biomol. Chem., 2017, 15, 2541–2550 RSC.
- A. N. Kazakova, R. O. Iakovenko, I. A. Boyarskaya, V. G. Nenajdenko and A. V. Vasilyev, J. Org. Chem., 2015, 80, 9506–9517 CrossRef CAS PubMed.
- A. N. Kazakova, R. O. Iakovenko, I. A. Boyarskaya, A. Y. Ivanov, M. S. Avdontceva, A. A. Zolotarev, T. L. Panikorovsky, G. L. Starova, V. G. Nenajdenko and A. V. Vasilyev, Org. Chem. Front., 2017, 2, 255–265 RSC.
- A. N. Kazakova, R. O. Iakovenko, V. M. Muzalevskiy, I. A. Boyarskaya, M. S. Avdontceva, G. L. Starova, A. V. Vasilyev and V. G. Nenajdenko, Tetrahedron Lett., 2014, 55, 6851–6855 CrossRef CAS.
- R. O. Iakovenko, A. N. Kazakova, V. M. Muzalevskiy, A. Yu. Ivanov, I. A. Boyarskaya, A. Chicca, V. Petrucci, J. Gertsch, M. Krasavin, G. L. Starova, A. A. Zolotarev, M. S. Avdontceva, V. G. Nenajdenko and A. V. Vasilyev, Org. Biomol. Chem., 2015, 13, 8827–8842 RSC.
- R. O. Iakovenko, A. Chicca, D. Nieri, I. Reynoso-Moreno, J. Gertsch, M. Yu. Krasavin and A. V. Vasilyev, Tetrahedron, 2019, 75, 624–632 CrossRef CAS.
- D. N. Zakusilo, D. S. Ryabukhin, I. A. Boyarskaya, O. S. Yuzikhin and A. V. Vasilyev, Tetrahedron, 2015, 71, 102–108 CrossRef CAS.
- M. A. Sandzhieva, A. N. Kazakova, I. A. Boyarskaya, A. Yu. Ivanov, V. G. Nenajdenko and A. V. Vasilyev, J. Org. Chem., 2016, 81, 5032–5045 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1875102, 1875106–1875119. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9qo00822e |
| This journal is © the Partner Organisations 2019 |