High-performance wide-bandgap copolymers with dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one units†
Yaxin
Gao‡
ab,
Dan
Li‡
bc,
Zuo
Xiao
 b,
Xin
Qian
b,
Junliang
Yang
b,
Xin
Qian
b,
Junliang
Yang
 *a,
Fangyang
Liu
*a,
Fangyang
Liu
 *d,
Shangfeng
Yang
*d,
Shangfeng
Yang
 *c and
Liming
Ding
*c and
Liming
Ding
 *b
*b
aHunan Key Laboratory for Super-microstructure and Ultrafast Process, School of Physics and Electronics, Central South University, Changsha 410083, China. E-mail: junliang.yang@csu.edu.cn
bCenter for Excellence in Nanoscience (CAS), Key Laboratory of Nanosystem and Hierarchical Fabrication (CAS), National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: ding@nanoctr.cn
cDepartment of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, China. E-mail: sfyang@ustc.edu.cn
dSchool of Metallurgy and Environment, Central South University, Changsha 410083, China. E-mail: liufangyang@csu.edu.cn
First published on 19th December 2018
Abstract
Two wide-bandgap copolymers (PBD and PBD2T) containing dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one units were designed. They have large optical bandgaps and deep HOMO levels. Solar cells based on them and a nonfullerene acceptor IT-M gave high open-circuit voltages up to 1.00 V and decent power conversion efficiencies up to 10.34%.
Nonfullerene organic solar cells (NFOSCs) have attracted great attention because of their higher performance and greater potential than their fullerene counterparts.1 Recently, record power conversion efficiencies (PCEs) of 14.62% and 17.36% were obtained from single-junction and tandem NFOSCs, respectively.2 To realize high-performance NFOSCs, using a wide-bandgap (WBG) donor–acceptor (D–A) copolymer donor to match the low-bandgap nonfullerene acceptor is an effective approach.3 WBG D–A copolymers show complementary absorption to that of nonfullerene acceptors, which can make full use of solar irradiation and increase the short-circuit current density (Jsc). The deep highest occupied molecular orbital (HOMO) levels of WBG copolymers can enhance the open-circuit voltage (Voc).4 However, efficient WBG copolymers for NFOSCs are still limited. The reported WBG copolymers include copolymers based on carboxylic ester-functionalized thiophene,5 copolymers based on benzo[1,2-c:4,5-c′]dithiophene-4,8-dione6 and copolymers based on benzo[d][1,2,3]triazole.7 New WBG copolymers for NFOSCs are highly desired.
D–A copolymers based on polycyclic aromatic lactam (PAL) units are efficient donor materials.8 The PAL units possess a strong electron-withdrawing ability and good planarity, endowing the copolymers with deep HOMO levels and high hole mobility. Over 10% PCEs have been achieved for PAL copolymer:fullerene solar cells.9 However, the performance of PAL copolymers in NFOSCs is less explored. Yu et al. first reported a tricyclic lactam unit, dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one (DTP), and the corresponding D–A copolymers, which gave PCEs up to 5.33% in fullerene solar cells.8c Ding et al. also reported a DTP-based D–A copolymer, PDTP4TFBT, which presented good performance in both fullerene and nonfullerene solar cells.10 NFOSCs based on PDTP4TFBT and a nonfullerene acceptor, ITIC,11 delivered a PCE of 7.58%. The optical bandgap (Eoptg) of PDTP4TFBT is small (1.62 eV) and the absorption spectrum of PDTP4TFBT largely overlaps with that of ITIC, thus limiting the device performance. Here, we report the development of two DTP-based WBG copolymers for NFOSCs. The copolymers PBD and PBD2T show large Eoptg and deep HOMO levels. Solar cells using them as the donors and IT-M12 as the acceptor afforded high Voc values up to 1.00 V and decent PCEs up to 10.34%.
The structures of PBD and PBD2T are shown in Fig. 1. Compared with PBD, PBD2T possesses two more 3-octylthiophene π-bridges in the backbone. PBD and PBD2T were obtained by copolymerization of (4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl)bis(trimethylstannane) (BDT monomer) with the corresponding DTP monomers via the Stille reaction (Scheme S1, ESI†). The details are given in the ESI.†PBD and PBD2T show good solubility in common solvents such as chloroform, toluene and chlorobenzene. The number-average molecular weights (Mn) for PBD and PBD2T are 31.2 kDa and 57.5 kDa, respectively, and the polydispersity indexes (PDIs) are 3.08 and 2.43, respectively.
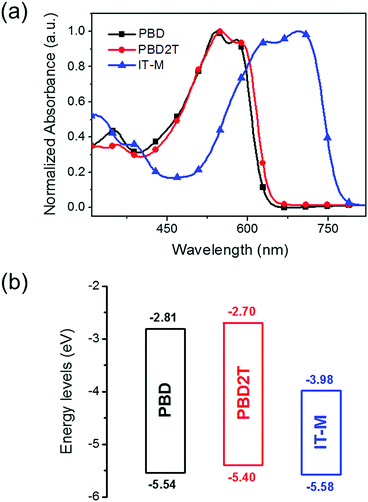
The absorption spectra for PBD and PBD2T in chloroform and as films are shown in Fig. S7 (ESI†) and Fig. 2a, respectively. In solution, PBD and PBD2T show a strong absorption band at 400–650 nm, with a low-energy peak at 578 nm and 580 nm, respectively, and shoulder absorption at 542 nm and 544 nm, respectively (Table 1). For films, the shoulder absorption intensifies and the spectra show slight bathochromic shifts. From solution to films, the redshifts for the low-energy peaks of PBD and PBD2T are 2 nm and 8 nm, respectively. The larger redshift of PBD2T suggests that introduction of 3-octylthiophene π-bridges enhances intermolecular interaction. The absorption onsets for the PBD and PBD2T films are 631 nm and 643 nm, respectively, corresponding to Eoptg values of 1.97 eV and 1.93 eV, respectively. An IT-M film shows intensive absorption at 500–790 nm, which is complementary to that of PBD and PBD2T. The energy levels for PBD and PBD2T were estimated by cyclic voltammetry (CV) (Fig. S8, ESI†).13 The HOMO and the lowest unoccupied molecular orbital (LUMO) energy levels for PBD and PBD2T were calculated from the onset potentials of oxidation (Eonox) and reduction (Eonred), respectively, i.e., HOMO = −(Eonox + 4.8) and LUMO = −(Eonred + 4.8). The HOMO levels for PBD and PBD2T are −5.54 eV and −5.40 eV, respectively, and the LUMO levels are −2.81 eV and −2.70 eV, respectively (Fig. 2b). The π-bridges lift both the HOMO and LUMO levels for PBD2T. The deep HOMO levels of PBD and PBD2T favor production of high Voc in solar cells. The acceptor IT-M has a HOMO at −5.58 eV and a LUMO at −3.98 eV.12
| Donor | λ sol [nm] | λ film [nm] | λ on [nm] |
E
optg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [eV] a [eV] |
E onox [V] | E onred [V] | HOMOb [eV] | LUMOc [eV] |
E
ecg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d [eV] d [eV] |
|---|---|---|---|---|---|---|---|---|---|
| a E optg = 1240/λon. b HOMO = −(Eonox + 4.8). c LUMO = −(Eonred + 4.8). d E ecg = LUMO–HOMO. | |||||||||
| PBD | 542 (sh), 578 | 542, 580 | 631 | 1.97 | 0.74 | −1.99 | −5.54 | −2.81 | 2.73 |
| PBD2T | 544 (sh), 580 | 550, 588 | 643 | 1.93 | 0.60 | −2.10 | −5.40 | −2.70 | 2.70 |
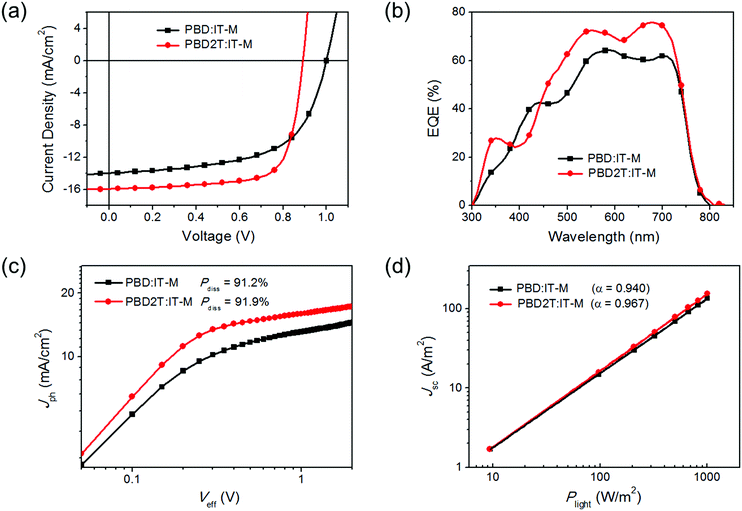
Solar cells with a structure of ITO/ZnO/donor:IT-M/MoO3/Ag were made to evaluate the performance of PBD and PBD2T.14J–V curves and external quantum efficiency (EQE) spectra are shown in Fig. 3a and b, respectively, and the performance data are listed in Table 2. The best PBD:IT-M cells gave a PCE of 8.33%, with a Voc of 1.00 V, a Jsc of 13.97 mA cm−2 and a fill factor (FF) of 59.6%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 (w/w), an active layer thickness of 106 nm and 0.6 vol% 1,8-diiodooctane (DIO) as the additive (Tables S1–S3, ESI†). The best PBD2T:IT-M cells gave a PCE of 10.34%, with a Voc of 0.89 V, a Jsc of 15.95 mA cm−2 and an FF of 72.8%. These cells have a D/A ratio of 1
1.8 (w/w), an active layer thickness of 106 nm and 0.6 vol% 1,8-diiodooctane (DIO) as the additive (Tables S1–S3, ESI†). The best PBD2T:IT-M cells gave a PCE of 10.34%, with a Voc of 0.89 V, a Jsc of 15.95 mA cm−2 and an FF of 72.8%. These cells have a D/A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
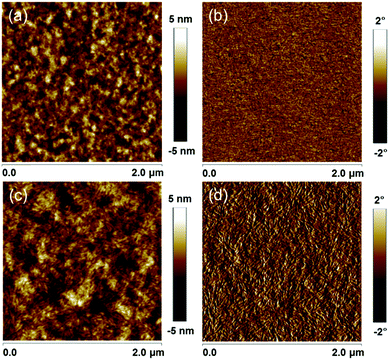
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), an active layer thickness of 103 nm and 0.2 vol% DIO as the additive (Tables S4–S6, ESI†). The Voc for the PBD2T cells is 0.11 V smaller than that of the PBD cells due to the higher HOMO level of PBD2T. In contrast, the Jsc and FF values for the PBD2T cells are much higher than those of the PBD cells. The PBD2T cells give higher EQEs at 300–380 nm and 442–740 nm than the PBD cells. The maximum EQEs for PBD and PBD2T cells are 64% and 76%, respectively. The integrated current densities from the EQE spectra are 13.10 mA cm−2 and 15.24 mA cm−2, respectively, which are consistent with the Jsc from the J–V measurements. Exciton dissociation probabilities (Pdiss) were evaluated (Fig. 3c). The Pdiss values for the PBD and PBD2T cells are 91.2% and 91.9%, respectively. The higher Pdiss for the PBD2T cells suggests more efficient charge generation, thus benefiting Jsc. We studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. 3d). The data were fitted to a power law: Jsc ∝ Pαlight. The α values for the PBD and PBD2T cells are 0.940 and 0.967, respectively, suggesting less charge recombination in the latter.15 The higher Jsc and FF and suppressed charge recombination for the PBD2T cells suggest a better charge-transporting ability of PBD2T than PBD. We measured hole mobilities (μh) using the space charge limited current (SCLC) method (Fig. S9, ESI†). Pure PBD and PBD2T films show μh of 9.7 × 10−5 cm2 V−1 s−1 and 1.3 × 10−4 cm2 V−1 s−1, respectively. The higher μh of PBD2T suggests that 3-octylthiophene π-bridges favor the polymer packing, thus improving the charge-transporting ability. μh and electron mobilities (μe) for PBD:IT-M and PBD2T:IT-M blend films were also measured (Fig. S10, S11 and Table S7, ESI†). The PBD2T:IT-M film shows higher μh and μe than the PBD:IT-M film, indicating more efficient charge transport in the PBD2T:IT-M film. The μh/μe values are 4.7 and 3.6 for the PBD:IT-M and PBD2T:IT-M films, respectively, suggesting that charge transport in the PBD2T:IT-M film is more balanced. In the XRD profiles, the PBD2T film shows a stronger (010) diffraction peak (2θ = ∼23.2°) than that of the PBD film, suggesting enhanced π–π stacking of PBD2T (Fig. S12, ESI†). The π–π stacking d-spacing is estimated to be ∼3.8 Å for PBD and PBD2T. We studied the morphologies of the active layers using atomic force microscopy (AFM) (Fig. 4). Compared with the PBD:IT-M blend film, the PBD2T:IT-M blend film presents much clearer nanofiber structures with ∼15 nm diameters. Such an optimal nanoscale phase separation favors exciton dissociation and charge carrier transport, thus leading to higher Jsc and FF values.
1 (w/w), an active layer thickness of 103 nm and 0.2 vol% DIO as the additive (Tables S4–S6, ESI†). The Voc for the PBD2T cells is 0.11 V smaller than that of the PBD cells due to the higher HOMO level of PBD2T. In contrast, the Jsc and FF values for the PBD2T cells are much higher than those of the PBD cells. The PBD2T cells give higher EQEs at 300–380 nm and 442–740 nm than the PBD cells. The maximum EQEs for PBD and PBD2T cells are 64% and 76%, respectively. The integrated current densities from the EQE spectra are 13.10 mA cm−2 and 15.24 mA cm−2, respectively, which are consistent with the Jsc from the J–V measurements. Exciton dissociation probabilities (Pdiss) were evaluated (Fig. 3c). The Pdiss values for the PBD and PBD2T cells are 91.2% and 91.9%, respectively. The higher Pdiss for the PBD2T cells suggests more efficient charge generation, thus benefiting Jsc. We studied bimolecular recombination by plotting Jsc against light intensity (Plight) (Fig. 3d). The data were fitted to a power law: Jsc ∝ Pαlight. The α values for the PBD and PBD2T cells are 0.940 and 0.967, respectively, suggesting less charge recombination in the latter.15 The higher Jsc and FF and suppressed charge recombination for the PBD2T cells suggest a better charge-transporting ability of PBD2T than PBD. We measured hole mobilities (μh) using the space charge limited current (SCLC) method (Fig. S9, ESI†). Pure PBD and PBD2T films show μh of 9.7 × 10−5 cm2 V−1 s−1 and 1.3 × 10−4 cm2 V−1 s−1, respectively. The higher μh of PBD2T suggests that 3-octylthiophene π-bridges favor the polymer packing, thus improving the charge-transporting ability. μh and electron mobilities (μe) for PBD:IT-M and PBD2T:IT-M blend films were also measured (Fig. S10, S11 and Table S7, ESI†). The PBD2T:IT-M film shows higher μh and μe than the PBD:IT-M film, indicating more efficient charge transport in the PBD2T:IT-M film. The μh/μe values are 4.7 and 3.6 for the PBD:IT-M and PBD2T:IT-M films, respectively, suggesting that charge transport in the PBD2T:IT-M film is more balanced. In the XRD profiles, the PBD2T film shows a stronger (010) diffraction peak (2θ = ∼23.2°) than that of the PBD film, suggesting enhanced π–π stacking of PBD2T (Fig. S12, ESI†). The π–π stacking d-spacing is estimated to be ∼3.8 Å for PBD and PBD2T. We studied the morphologies of the active layers using atomic force microscopy (AFM) (Fig. 4). Compared with the PBD:IT-M blend film, the PBD2T:IT-M blend film presents much clearer nanofiber structures with ∼15 nm diameters. Such an optimal nanoscale phase separation favors exciton dissociation and charge carrier transport, thus leading to higher Jsc and FF values.
Conclusions
In summary, two WBG copolymer donors, PBD and PBD2T, were developed. They have large Eoptg and deep HOMO levels, offering high Voc values up to 1.00 V and decent PCEs up to 10.34% in solar cells. PBD2T with 3-octylthiophene π-bridges has enhanced π–π stacking in the solid state and higher hole mobility, and endows the active layer with a good morphology. As a result, PBD2T:IT-M cells gave higher Jsc, FF and PCE values than PBD:IT-M cells. This work indicates that WBG copolymers based on polycyclic aromatic lactam units are promising donor materials for nonfullerene solar cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Key Research and Development Program of China (2017YFA0206600), the National Natural Science Foundation of China (U1401244, 21572041, 51503050, 51773045, 21772030 and 21704021) and the Youth Association for Promoting Innovation (CAS) for financial support.References
- (a) C. Yan, S. Barlow, Z. Wang, H. Yan, A. K.-Y. Jen, S. R. Marder and X. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef CAS; (b) D. Baran, T. Kirchartz, S. Wheeler, S. Dimitrov, M. Abdelsamie, J. Gorman, R. S. Ashraf, S. Holliday, A. Wadsworth, N. Gasparini, P. Kaienburg, H. Yan, A. Amassian, C. J. Brabec, J. R. Durrant and I. McCulloch, Energy Environ. Sci., 2016, 9, 3783 RSC; (c) Z. Xiao, F. Liu, X. Geng, J. Zhang, S. Wang, Y. Xie, Z. Li, H. Yang, Y. Yuan and L. Ding, Sci. Bull., 2017, 62, 1331 CrossRef CAS; (d) Z. Xiao, X. Jia, D. Li, S. Wang, X. Geng, F. Liu, J. Chen, S. Yang, T. P. Russell and L. Ding, Sci. Bull., 2017, 62, 1494 CrossRef CAS; (e) Z. Xiao, X. Jia and L. Ding, Sci. Bull., 2017, 62, 1562 CrossRef CAS; (f) Z. Xiao, S. Yang, Z. Yang, J. Yang, H.-L. Yip, F. Zhang, F. He, T. Wang, J. Wang, Y. Yuan, H. Yang, M. Wang and L. Ding, Adv. Mater., 2018 DOI:10.1002/adma.201804790.
- (a) H. Li, Z. Xiao, L. Ding and J. Wang, Sci. Bull., 2018, 63, 340 CrossRef CAS; (b) L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094 CrossRef CAS PubMed.
- H. Fu, Z. Wang and Y. Sun, Angew. Chem., Int. Ed., 2018 DOI:10.1002/anie.201806291.
- B. P. Rand, D. P. Burk and S. R. Forrest, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 115327 CrossRef.
- (a) Y. Qin, M. A. Uddin, Y. Chen, B. Jang, K. Zhao, Z. Zheng, R. Yu, T. J. Shin, H. Y. Woo and J. Hou, Adv. Mater., 2016, 28, 9416 CrossRef CAS PubMed; (b) S. Li, L. Ye, W. Zhao, H. Yan, B. Yang, D. Liu, W. Li, H. Ade and J. Hou, J. Am. Chem. Soc., 2018, 140, 7159 CrossRef CAS PubMed.
- (a) Y. Lin, F. Zhao, Q. He, L. Huo, Y. Wu, T. C. Parker, W. Ma, Y. Sun, C. Wang, D. Zhu, A. J. Heeger, S. R. Marder and X. Zhan, J. Am. Chem. Soc., 2016, 138, 4955 CrossRef CAS PubMed; (b) W. Zhao, D. Qian, S. Zhang, S. Li, O. Inganas, F. Gao and J. Hou, Adv. Mater., 2016, 28, 4734 CrossRef CAS PubMed.
- (a) Y. Lin, F. Zhao, S. K. K. Prasad, J. Chen, W. Cai, Q. Zhang, K. Chen, Y. Wu, W. Ma, F. Gao, J. Tang, C. Wang, W. You, J. M. Hodgkiss and X. Zhan, Adv. Mater., 2018, 30, 1706363 CrossRef PubMed; (b) Y. Yang, Z. Zhang, H. Bin, S. Chen, L. Gao, L. Xue, C. Yang and Y. Li, J. Am. Chem. Soc., 2016, 138, 15011 CrossRef CAS PubMed.
- (a) J. Cao, Q. Liao, X. Du, J. Chen, Z. Xiao, Q. Zuo and L. Ding, Energy Environ. Sci., 2013, 6, 3224 RSC; (b) J. Cao, L. Qian, F. Lu, J. Zhang, Y. Feng, X. Qiu, H. L. Yip and L. Ding, Chem. Commun., 2015, 51, 11830 RSC; (c) A. M. Schneider, L. Lu, E. F. Manley, T. Zheng, V. Sharapov, T. Xu, T. J. Marks, L. X. Chen and L. Yu, Chem. Sci., 2015, 6, 4860 RSC; (d) W. Gao, T. Liu, M. Hao, K. Wu, C. Zhang, Y. Sun and C. Yang, Chem. Sci., 2016, 7, 6167 RSC.
- D. Li, Z. Xiao, S. Wang, X. Geng, S. Yang, J. Fang, H. Yang and L. Ding, Adv. Energy Mater., 2018, 8, 1800397 CrossRef.
- M. An, F. Xie, X. Geng, J. Zhang, J. Jiang, Z. Lei, D. He, Z. Xiao and L. Ding, Adv. Energy Mater., 2017, 7, 1602509 CrossRef.
- Y. Lin, J. Wang, Z. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423 CrossRef CAS PubMed.
- Z. Xiao, G. Ye, Y. Liu, S. Chen, Q. Peng, Q. Zuo and L. Ding, Angew. Chem., Int. Ed., 2012, 51, 9038 CrossRef CAS PubMed.
- Z. Xiao, X. Geng, D. He, X. Jia and L. Ding, Energy Environ. Sci., 2016, 9, 2114 RSC.
- H. Li, J. Cao, Q. Zhou, L. Ding and J. Wang, Nano Energy, 2015, 15, 125 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Material preparation and characterization, and solar cell fabrication and measurements. See DOI: 10.1039/c8qm00604k |
| ‡ Y. Gao and D. Li contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |