Influence of sulfonyl substituents on the decomposition of N-sulfonylhydrazones at room temperature†
Zhaohong
Liu
 a,
Kaki
Raveendra Babu
a,
Feng
Wang
b,
Yang
Yang
a and
Xihe
Bi
a,
Kaki
Raveendra Babu
a,
Feng
Wang
b,
Yang
Yang
a and
Xihe
Bi
 *ac
*ac
aJilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Department of Chemistry, Northeast Normal University, 5268 Renmin Street, 130024 Changchun, China. E-mail: bixh507@nenu.edu.cn
bSchool of Chemical Engineering, Changchun University of Technology, Changchun 130012, China
cState Key Laboratory of Elemento-Organic Chemistry, Nankai University, 300071 Tianjin, China
First published on 5th December 2018
Abstract
A detailed study of the dissociation performance of structurally varied N-sulfonylhydrazones was described, and N-tfsylhydrazone was proved to be the best substrate to give the corresponding diazo compound at room temperature. N-Tfsylhydrazone is capable of releasing nonstabilized aryldiazomethane in a wide range of solvents in the presence of various commonly used bases under mild reaction conditions (−25 °C to 40 °C). The utility of the strategy is established by late-stage derivatization of bioactive molecules and several typically sensitive reactions, in which highly purified diazo compounds are required.
Diazo compounds are highly useful reactive substrates in organic synthesis. These have been widely used in a variety of organic transformations.1 However, the utility of diazo compounds is often limited by their toxicity, instability, and unpredictable explosive behaviour, particularly in the case of electron-rich aryldiazomethanes. Thus, identifying sustainable and safe surrogates for the generation of diazo compounds has attracted great attention.2 Among these diazo surrogates, N-tosylhydrazones are of particular importance as witnessed by the rapidly expanded repertoire of transformations in the past decades.3 However, the relative high temperature required for their dissociation into diazo species limited the applications of N-tosylhydrazones, especially in the total synthesis of natural products,4 asymmetric synthesis,5 and other reactions involving temperature sensitive substrates, reagents and products.6 To overcome these challenges, several methods have been developed for in situ generation of aryldiazomethanes at low-temperature via batch7 and continuous flow8 one-pot processes. For example, the Aggarwal group described the phase-transfer catalyst (PTC) promoted decomposition of sodium tosylhydrazone salts to form diazo compounds under mild conditions (30–60 °C).9 Recently, Charette et al. demonstrated that sterically congested mesityl- and triisopropylphenyl sulfonylhydrazones undergo elimination more readily than N-tosylhydrazones.10 Moreover, we have also reported the room temperature decomposition of N-nosylhydrazones to give the aryldiazo compounds.11 These methods hint that the steric hindrance and electron properties of substituents on the aryl ring have great influence on the decomposition of N-sulfonylhydrazones. However, to the best of our knowledge, the systematic study on the decomposition of N-sulfonylhydrazones with different substituted arylsulfonyl groups has not been reported. In continuation of our interest in the low-temperature decomposition of N-sulfonylhydrazone,11,12 herein we report a comprehensive study of N-sulfonylhydrazones with varying arylsulfonyl substituents, and discover that N-2-(trifluoromethyl)benzenesulfonylhydrazone (N-tfsylhydrazone) was the best one to give the corresponding diazo compound under mild conditions (−25 °C to 40 °C). Besides, the decomposition of N-tfsylhydrazone showed high tolerance to a variety of commonly used solvents and bases, which exhibited the great potential of this protocol to exploit the non-stabilized diazo compounds at room temperature.
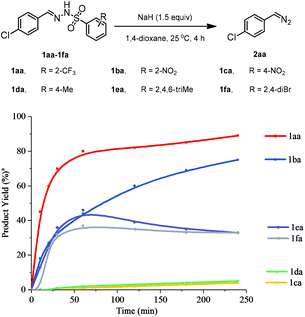
Our initial investigation was commenced with dissociation kinetics of 4-chloro-phenyl N-sulfonylhydrazones bearing different N-arylsulfonyl substituents in the presence of NaH in 1,4-dioxane at room temperature (Fig. 1, see the ESI† for details). ortho-Substituted strong electron-withdrawing groups on the N-arylsulfonyl group were able to produce the desired 4-chlorophenyl-diazomethane 2aa. For instance, when the 2-trifluoromethyl group (1aa) was employed, 2aa was obtained in 80% yield within 1 h. The 2-nitro-substituted substrate11 (1ba) gave the desired product with similar high yield, albeit with a relatively low reaction rate compared with 1aa, while sterically bulkier N-mesitylhydrazone10 (1ea) and N-dibromophenylhydrazone13 (1fa) provided moderate reactivity. In contrast, para-substituted N-sulfonylhydrazones, either with electron-donating (Me) or electron-withdrawing substituents (NO2 and CF3, for more details see Table S1 in the ESI†), displayed poor reactivity. These results clearly indicate that both position and electronic characteristics of the substituents strongly influence the dissociation reactivity of N-sulfonylhydrazones.
Next, a wide range of solvents, such as DMSO, DMF, CH3CN, etheric solvents (1,4-dioxane and THF), halogen-containing solvents (PhCl, DCM, and 1,1-dichloroethane), and toluene, were employed to assess the effect of solvent on the decomposition of N-tfsylhydrazone 1aa (Table 1). To our delight, almost all the solvents provided satisfactory results, and afforded 4-chlorophenyl diazomethane in good to excellent yields (entries 2–9). DMSO was an exception and only 20% yield was obtained (entry 1).
| Entry | Solvent | NMR yieldb (%) | |
|---|---|---|---|
| Recovery (1aa) | Yield (2aa) | ||
| a Reaction conditions: 1aa (0.5 mmol), NaH (0.75 mmol), in solvent (10.0 mL) for 4 h under an N2 atmosphere. b Yield calculated from 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as the internal standard. | |||
| 1 | DMSO | 14 | 20 |
| 2 | DMF | 0 | 70 |
| 3 | CH3CN | 0 | 86 |
| 4 | 1,4-Dioxane | 0 | 89 |
| 5 | THF | 5 | 76 |
| 6 | PhCl | 24 | 68 |
| 7 | DCE | 20 | 65 |
| 8 | DCM | 24 | 69 |
| 9 | Toluene | 0 | 70 |
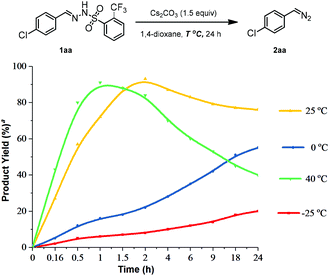
The effect of common bases on the decomposition in 1,4-dioxane at room temperature was investigated. As summarized in Table 2, the conversions were generally good, and Cs2CO3 was proved to be the best base (93%, entry 4), whereas in the case of LiOtBu, product 2aa was found with low yield (11%, entry 7). Furthermore, the kinetic results were obtained at different temperatures (−25 °C, 0 °C, 25 °C, and 40 °C) (Fig. 2). For example, 4-chlorophenyl diazomethane (2aa) was achieved in 91% yield at 40 °C within 1 h and in 93% yield at 25 °C within 2 h, respectively. Notably, the obtained product (2aa) underwent degradation much faster at 40 °C. More importantly, N-tfsylhydrazone could undergo decomposition at 0 °C and −25 °C, albeit with relatively lower efficiency. However, this provided the opportunity for diazo compounds to sustain for a longer time in the reaction mixture to react with substrates, instead of undergoing quick decomposition. Therefore, the optimal reaction condition for the decomposition of N-tfsylhydrazone to give diazo compounds is identified as use of Cs2CO3 as a base in 1,4-dioxane at 25 °C for 2 hours.
| Entry | Base | NMR yieldb (%) | |
|---|---|---|---|
| Recovery (1aa) | Yield (2aa) | ||
| a Reaction conditions: 1aa (0.5 mmol), base (0.75 mmol), in 1,4-dioxane (10.0 mL) for 4 h under an N2 atmosphere. b Yield calculated from 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as the internal standard. | |||
| 1 | NaH | 0 | 89 |
| 2 | K3PO4 | 12 | 75 |
| 3 | K2CO3 | 15 | 63 |
| 4 | Cs2CO3 | 0 | 93 |
| 5 | KOH | 0 | 65 |
| 6 | KOtBu | 10 | 43 |
| 7 | LiOtBu | 5 | 11 |
| 8 | LiOMe | 0 | 75 |
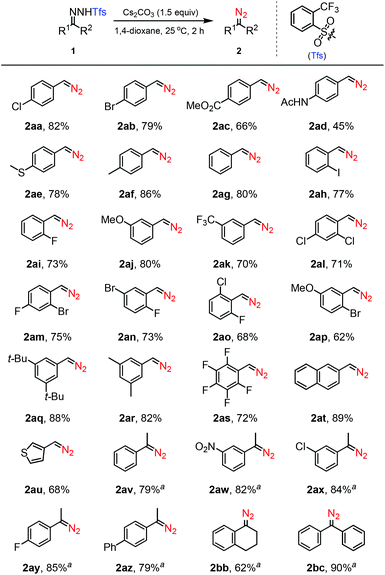
With the optimized reaction conditions in hand, the room temperature dissociation reaction scope was explored with a range of structurally varied N-tfsylhydrazones (Scheme 1). It was found that a wide range of N-tfsylhydrazones possessing both strong electron-withdrawing or electron-donating groups underwent efficient dissociation to afford the corresponding diazo products in moderate to high yields (2aa–2as). A variety of functional groups, such as –CO2Me, –NHAc, –CF3, –OMe, t-Bu, –SMe, and –NO2, as well as halo groups (F, Cl, Br, I), were compatible with this dissociation process. Remarkably, the reaction with N-tfsylhydrazones derived from highly substituted benzaldehydes could afford the corresponding diazo derivatives in moderate to high yields (2al–2as, 62–88%). Also, N-tfsylhydrazones having other aromatic functionalities, including naphthyl and thiophenyl groups, proceeded rapidly to afford the corresponding diazo derivatives (2at and 2au) in good yields. In addition, aryl–alkyl N-tfsylhydrazones and diaryl N-tfsylhydrazones were readily accommodated in this protocol and rapidly converted into the corresponding aryl–alkyl diazomethanes (2av–2az and 2bb) and diaryl diazomethane (2bc) in high yields, thus demonstrating the excellent functional group tolerance. Note that slightly elevated temperature (50 °C) is necessary for the dissociation of ketone-derived N-tfsylhydrazones.
A particularly useful application of this method is in the late-stage derivatization of bioactive molecules. For example, fenofibrate, which is used to decrease the risk of cardiovascular disease, was readily converted to the corresponding esterified derivative 3bd in 62% total yield via a three-step procedure (Scheme 2). Notably, both ether and ester groups were compatible with steps of both diazotization and esterification.
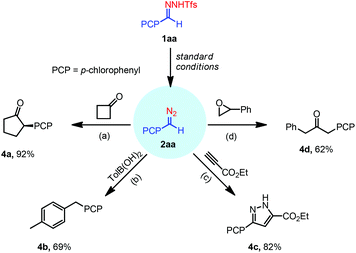
The above facile preparation of donor diazo compounds using N-tfsylhydrazones at room temperature inspired us to assess their compatibility in various transformations (Scheme 3). The scandium-catalyzed homologation reaction of cycloalkanones with aryldiazomethanes failed when the diazo solution was not of high purity.14 To our delight, when 4-chlorophenyl-diazomethane 2aa, which is prepared through our method and washed with ammonia chloride, was reacted with cyclobutanone in the presence of Sc(OTf)3 the ring expansion product cyclopentanone derivative 4a was afforded in 92% yield. Next, the coupling reaction between 2aa and boronic acid gave the desired coupling product 4b in 69% yield under low temperature conditions, whereas previous coupling reactions used high reaction temperatures with lower yields.15 The multi-operation flow process which requires special equipment has been reported for the cycloaddition reaction of diazo compounds with alkynes,16 whereas our protocol is quite simple, through which the [3 + 2] cycloaddition reaction of 2aa with ethyl propiolate provided the pyrazole derivative 4c in 82% yield under mild reaction conditions. Finally, the reaction of 2aa with epoxide in the presence of Yb(OTf)3 afforded the ketone 4d in 62% yield.
In summary, we have systematically investigated the dissociation performance of structurally varying N-sulfonylhydrazones, and for the first time discovered N-tfsylhydrazone as a new and efficient diazo surrogate at room temperature. It is capable of providing highly pure aryldiazomethane in various solvents with commonly used bases under mild reaction conditions (−25 °C to 40 °C). This finding will further stimulate the synthetic exploration of N-sulfonylhydrazones as un-stabilized diazo precursors at room temperature.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the NSFC (21522202, 21502017).Notes and references
-
(a)
M. P. Doyle, M. A. McKervey and T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds, Wiley, New York, 1998 Search PubMed
; (b) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704–724 CrossRef CAS PubMed
; (c) X. Zhao, Y. Zhang and J. Wang, Chem. Commun., 2012, 48, 10162–10173 RSC
; (d) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS PubMed
; (e) K. A. Mix, M. R. Aronoff and R. T. Raines, ACS Chem. Biol., 2016, 11, 3233–3244 CrossRef CAS PubMed
.
-
(a) G. Maas, Angew. Chem., Int. Ed., 2009, 48, 8186–8195 CrossRef CAS
; (b) M. Jia and S. Ma, Angew. Chem., Int. Ed., 2016, 55, 9134–9166 CrossRef CAS
.
-
(a) J. Barluenga and C. Valdés, Angew. Chem., Int. Ed., 2011, 50, 7486–7500 CrossRef CAS PubMed
; (b) Z. Shao and H. Zhang, Chem. Soc. Rev., 2012, 41, 560–572 RSC
; (c) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236–247 CrossRef CAS
; (d) Y. Xia and J. Wang, Chem. Soc. Rev., 2017, 46, 2306–2362 RSC
.
- C. Yuan, B. Du, L. Yang and B. Liu, J. Am. Chem. Soc., 2013, 135, 9291–9294 CrossRef CAS
.
-
(a) J. Feng, B. Li, Y. He and Z. Gu, Angew. Chem., Int. Ed., 2016, 55, 2186–2190 CrossRef CAS
; (b) Y. Wang, X. Wen, X. Cui, L. Wojtas and X. P. Zhang, J. Am. Chem. Soc., 2017, 139, 1049–1052 CrossRef CAS
.
- A. R. Reddy, F. Hao, K. Wu, C. Zhou and C. Che, Angew. Chem., Int. Ed., 2016, 55, 1810–1815 CrossRef CAS
.
-
(a) C. Soldi, K. N. Lamb, R. A. Squitieri, M. González-López, M. J. Di Maso and J. T. Shaw, J. Am. Chem. Soc., 2014, 136, 15142–15145 CrossRef CAS
; (b) J. R. Fulton, V. K. Aggarwal and J. de Vicente, Eur. J. Org. Chem., 2005, 1479–1492 CrossRef CAS
.
-
(a) D. N. Tran, C. Battilocchio, S.-B. Lou, J. M. Hawkins and S. V. Ley, Chem. Sci., 2015, 6, 1120–1125 RSC
; (b) C. Battilocchio, F. Feist, A. Hafner, M. Simon, D. N. Tran, D. M. Allwood, D. C. Blakemore and S. V. Ley, Nat. Chem., 2016, 8, 360–367 CrossRef CAS
.
-
(a) V. K. Aggarwal, E. Alonso, G. Hynd, K. M. Lydon, M. J. Palmer, M. Porcelloni and J. R. Studley, Angew. Chem., Int. Ed., 2001, 40, 1430–1433 CrossRef CAS
; (b) V. K. Aggarwal, J. R. Fulton, C. G. Sheldon and J. de Vicente, J. Am. Chem. Soc., 2003, 125, 6034–6035 CrossRef CAS
.
- P. Lévesque, S. T. Laporte and A. B. Charette, Angew. Chem., Int. Ed., 2017, 56, 837–841 CrossRef
.
- Z. Liu, Q. Li, P. Liao and X. Bi, Chem. – Eur. J., 2017, 23, 4756–4760 CrossRef CAS
.
-
(a) Z. Liu, X. Zhang, G. Zanoni and X. Bi, Org. Lett., 2017, 19, 6646–6649 CrossRef CAS
; (b) Y. Yang, Z. Liu, A. Porta, G. Zanoni and X. Bi, Chem. – Eur. J., 2017, 23, 9009–9013 CrossRef CAS
; (c) Z. Liu, Q. Li, Y. Yang and X. Bi, Chem. Commun., 2017, 53, 2503–2506 RSC
; (d) Z. Liu, B. Liu, X. Zhao, Y. Wu and X. Bi, Eur. J. Org. Chem., 2017, 928–932 CrossRef CAS
; (e) Z. Liu, X. Zhang, M. Virelli, G. Zanoni, E. A. Anderson and X. Bi, iScience, 2018, 54–60 CrossRef
.
- Y. Jiang, A. B. Diagne, R. J. Thomson and S. E. Schaus, J. Am. Chem. Soc., 2017, 139, 1998–2005 CrossRef CAS
.
- D. C. Moebius and J. S. Kingsbury, J. Am. Chem. Soc., 2009, 131, 878–879 CrossRef CAS
.
-
(a) J. Barluenga, M. Tomás-Gamasa, F. Aznar and C. Valdés, Nat. Chem., 2009, 1, 494–499 CrossRef CAS
; (b) C. Peng, W. Zhang, G. Yan and J. Wang, Org. Lett., 2009, 11, 1667–1670 CrossRef CAS
.
- C. Audubert, O. J. G. Marin and H. Lebel, Angew. Chem., Int. Ed., 2017, 56, 6294–6297 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qo00802g |
| This journal is © the Partner Organisations 2019 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yield calculated from 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as the internal standard.
Yield calculated from 1H-NMR spectroscopy with 1,3,5-trimethoxybenzene as the internal standard.