Pd(TFA)2-catalyzed direct arylation of quinoxalinones with arenes†
Abstract
The direct C-3 arylation of quinoxalin-2-ones with arenes is achieved by a Pd(TFA)2-catalyzed cross-dehydrogenative coupling (CDC) reaction. A wide array of functionalities, including highly sensitive allyl- and benzyl group substituted quinoxalin-2(H)-ones, are readily assembled with arenes containing electron-donating and electron-withdrawing groups in this CDC reaction. Highly regioselective products are produced in good yields with stronger electron-donating groups than toluene and densely substituted arenes as coupling partners. This protocol represents an efficient technique to access various 3-arylbenzo[g]quinoxalinones and 2-aryl-4-methylpyrido[3,4-b]pyrazin-3(4H)-ones under mild conditions.
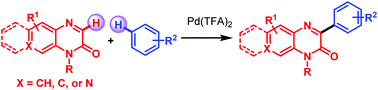


 Please wait while we load your content...
Please wait while we load your content...