Transition metal-free base-promoted arylation of sulfenate anions with diaryliodonium salts†
Lei
Wang
a,
Mingjie
Chen
a and
Junliang
Zhang
 *ab
*ab
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062, China. E-mail: jlzhang@chem.ecnu.edu.cn
bDepartment of Chemistry, Fudan University, 2005 Songhu Road, Shanghai 200438, China. E-mail: junliangzhang@fudan.edu.cn
First published on 1st November 2018
Abstract
A transition metal-free, base-promoted arylation of general sulfenate anions with diaryliodonium salts as an aryl source was developed under mild conditions. Retro-Michael reaction and C–S bond formation were involved in this transformation leading to diaryl and aryl alkyl sulfoxides in good yields. This procedure has general substrate scope and is easy to scale-up.
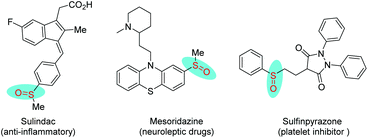
The sulfoxide motif is prevalent in a large number of bioactive compounds, natural products, and marketed pharmaceuticals such as sulindac, mesoridazine and sulfinpyrazone (Fig. 1).1 Several procedures have been developed for the synthesis of sulfoxide analogues. Among them, the direct oxidation of sulfides,2 and the nucleophilic substitution of sulfinyl derivatives with organometallic reagents3 have been widely utilized in the synthesis of sulfoxides.
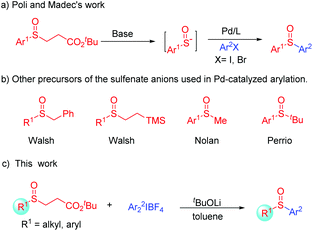
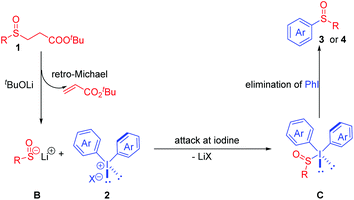
Recently, an alternative strategy has been developed involving the generation and trapping of aryl sulfenate anions (ArSO−).4 Transition-metal-catalyzed cross-coupling between sulfenate anions and C (sp3 or sp2) centers has also been proved to be one of the most powerful tools to access sulfoxides. For example, Bolm and co-workers successfully achieved the copper-catalyzed coupling of in situ generated sulfenate anions with oxidatively activated methylarenes.5 Besides, the palladium-catalyzed C(sp2)–S bond formation is particularly effective in the arylation of sulfenate anions (RSO−) using aryl halides. In 2006, Poli and co-workers firstly reported the synthesis of bisarylated sulfoxides through C–S bond formation involving in situ generated sulfenate anions (Scheme 1a).6 Later, Walsh,7 Nolan,8 and Perrio9 successfully developed various different precursors for the in situ generation of sulfenate anions and cross-coupling with aryl halides by a Pd-catalyzed arylation reaction (Scheme 1b). Despite these significant advances, noble transition-metal catalysts and high temperature were required in these transformations. In view of the importance of sulfoxides and the sustainable synthesis, the development of a novel procedure for the generation of sulfoxides under mild and metal-free conditions was in high demand. With this in mind, we developed a transition-metal-free formal arylation of general sulfenate anions with diaryliodonium salts (up to 91%).
Based on our interest in the enantioselective arylation of sulfenate anions10a and related reactions,10b we started our study by choosing tert-butyl 3-(p-tolylsulfinyl) propanoate 1a and diaryliodonium salt 2a as the model substrates. We hypothesized that a diaryliodonium salt reacted with a Cu catalyst to generate an aromatic electrophile equivalent in the formation of a putative Cu(III)–aryl intermediate to react with sulfenate anions which finally led to 1-methyl-4-(phenylsulfinyl)benzene 3a (Table 1). A brief screening of copper salts with 3 equiv. of Cs2CO3 as the base in toluene at 80 °C for 12 h was conducted (Table 1, entries 1–4). To our delight, the desired product 1-methyl-4-(phenylsulfinyl)benzene (3a) was obtained in 34% yield (Table 1, entry 1). Replacing CuI with other copper sources showed no improvement in the yield. The arylation product could also be obtained in the absence of any copper salts (Table 1, entry 5). Other common bases, such as Na2CO3, KOH and K2CO3, were tested, and K2CO3 was found to be the best choice with the yield of 3a increasing to 67% (Table 1, entry 8). The yield dramatically increased to 85% by using tBuOLi as the base at room temperature (Table 1, entry 11). However, further screening of solvents did not give better results (Table 1, entries 12–15).
| Entry | Catalyst | Base | Temp. (°C) | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol, 1.5 equiv.), catalyst (10 mol%), in the solvent (2.0 mL) at 80 °C under air for 12 h. b Isolated yield. c At r.t. under air for 4 h. | |||||
| 1 | CuI | Cs2CO3 | 80 | Toluene | 34 |
| 2 | CuCl | Cs2CO3 | 80 | Toluene | 16 |
| 3 | CuOAc | Cs2CO3 | 80 | Toluene | 21 |
| 4 | CuBr | Cs2CO3 | 80 | Toluene | 17 |
| 5 | — | Cs2CO3 | 80 | Toluene | 19 |
| 6 | — | Na2CO3 | 80 | Toluene | <5 |
| 7 | — | KOH | 80 | Toluene | 27 |
| 8 | — | K2CO3 | 80 | Toluene | 67 |
| 9c | — | t BuONa | r.t. | Toluene | 32 |
| 10c | — | t BuOK | r.t. | Toluene | 49 |
| 11c | — | t BuOLi | r.t. | Toluene | 85 |
| 12c | — | t BuOLi | r.t. | CH3CN | 80 |
| 13c | — | t BuOLi | r.t. | THF | 77 |
| 14c | — | t BuOLi | r.t. | 1,4-Dioxane | 72 |
| 15c | — | t BuOLi | r.t. | DME | 71 |
After the establishment of the optimal reaction conditions, the substrate scope of sulfenate anions in the arylation reaction with diaryliodonium salts 2a was examined, as listed in Scheme 2. Generally, aryl sulfenate anions 1 with various electron-neutral, -donating or -withdrawing substituents at the para-, meta-, or ortho-positions of the phenyl group were all well-tolerated, affording the corresponding diarylsulfoxides 3a–3i in good yields (78–91%). The steric hindrance had little effect on the reaction. 3d could be obtained in 86% yield. Heteroaryl sulfoxides could exhibit anticancer, antifungal and anti-inflammatory activities.1b,f,h Under our procedure, 2-thiophenyl- and 2-pyridinyl-containing sulfoxides 3j and 3k were synthesized in 78% and 84% yields, respectively. A variety of alkyl sulfenate anions were then investigated. The corresponding alkylaryl sulfoxides were obtained in good to moderate yields (3l–3u).
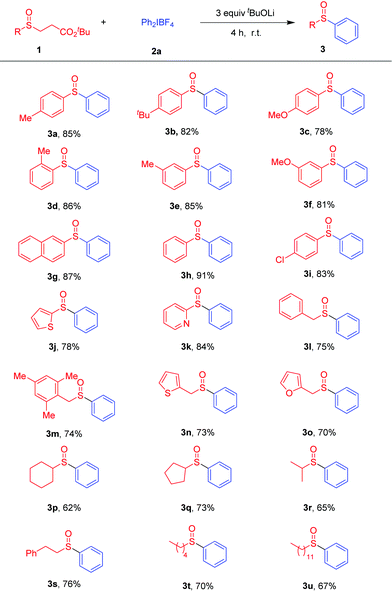 | ||
Scheme 2 Scope of sulfenate anions in the arylation with diaryliodonium salts 2a. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (0.2 mmol) and 2a (0.3 mmol, 1.5 equiv.) in toluene (2.0 mL) at r.t. under air for 4 h. 1 (0.2 mmol) and 2a (0.3 mmol, 1.5 equiv.) in toluene (2.0 mL) at r.t. under air for 4 h. | ||
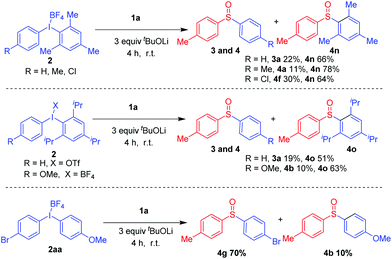
Encouraged by these promising results, we next examined the substrate scope of diaryliodonium salts. A wide range of substrates exhibited excellent reactivities, as shown in Scheme 3. Diaryliodonium salts with electron-donating groups such as methyl, methoxy, and tert-butyl groups at the para-position of the phenyl group were good reaction partners and the corresponding products 4a–4c were isolated in 77–87% yields, respectively. Besides, substrates with electron-withdrawing groups such as trifluoromethoxy, fluoro, chloro, bromide, ethoxy carbonyl or trifluoromethyl groups were also suitable coupling partners, furnishing 4d–4k in 65–89% yields.
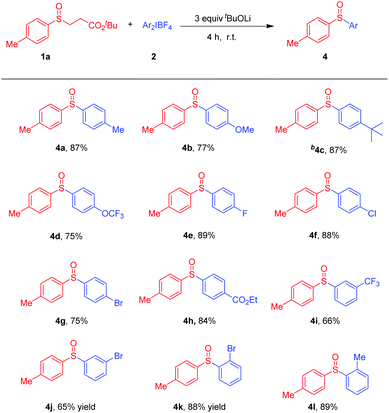 | ||
Scheme 3 Scope of diaryliodonium salts 2 in arylation of sulfenate anions. a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1a (0.2 mmol) and 2 (0.3 mmol, 1.5 equiv.) in toluene (2.0 mL) at r.t. under air for 4 h. b 1a (0.2 mmol) and 2 (0.3 mmol, 1.5 equiv.) in toluene (2.0 mL) at r.t. under air for 4 h. b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar2IOTf was used. Ar2IOTf was used. | ||
Notably, halogens were also tolerable, which made further functionalizations possible. Interestingly, the reaction is inert to steric hindrance, as the desired diaryl sulfoxides 4k and 4l could be afforded in 88% and 89% yields, respectively. Various unsymmetrical diaryliodonium salts were then tested.13 The main products 4n and 4o were formed in good yields. When 2aa was used as the substrate, 70% yield of 4g and 10% yield of 4b were obtained, respectively (Scheme 4).
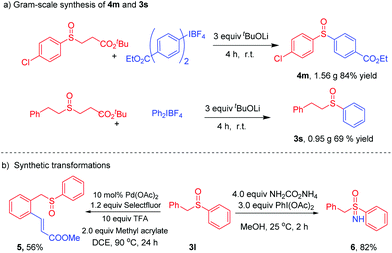
The practicability of our procedure was demonstrated by performing a gram scale reaction, delivering 4m in 84% yield (1.56 g) and 3s in 69% yield (0.95 g), respectively (Scheme 5a). Olefination of 3l produced 5 in 56% yield. The sulfoximine 6 could be efficiently prepared in 82% yield following simple procedures (Scheme 5b).11
Based on the above results and previous work,12 a plausible mechanism was proposed as depicted in Scheme 6. Base-mediated deprotonation of substrate 1 gave the corresponding ester enolate which underwent a retro-Michael procedure and generated sulfenate anion B. Then, a sulfenate anion B nucleophilic attack on diaryliodonium salts 2 afforded hypervalent iodine intermediate C. Finally, the reductive elimination of intermediate C delivered the desired sulfoxide 3 or 4.
In conclusion, we have developed an efficient arylation of general sulfenate anions with diaryliodonium salts under transition metal-free conditions. This method provides a novel route towards a wide range of functionalized diaryl and aryl alkyl sulfoxides in good yields. The salient features of this transformation including mild reaction conditions, general substrate scope, good functional group tolerance and good yields. Further applications of this method and asymmetric synthesis of optically active sulfoxides are currently underway.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Natural Science Foundation of China (21672067 and 21425205), 973 Program (2015CB856600), Innovative Research Team in University and Shanghai Sailing Program (15YF1403600).Notes and references
- For reviews, see: (a) M. C. Carreño, Chem. Rev., 1995, 95, 1717 CrossRef; (b) I. Fernández and N. Khiar, Chem. Rev., 2003, 103, 3651 CrossRef; (c) R. Bentley, Chem. Soc. Rev., 2005, 34, 609 RSC; (d) I. Dini, G. C. Tenore and A. J. Dini, Nat. Prod., 2008, 71, 2036 CrossRef CAS; (e) M. El-Aasr, Y. Fujiwara, T. Takeya, S. Tsukamoto, M. Ono, D. Nakamo, M. Okawa, J. Kinjo, H. Yoshimitsu and T. J. Nohara, Nat. Prod., 2010, 73, 1306 CrossRef CAS; (f) T. Nohara, Y. Fujiwara, Y. Komota, Y. Kondo, T. Saku, K. Yamaguchi, Y. Komohara and M. Takeya, Chem. Pharm. Bull., 2015, 63, 117 CrossRef CAS; (g) R. D. Mahale, M. R. Rajput, G. C. Maikap and M. K. Gurjar, Org. Process Res. Dev., 2010, 14, 1264 CrossRef CAS; (h) J. M. Shin, Y. M. Cho and G. Sachs, J. Am. Chem. Soc., 2004, 126, 7800 CrossRef CAS.
- For selected examples, see: (a) P. Pitchen, E. Dunach, M. N. Deshmukh and H. B. Kagan, J. Am. Chem. Soc., 1984, 106, 8188 CrossRef CAS; (b) F. Di Furia, G. Modena and R. Seraglia, Synthesis, 1984, 325 CrossRef CAS; (c) J. Legros, J. R. Dehli and C. Bolm, Adv. Synth. Catal., 2005, 347, 19 CrossRef CAS; (d) G. E. O'Mahony, P. Kelly, S. E. Lawrence and A. R. Maguire, ARKIVOC, 2011, 1, 1–110 Search PubMed; (e) C. Bolm, Coord. Chem. Rev., 2003, 237, 245 CrossRef CAS; (f) E. Wojaczyńska and J. Wojaczyński, Chem. Rev., 2010, 110, 4303 CrossRef; (g) X. Ye, A. M. P. Moeljadi, K. F. Chin, H. Hirao, L. Zong and C.-H. Tan, Angew. Chem., Int. Ed., 2016, 55, 7101 CrossRef CAS; (h) L. Zong, C. Wang, A. M. P. Moeljadi, X. Ye, R. Ganguly, Y. Li, H. Hirao and C.-H. Tan, Nat. Commun., 2016, 7, 13455 CrossRef; (i) Y. Li, M. Wang and X. Jiang, ACS Catal., 2017, 7, 7587–7592 CrossRef CAS.
- (a) K. K. Andersen, Tetrahedron Lett., 1962, 3, 93 CrossRef; (b) K. K. Andersen, W. Gaffield, N. E. Papanikolaou, J. W. Foley and R. I. Perkins, J. Am. Chem. Soc., 1964, 86, 5637 CrossRef CAS; (c) Z. Han, D. Krishnamurthy, P. Grover, H. S. Wilkinson, Q. K. Fang, X. Su, Z.-H. Lu, D. Magiera and C. H. Senanayake, Angew. Chem., Int. Ed., 2003, 42, 2032 CrossRef CAS PubMed; (d) Z. Han, J. J. Song, N. K. Yee, Y. Xu, W. Tang, J. T. Reeves, Z. Tan, X.-J. Wang, B. Lu, D. Krishnamurthy and C. H. Senanayake, Org. Process Res. Dev., 2007, 11, 605 CrossRef CAS.
- For reviews, see: (a) C. Caupène, C. Boudou, S. Perrio and P. Metzner, J. Org. Chem., 2005, 70, 2812 CrossRef; (b) F. Gelat, J. Jayashankaran, J. Lohier, A. Gaumont and S. Perrio, Org. Lett., 2011, 13, 3170 CrossRef CAS; (c) F. Gelat, A. Gaumont and S. Perrio, J. Sulfur Chem., 2013, 34, 596 CrossRef CAS; (d) J. S. O'Donnell and A. L. J. Schwan, J. Sulfur Chem., 2004, 25, 183 CrossRef; (e) A. L. Schwan and S. C. Söderman, Phosphorus, Sulfur Silicon Relat. Elem., 2013, 188, 275 CrossRef CAS; (f) L. Zong, X. Ban, C. W. Kee and C.-H. Tan, Angew. Chem., Int. Ed., 2014, 53, 11849 CrossRef CAS.
- H. Yu, Z. Li and C. Bolm, Org. Lett., 2018, 20, 2076 CrossRef CAS.
- (a) G. Maitro, S. Vogel, G. Prestat, D. Madec and G. Poli, Org. Lett., 2006, 8, 5951 CrossRef CAS; (b) G. Maitro, S. Vogel, M. Sadaoui, G. Prestat, D. Madec and G. Poli, Org. Lett., 2007, 9, 5493 CrossRef CAS; (c) E. Bernoud, G. L. Duc, X. Bantreil, G. Prestat, D. Madec and G. Poli, Org. Lett., 2010, 12, 320 CrossRef CAS; (d) G. Maitro, G. Prestat, D. Madec and G. Poli, Tetrahedron: Asymmetry, 2010, 21, 1075 CrossRef CAS.
- (a) T. Jia, A. Bellomo, S. Montel, M. Zhang, K. E. L. Baina, B. Zheng and P. J. Walsh, Angew. Chem., Int. Ed., 2014, 53, 260 CrossRef CAS; (b) T. Jia, M. Zhang, I. K. Sagamanova, C. Y. Wang and P. J. Walsh, Org. Lett., 2015, 17, 1168 CrossRef CAS; (c) T. Jia, M. Zhang, H. Jiang, C. Y. Wang and P. J. Walsh, J. Am. Chem. Soc., 2015, 137, 13887 CrossRef CAS; (d) H. Jiang, T. Jia, M. Zhang and P. J. Walsh, Org. Lett., 2016, 18, 972 CrossRef CAS; (e) T. Jia, M. Zhang, H. Jiang, S. P. McCollom, A. Bellomo, S. Montel, J. Mao, S. D. Dreher, C. J. Welch, E. L. Regalado, R. T. Williamson, B. C. Manor, N. C. Tomson and P. J. Walsh, J. Am. Chem. Soc., 2017, 139, 8337 CrossRef CAS.
- F. Izquierdo, A. Chartoire and S. P. Nolan, ACS Catal., 2013, 3, 2190 CrossRef CAS.
- F. Gelat, J.-F. Lohier, A.-C. Gaumont and S. Perrio, Adv. Synth. Catal., 2015, 357, 2011 CrossRef CAS.
- (a) L. Wang, M. Chen, P. Zhang, W. Li and J. Zhang, J. Am. Chem. Soc., 2018, 140, 3467 CrossRef CAS; (b) Q. Dai and J. Zhang, Adv. Synth. Catal., 2018, 360, 1123 CrossRef CAS.
- (a) B. Wang, C. Shen, J. Yao, H. Yin and Y. Zhang, Org. Lett., 2014, 16, 46 CrossRef CAS; (b) M. Zenzola, R. Doran, L. Degennaro, R. Luisi and J. Bull, Angew. Chem., Int. Ed., 2016, 55, 7203 CrossRef CAS.
- (a) M. S. Yusubov, A. V. Maskaev and V. V. Zhdankin, ARKIVOC, 2011, 1, 370 Search PubMed; (b) Q. Y. Toh, A. McNally, S. Vera, N. Erdmann and M. J. Gaunt, J. Am. Chem. Soc., 2013, 135, 3772 CrossRef CAS; (c) L. Chan, A. McNally, Q. Y. Toh, A. Mendoza and M. J. Gaunt, Chem. Sci., 2015, 6, 1277 RSC.
- (a) J.-H. Chun, S. Lu, Y.-S. Lee and V. W. Pike, J. Org. Chem., 2010, 75, 3332 CrossRef CAS; (b) N. Umierski and G. Manolikakes, Org. Lett., 2013, 15, 188 CrossRef CAS; (c) A. Monastyrskyi, N. Namelikonda and R. Manetsch, J. Org. Chem., 2015, 80, 2513 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qo00914g |
| This journal is © the Partner Organisations 2019 |