Pyrene-fused PDI based ternary solar cells: high power conversion efficiency over 10%, and improved device thermal stability†
Yanbin
Gong‡
a,
Kai
Chang‡
a,
Cheng
Chen
a,
Mengmeng
Han
a,
Xuejun
Zhan
a,
Jie
Min
 *b,
Xuechen
Jiao
cd,
Qianqian
Li
*b,
Xuechen
Jiao
cd,
Qianqian
Li
 a and
Zhen
Li
a and
Zhen
Li
 *ae
*ae
aDepartment of Chemistry, Hubei Key Lab on Organic and Polymeric Opto Electronic Materials, Wuhan University Wuhan, 430072, China. E-mail: lizhen@whu.edu.cn; lichemlab@163.com; Fax: +86-27-68755767
bThe Institute for Advanced Studies, Wuhan University, Wuhan 430072, China. E-mail: min.jie@whu.edu.cn
cDepartment of Materials Science and Engineering, Monash University, Victoria, Australia
dAustralian Synchrotron, ANSTO, Clayton, Victoria, 3168, Australia
eInstitute of Molecular Aggregation Science, Tianjin University, Tianjin 300072, China
First published on 29th October 2018
Abstract
Although great advances have been achieved in the field of organic solar cells (OSCs) with the rapid development of nonfullerene acceptors (NFAs), devices with simultaneous high power conversion efficiency (PCE) and thermal stability remain a big challenge, particular small molecule NFA-based devices. In this paper, we designed and synthesized a class of pyrene-fused perylene diimides (PDIs) for fabricating ternary OSCs, which exhibited much better device performance than those of the parent PDI counterpart based devices due to the better miscibility and crystallization of the ternary films. Excitingly, Th-PYPDI and O-PYPDI based ternary OSCs showed improved thermal stability, as a result of the better morphology stability under the same annealing conditions. Notably, the O-PYPDI based ternary device exhibited the best PCE of 10.96% and good thermal stability with the PCE remaining at 82% of the initial value after thermal annealing at 80 °C for 72 h.
Introduction
Impressive progress has been made in the field of solution-processed bulk heterojunction (BHJ) organic solar cells (OSCs) with the highest power conversion efficiency (PCE) of 17.3%, revealing their potential prospects of promising and low-cost renewable energy.1 The prerequisite to achieve high PCEs and stable device performance is to screen well matched donors (D) and acceptors (A) of the active layer, which is the most important part of cell devices.2 In earlier studies, numerous polymer donors were designed and synthesized to blend together with fullerene acceptors (FAs) for the fabrication of OSC devices.3 However, some drawbacks hampered the commercial process seriously, such as high cost, weak absorption in the visible region, limited energy-level tunability and so on.4 Fortunately, great efforts have been made toward the development of nonfullerene acceptors (NFAs), leading to higher open-circuit voltage (VOC), larger photocurrent and correspondingly much higher PCEs than those of FA-OSCs, thanks to their advantages of tunable energy level and better absorption.5 Nowadays, research on small molecular NFAs has stimulated a new development of this field to some degree.1a–c To construct highly efficient NFAs, perylene diimide (PDI) is one of the most investigated electron-withdrawing building units due to its appropriate energy level, strong absorption, high mobility and excellent photochemical and thermal stability.6 Yan's group reported a PDI-NFA with upshifting lowest unoccupied molecular orbital (LUMO) level, and a high VOC exceeding 1.1 V was realized and the highest PCE reached 10.58%.7 In our previous work, we reported two pyrene-fused PDI-NFAs, which exhibited moderate PCEs but an ultrahigh VOC of 1.26 V.8 This inspired us to develop more pyrene-fused PDI-NFAs with high VOC.Generally, the active layer can be classified into binary and ternary blends. Binary blends are made of D1 and A1, while D2 or A2 blends together with a binary system to form a ternary blend. Relatively, ternary OSCs can improve the performance of OSCs owing to their extended light absorption range and better photovoltaic properties rather than their binary counterparts.9 Accordingly, numerous ternary devices have been fabricated to explore material combinations with high performance. However, the literature reports concerning PDI-NFA based ternary OSCs are very scarce regardless of the possible significance.10 Recently, ternary OSCs exhibited that a second acceptor with higher VOC could be mixed into a host binary blend solution to improve VOC gradually with the increasing content of the second acceptor.1a,10c,11 Thus, it should be very meaningful to fabricate ternary OSCs by utilizing our pyrene-fused PDI-NFAs, possibly resulting in the improvement of the device VOC.
According to the above considerations, in this paper, three pyrene-fused PDI-NFAs were rationally designed, while their PDI counterparts were also synthesized for comparison (Scheme S1 in the ESI†). These six NFAs were intermixed together with a mature host binary system of J71 and ITIC, to investigate their ternary OSC performance. As expected, the VOC of the ternary OSCs increases gradually along with the increasing content of pyrene-fused PDI-NFAs, as a result of the varied organic alloy states. Notably, pyrene-fused PDI-NFA based devices exhibit much better performance than their PDI counterparts with the same incorporation content, due to the overall enhanced VOC, short circuit current density (JSC) and fill factor (FF). The J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O-PYPDI (1
O-PYPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) device exhibits the best performance with a PCE of 10.96%, a VOC of 0.945 V, a JSC of 16.59 mA cm2 and an FF of 69.92%, which is comparable to that of the host binary device. Meanwhile, it should be highlighted that 10% content of O-PYPDI based ternary devices shows improved (about 10%) thermal stability compared to that of the host binary device, thanks to the higher morphology stability under the same annealing conditions. Thus, the obtained experimental results demonstrate that pyrene-fused PDI-NFAs are good candidates for fabricating ternary OSC devices, due to the complementary absorption in the short wavelength region, high VOC and the ability to improve the thermal stability of ternary devices.
0.1) device exhibits the best performance with a PCE of 10.96%, a VOC of 0.945 V, a JSC of 16.59 mA cm2 and an FF of 69.92%, which is comparable to that of the host binary device. Meanwhile, it should be highlighted that 10% content of O-PYPDI based ternary devices shows improved (about 10%) thermal stability compared to that of the host binary device, thanks to the higher morphology stability under the same annealing conditions. Thus, the obtained experimental results demonstrate that pyrene-fused PDI-NFAs are good candidates for fabricating ternary OSC devices, due to the complementary absorption in the short wavelength region, high VOC and the ability to improve the thermal stability of ternary devices.
Results and discussion
Synthesis and characterization
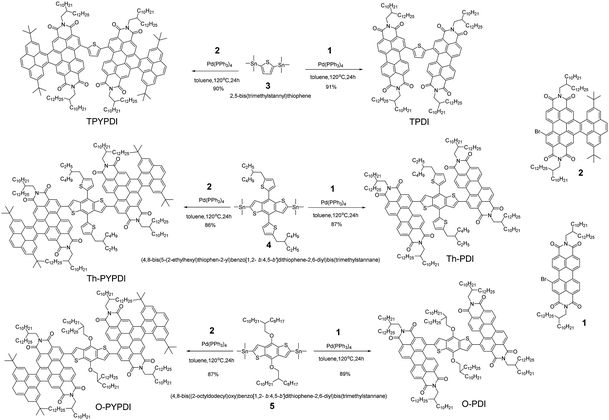
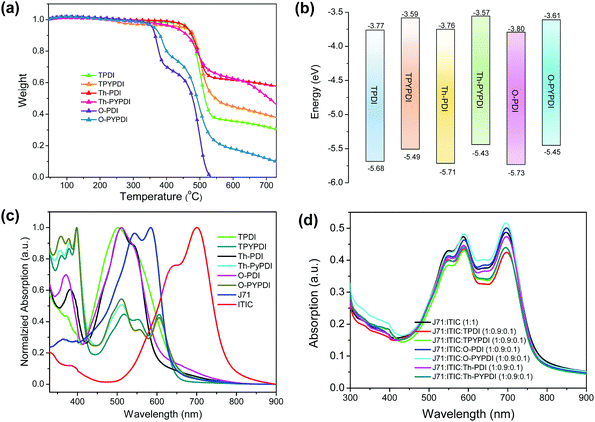
The chemical structures of all pyrene-fused PDI-NFAs are depicted in Scheme 1. The target six NFAs, TPDI, Th-PDI, O-PDI, TPYPDI, Th-PYPDI and O-PYPDI were synthesized via a Stille coupling reaction with high yields (Scheme 1). The important intermediates of 1 and 2 were prepared according to the previous work of our group and three bis(trimethylstannyl) reagents were obtained from a commercial route.12 All of the six NFAs were characterized by 1H and 13C NMR, and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) analyses. Thanks to the long alkyl side chains, these six NFAs show very good solubility in common organic solvents such as chloroform, dichloromethane (DCM) and toluene, ensuring the easy fabrication of the solution-processed OSC devices. Their thermal stability was investigated by using thermogravimetric analysis (TGA) (Fig. 1a). TPDI, TPYPDI, Th-PDI and Th-PYPDI show excellent thermal stability with decomposition temperatures (Td, 5% weight loss) of 463.1 °C, 450.7 °C, 460.9 °C and 423.2 °C, respectively. O-PDI and O-PYPDI were also thermally stable with relatively low Td values of 356.6 °C and 362.4 °C, respectively.Cyclic voltammetry (CV) measurements were performed to investigate the energy levels of the synthesized NFAs. As shown in Fig. S2 (ESI†), O-PYPDI displayed two reversible reduction waves, while only one for the other five NFAs. The LUMO energies of the six NFAs estimated from their onset reduction potentials were approximately −3.57 eV to −3.80 eV (Fig. 1b). Combined with the optical bandgaps, their HOMO energy levels were calculated to be in the range of −5.43 to −5.46 eV. Notably, in comparison with three parent PDIs, three pyrene-fused PDIs show significantly upshifted LUMO and highest occupied molecular orbital (HOMO) levels, due to the electron-donating effect of the pyrene unit. According to the literature reports, a higher LUMO energy level could result in a much larger offset between the HOMO of the donor polymer (J71, −5.40 eV) and the LUMO of the acceptor, and thus, a high VOC could be anticipated.7,8,10b
The UV-vis absorption spectra of the six NFAs in chloroform solution and as films were measured (Fig. S1 in the ESI† and Fig. 1c). The absorptions in films show broader and redshifted absorption, in comparison with their solutions. In films, the three PDI-NFAs show similar absorption located at 400–600 nm. After ring-fusion with pyrene, the absorption edges redshifted relative to their PDI counterparts, due to the enlarged molecular conjugation. Interestingly, the most intense absorption band of the three pyrene-fused PDI NFAs located at 300–400 nm, while the relatively weak absorption band was from 450 to 650 nm, in which, the absorption peak at approximately 605 nm should be attributed to the intramolecular charge transfer caused by the ring-fusion with pyrene. As shown in Fig. 2b, the absorption spectrum of J71-ITIC film covers a broad absorption range from 300 nm to 900 nm, and there is an apparent weak absorption band from 300 to 450 nm. Thus, it is really very good that this weak absorption band could be enhanced by the incorporation of three pyrene-fused PDI-NFAs (Fig. 1d). The optical bandgaps of these six NFAs were calculated to be in the range of 1.84–1.95 eV from the absorption edge. Compared to PDI NFAs, pyrene-fused PDI NFAs have a slightly narrow bandgap induced by larger π-conjugation, possibly leading to the decreased energy loss. The decomposition temperatures and basic photophysical properties of the six NFAs are summarized in Table 1.
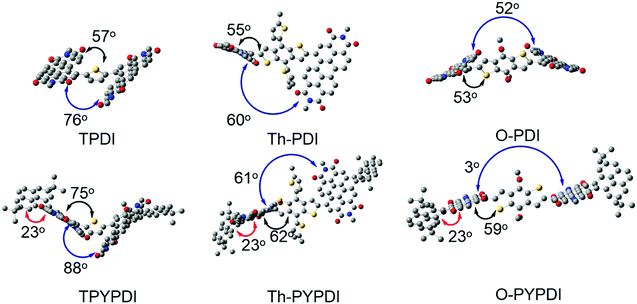 | ||
| Fig. 2 The optimal geometries of TPDI, Th-PDI, O-PDI, TPYPDI, Th-PYPDI and O-PYPDI calculated by the Gaussian09 program at the B3LYP/6-31G level. Yellow S, gray C, red O, blue N. | ||
| Compound | T d (°C) | λ max,onset (nm) | ε max (M−1 cm−1) | E g (eV) | E LUMO (eV) | E HOMO (eV) | λ max,abs | |
|---|---|---|---|---|---|---|---|---|
| Solv.g (nm) | Filmh (nm) | |||||||
| a Five percent weight loss temperature measured by TGA under N2. b Observed from the absorption spectra in the films. c Molar extinction coefficient at λmax in solution. d Bandgap estimated from the optical absorption band edges of the films. e Calculated from the onset reduction potentials of the compounds. f Estimated using empirical equations EHOMO = ELUMO − Eg. g Absorption maximum in chloroform solution. h Absorption maximum in films. | ||||||||
| TPDI | 463.1 | 649 | 0.66 × 105 | 1.91 | −3.77 | −5.68 | 528 | 502 |
| TPYPDI | 450.7 | 653 | 1.20 × 105 | 1.90 | −3.59 | −5.49 | 397 | 399 |
| Th-PDI | 460.9 | 636 | 0.70 × 105 | 1.95 | −3.76 | −5.71 | 528 | 511 |
| Th-PYPDI | 423.2 | 667 | 1.34 × 105 | 1.86 | −3.57 | −5.43 | 397 | 399 |
| O-PDI | 356.6 | 642 | 0.71 × 105 | 1.93 | −3.80 | −5.73 | 528 | 511 |
| O-PYPDI | 362.4 | 674 | 1.26 × 105 | 1.84 | −3.61 | −5.45 | 397 | 398 |
Theoretical calculations
The optimized geometries of each parent PDI and pyrene-fused PDI were calculated by density functional theory (DFT) using Gaussian at the B3LYP/6-31G level (Fig. 2). The dihedral angles between the central donor and PDI are 57°, 55°, and 52° for TPDI, Th-PDI and O-PDI, respectively. Meanwhile, the dihedral angles between two PDI subunits decrease from 76°, to 60° to 53° for TPDI, Th-PDI and O-PDI, respectively. These observations suggest that the overall planarity of the molecular geometry was gradually increased with decreasing the steric hindrance of the central donor. Similarly, as the steric hindrance of the central donor decreases, the planarity of the pyrene-fused PDIs increases gradually. Additionally, the twist angles between pyrene and adjacent PDI are 23° for the three pyrene-fused PDIs. Notably, the overall geometry of O-PYPDI exhibits highly planarity with an almost parallel arrangement (3°) between two PDI subunits. Furthermore, the electron distribution of frontier orbitals was also affected by the pyrene fusing through adjusting LUMO and HOMO delocalization over the whole π-system (Fig. S3, ESI†). The LUMO levels of TPDI, Th-PDI, O-PDI, TPYPDI, Th-PYPDI and O-PYPDI were calculated to be −3.57, −3.50, −3.54, −3.23, −3.15 and −3.19 eV, respectively. And the calculated LUMO levels of pyrene-fused PDIs are higher than those of PDI counterparts, in agreement with the experimental results.Photovoltaic properties
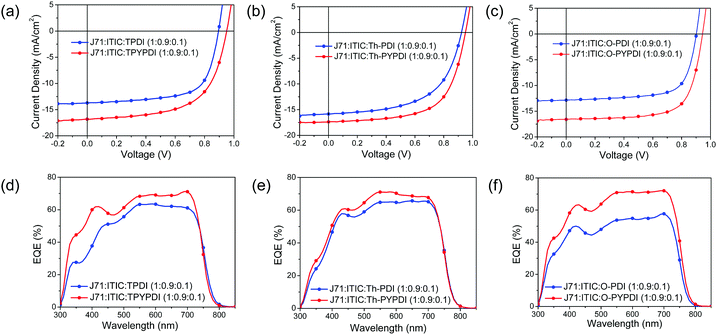
J71:ITIC was chosen as the host binary system and blended with the synthesized NFAs together to explore the ternary OSC performance. On one hand, ITIC is a famous NFA and industrially relevant developed by Zhan's group, which has been fully investigated to fabricate OSC devices with many polymer donors with PCEs surpassing 10%.13 On the other hand, J71 is an efficient polymer donor and commercially available.14 The binary OSCs based on J71:ITIC exhibit PCEs surpassing 11% with simultaneously high VOC and high short circuit current density (JSC). In addition, the solvent additive is not required to achieve the best device performance in the J71-ITIC system, which emphasizes the impact of ternary film morphology on other factors rather than solvent additive.As shown in Fig. S4a (ESI†), all the OSC devices in this study were fabricated with a forward configuration of ITO/PEDOT:PSS/Active Layer/PDINO/Al and all the device parameters from ten devices are listed in Table S1 (ESI†). For ITIC:J71 based binary OSCs, a PCE of 11.08% was achieved with a VOC of 0.939 V, a JSC of 17.55 mA cm−2, and a FF of 67.28%, which is comparable to that reported in the literature (≈11.4%).14 However, J71:PDI-NFAs based binary OSCs showed poor PCEs (0.36–0.47%) with lower VOC (0.652–0.833 V), JSC and fill factor (FF). For J71:pyrene-fused PDI-NFAs based binary OSCs, the PCEs (0.37–1.47%) were slightly higher than that of J71:PDI-NFAs based binary OSCs, mainly due to the improved VOC (1.013–1.047 V) derived from the upshifting LUMO energy level of pyrene-fused PDI-NFAs. As expected, the pyrene-fused PDI exhibited significantly improved VOC than that of the parent PDI counterparts. However, the weak JSC (1.26–4.22 mA cm−2) led to the limited PCEs of J71:pyrene-fused PDI-NFA binary devices severely, partially caused by the blue shift of the main absorption band (300–400 nm). Based on the special properties of pyrene-fused PDI-NFAs, it can be used as a second acceptor to fabricate ternary OSCs and improve the VOC as well as the absorption in the short wavelength region of ternary devices.
Ternary active layers of J71:ITIC:NFAs with various NFA contents were blended together, where the NFAs were three target parent PDIs and three pyrene-fused PDIs. The overall ratio of donor to acceptor was kept at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in this study. According to the device performance of J71:ITIC:NFAs with different NFA content (Table S1 in the ESI†), 10% was chosen as the optimal content to explore the influence of the addition of second NFAs on the absorption, film morphology, device performance and thermal stability of J71:ITIC:NFA ternary devices. Current density–voltage (J–V) characteristics of the best-performing devices are shown in Fig. 3a–c, while the corresponding external quantum efficiency (EQE) spectra are demonstrated in Fig. 3d–f, with the best-performing device parameters summarized in Table 2. The J71
1 in this study. According to the device performance of J71:ITIC:NFAs with different NFA content (Table S1 in the ESI†), 10% was chosen as the optimal content to explore the influence of the addition of second NFAs on the absorption, film morphology, device performance and thermal stability of J71:ITIC:NFA ternary devices. Current density–voltage (J–V) characteristics of the best-performing devices are shown in Fig. 3a–c, while the corresponding external quantum efficiency (EQE) spectra are demonstrated in Fig. 3d–f, with the best-performing device parameters summarized in Table 2. The J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O-PYPDI (1
O-PYPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) device exhibits the best performance with a PCE of 10.96%, a VOC of 0.945 V, a JSC of 16.59 mA cm2 and an FF of 69.92%, which is comparable to that of the host binary device. Notably, the addition of PDI-NFAs yielded declined PCEs (6.46–8.91%), while the addition of pyrene-fused PDI-NFAs improved the PCEs (9.80–10.96%) with overall enhanced VOC, JSC and FF.
0.1) device exhibits the best performance with a PCE of 10.96%, a VOC of 0.945 V, a JSC of 16.59 mA cm2 and an FF of 69.92%, which is comparable to that of the host binary device. Notably, the addition of PDI-NFAs yielded declined PCEs (6.46–8.91%), while the addition of pyrene-fused PDI-NFAs improved the PCEs (9.80–10.96%) with overall enhanced VOC, JSC and FF.
| Devices | V OC [V] | J SC [mA cm−2] | FF [%] | PCEa [%] | |
|---|---|---|---|---|---|
| Best | Average | ||||
| a The values are calculated from the statistical values from ten devices. | |||||
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPDI (1 TPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.913 | 13.55 | 62.03 | 7.67 | 7.34 |
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPyPDI (1 TPyPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.951 | 15.54 | 66.27 | 9.8 | 9.52 |
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th-PDI (1 Th-PDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.93 | 15.23 | 62.82 | 8.91 | 8.71 |
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th-PyPDI (1 Th-PyPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.955 | 16.66 | 65.34 | 10.63 | 10.42 |
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O-PDI (1 O-PDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.916 | 11.96 | 59.46 | 6.46 | 6.46 |
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O-PyPDI (1 O-PyPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) 0.1) |
0.945 | 16.59 | 69.92 | 10.96 | 10.71 |
J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC (1 ITIC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.939 | 17.55 | 67.28 | 11.08 | 10.83 |
It is a clear trend that the VOC of ternary OSCs increases gradually along with the content of pyrene-fused PDI-NFAs. On one hand, pyrene-fused PDI-NFAs have higher LUMO energy levels than that of ITIC, which gives a larger offset energy referencing to the HOMO of the donor material. On the other hand, ITIC and pyrene-fused PDI-NFAs could form close contact mixtures or new electronic alloys, leading to tunable VOCs in ternary OSCs caused by the charge transfer state.
Compared to PDI-NFAs, the increased JSCs of pyrene-fused PDI-NFA based devices are consistent with comparable changes in the corresponding EQE spectra, which should be partially originated from the enhanced absorption of pyrene-fused PDI-NFAs and improved charge separation or collection. As shown in Table 2, the JSC of the J71:ITIC binary device is 17.55 mA cm−2, meanwhile the JSC is 15.54, 16.66 and 16.59 mA cm−2 for J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TPYPDI (1
TPYPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), J71
0.1), J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Th-PYPDI (1
Th-PYPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), and J71
0.1), and J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O-PYPDI (1
O-PYPDI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), respectively. Thus, the 10% content incorporation of pyrene-fused PDI-NFAs resulted in a small decrease, which should be derived from two aspects. On one hand, pyrene is a good hole transport molecule, and thus the ring-fusion with pyrene would decrease the electron transport mobility of pyrene-fused PDI derivatives, resulting in the low JSC. In our previous and this work, we reported five pyrene-fused PDI-NFAs, all of which exhibited low JSC (<6.83 mA cm−2) in the binary devices. On the other hand, by replacing 10% content of ITIC with pyrene-fused PDI-NFAs, the absorption at 300–400 nm increased with the sacrifice of absorption at 650–800 nm, leading to a possible small decrease of the overall absorption in the visible region. Taking these two aspects together, the JSC of J71
0.1), respectively. Thus, the 10% content incorporation of pyrene-fused PDI-NFAs resulted in a small decrease, which should be derived from two aspects. On one hand, pyrene is a good hole transport molecule, and thus the ring-fusion with pyrene would decrease the electron transport mobility of pyrene-fused PDI derivatives, resulting in the low JSC. In our previous and this work, we reported five pyrene-fused PDI-NFAs, all of which exhibited low JSC (<6.83 mA cm−2) in the binary devices. On the other hand, by replacing 10% content of ITIC with pyrene-fused PDI-NFAs, the absorption at 300–400 nm increased with the sacrifice of absorption at 650–800 nm, leading to a possible small decrease of the overall absorption in the visible region. Taking these two aspects together, the JSC of J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrene-fused PDI-NFA (1
pyrene-fused PDI-NFA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) devices exhibited a small decrease.
0.1) devices exhibited a small decrease.
In comparison with PDI-NFAs, the enhanced FF of pyrene-fused PDI-NFA based OSC devices may be connected with the charge mobility and film morphology, which will be discussed in the following sections.
Hole and electron mobilities of J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NFAs (1
NFAs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) ternary devices were measured using the space charge limited current (SCLC) method. The structure of the hole only devices was Glass/ITO/PEDOT:PSS/Active Layer/MoOx/Ag. As for electron only devices, the structure was Glass/ITO/ZnO/Active Layer/Ca/Al. The hole mobilities range from 0.92 × 10−3 (10 wt% TPDI) to 2.84 × 10−3 cm2 V−1 s−1 (10 wt% O-PYPDI), while the electron mobilities are in the range of 1.02 × 10−3–2.39 × 10−3 cm2 V−1 s−1 (10 wt% O-PYPDI), with the μh, μe and μh/μe values presented in Table S2 (ESI†). Notably, the μh/μe values in these pyrene-fused PDI-NFA based devices were more balanced, in comparison with those of their corresponding parent PDI-NFA based devices (from 0.49 to 1.27, 1.68 to 0.80, and 1.60 to 1.19). The more balanced μh/μe contributes to the higher FF of pyrene-fused PDI-NFA based devices, and the highest FF value (69.92%) of the O-PYPDI based device results from the simultaneously higher mobility and more balanced μh/μe.
0.1) ternary devices were measured using the space charge limited current (SCLC) method. The structure of the hole only devices was Glass/ITO/PEDOT:PSS/Active Layer/MoOx/Ag. As for electron only devices, the structure was Glass/ITO/ZnO/Active Layer/Ca/Al. The hole mobilities range from 0.92 × 10−3 (10 wt% TPDI) to 2.84 × 10−3 cm2 V−1 s−1 (10 wt% O-PYPDI), while the electron mobilities are in the range of 1.02 × 10−3–2.39 × 10−3 cm2 V−1 s−1 (10 wt% O-PYPDI), with the μh, μe and μh/μe values presented in Table S2 (ESI†). Notably, the μh/μe values in these pyrene-fused PDI-NFA based devices were more balanced, in comparison with those of their corresponding parent PDI-NFA based devices (from 0.49 to 1.27, 1.68 to 0.80, and 1.60 to 1.19). The more balanced μh/μe contributes to the higher FF of pyrene-fused PDI-NFA based devices, and the highest FF value (69.92%) of the O-PYPDI based device results from the simultaneously higher mobility and more balanced μh/μe.
To investigate the charge recombination in the J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NFA (1
NFA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
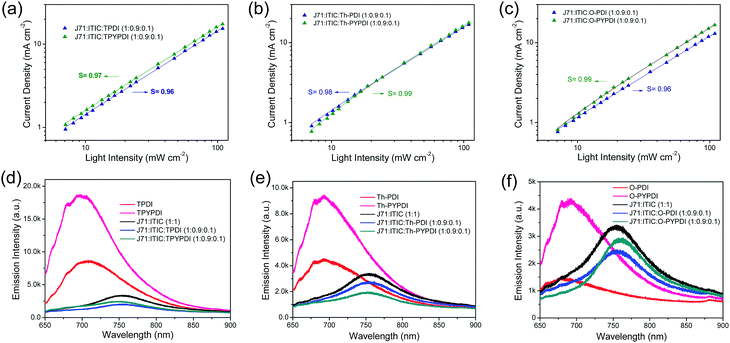
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) ternary devices, measurements of light intensity dependent JSC were conducted. JSC as a function of incident light intensity (P) is described in Fig. 4a, b and c, respectively. The relationship between JSC and P can be expressed as JSC ∝ PS. The bimolecular recombination can be neglected in ternary films, if the S value is close to 1. The fitted S values are approximately 0.96, 0.97, 0.98, 0.99, 0.96 and 0.99 for the TPDI, TPYPDI, Th-PDI, Th-PYPDI, O-PDI and O-PYPDI based ternary active layers, respectively, suggesting that bimolecular recombination was hardly observed in ternary devices with the incorporation of 10% NFAs. Considering that the fitted S values of the pyrene-fused PDI-NFA based devices were slightly closer to 1 rather than those of the PDI counterpart based devices, the bimolecular recombination can be slightly suppressed in the devices of J71
0.1) ternary devices, measurements of light intensity dependent JSC were conducted. JSC as a function of incident light intensity (P) is described in Fig. 4a, b and c, respectively. The relationship between JSC and P can be expressed as JSC ∝ PS. The bimolecular recombination can be neglected in ternary films, if the S value is close to 1. The fitted S values are approximately 0.96, 0.97, 0.98, 0.99, 0.96 and 0.99 for the TPDI, TPYPDI, Th-PDI, Th-PYPDI, O-PDI and O-PYPDI based ternary active layers, respectively, suggesting that bimolecular recombination was hardly observed in ternary devices with the incorporation of 10% NFAs. Considering that the fitted S values of the pyrene-fused PDI-NFA based devices were slightly closer to 1 rather than those of the PDI counterpart based devices, the bimolecular recombination can be slightly suppressed in the devices of J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pyrene-fused PDI-NFAs (1
pyrene-fused PDI-NFAs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) compared to J71
0.1) compared to J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI-NFAs (1
PDI-NFAs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1).
0.1).
To confirm the energy transfer in the donor–acceptor interface, the photoluminescence (PL) quenching measurements were carried out. As shown in Fig. S6 (ESI†), the neat films of both J71 and ITIC exhibit broad emission bands, with the peaks at 695 and 765 nm, respectively. The emission peak in the J71:ITIC binary film red shifted to 750 nm with a PL quenching efficiency of 0.82 as compared to the respective neat J71 film, indicating conspicuous energy transfer in the J71-ITIC interface. The PL spectra of neat films of each pure NFA and the corresponding ternary films are shown in Fig. 4d–f, and the pyrene-fused PDI-NFA neat films exhibit much stronger PL intensity compared to those of PDI counterpart neat films. Notably, with the incorporation of 10% content of NFAs in ternary films, the PL quenching efficiencies were improved to 0.89, 0.87, 0.85, 0.90, 0.87 and 0.85 for TPDI, TPYPDI, Th-PDI, Th-PYPDI, O-PDI and O-PYPDI, respectively, indicating the better energy transfer in the 10% content NFA based ternary films relative to the J71:ITIC binary film.
Film morphology
Atomic force microscopy (AFM) was used to study the morphology of the host binary and corresponding 10% content of NFA based ternary blend films. As shown in Fig. S7 (ESI†), the root-mean-square (RMS) roughness of the J71:ITIC binary film is 0.59 nm, indicating a smooth and uniform surface. When 10% content of ITIC was changed to pyrene-fused PDI-NFAs, the RMS roughnesses declined to 0.55, 0.57 and 0.57 nm for TPYPDI, Th-PYPDI and O-PYPDI, respectively. In contrast, by replacing 10% content of ITIC with PDI-NFAs, the RMS roughnesses increased to 0.70, 0.62 and 0.76 nm for TPDI, Th-PDI and O-PDI, respectively. Notably, large domains can be easily observed in J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PDI-NFAs (1
PDI-NFAs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) ternary blend films, since PDI derivatives are known to aggregate severely.7 The results revealed that the addition of 10% content of pyrene-fused PDI-NFAs is in favor of better miscibility of the ternary blend, whereas PDI NFAs tend to deteriorate the film morphology more or less.
0.1) ternary blend films, since PDI derivatives are known to aggregate severely.7 The results revealed that the addition of 10% content of pyrene-fused PDI-NFAs is in favor of better miscibility of the ternary blend, whereas PDI NFAs tend to deteriorate the film morphology more or less.
It is an accepted notion that materials with a similar surface energy lead to close contact.10c,15 The smaller the difference in surface energy is, the better the miscibility. Thus, to gain deeper insight into the miscibility of the ternary films, the contact angle and surface energy of the used materials were investigated. First, the contact angle measurements on thin films of each pure NFA were performed using two different liquids (water, ethylene glycol (EG)), to obtain two pairs of contact angles for each NFA. The surface energy of each pure NFA was calculated directly by instruments using the model of Wu.16 As shown in Fig. 5b, the surface energy of J71 (22.0 mN m−1) is similar to that of ITIC (24.1 mN m−1), and the small surface energy difference (2.1 mN m−1) between J71 and ITIC reveals their close contact and good miscibility in films, which corresponds well to the AFM result of the binary film. Compared to the binary film of J71-ITIC, it should be highlighted that the surface energy difference between pyrene-fused PDI-NFAs and J71 is smaller (0.4, 1.6 and 1.2 mN m−1 for TPYPDI, Th-PYPDI and O-PYPDI, respectively). Thus, in comparison with the binary film, better miscibility can be obtained in the pyrene-fused NFA based ternary films. In contrast, PDI-NFAs exhibit larger surface energy difference with ITIC and J71, compared to that of pyrene-fused PDI counterparts, indicating the worse miscibility in PDI-NFA based ternary films. The results also explain the AFM profiles aforementioned well.
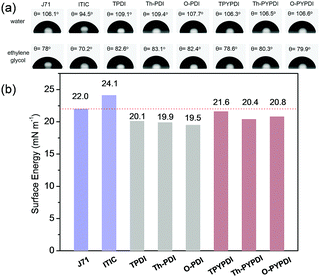 | ||
| Fig. 5 (a) Contact angle images and contact angle value of the pure materials used in this work. (b) Surface energy of the pure materials used in this work. | ||
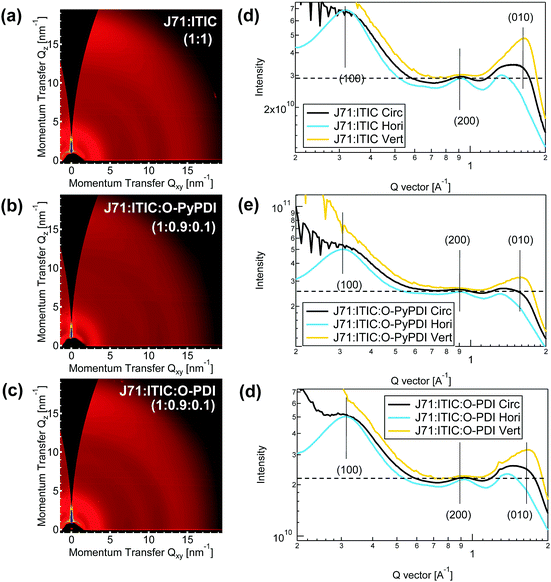
Grazing incidence wide angle X-ray scattering (GIWAXS) measurements were employed to characterize the molecular packing and crystallinity of the neat and blended films. As shown in Fig. S10 (ESI†), in the horizontal (in-plane) direction, the neat films of O-PDI show strong lamellar molecular packing with visible (200) and (300) peaks and a sharp (400) peak, indicating a dominant face-on orientation for better electron transport. In the similar diffraction vector (q) value, the slightly lower peaks were observed in the neat film of Th-PDI. In contrast, the (300) and (400) peaks almost disappeared in the neat film of TPDI. Such a trend of decreased in-plane lamellar packing in the neat film of PDI-NFAs should result from the planarity of the whole molecules. The same trend also exists in the neat film of pyrene-fused PDI-NFAs. Additionally, compared to the in-plane lamellar and vertical (out-of-plane) π–π stacking intensity of PDI-NFAs in neat films, pyrene-fused PDI-NFAs show much lower intensity, caused by the twist angle between pyrene and the adjacent PDI unit along with the reduced planarity of the whole molecules. Both neat films of J71 and ITIC exhibit face-on crystallites, as evidenced by the simultaneous appearance of in-plane lamellar stacking and out-of-plane π–π stacking peaks. Similar GIWAXS profiles of the neat J71 film and the binary J71:ITIC film indicate that the molecular arrangement and crystallinity of J71 can be kept well in the blend film (Fig. 6a).
In J71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ITIC
ITIC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NFA (1
NFA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9
0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) ternary films, each set of parent PDIs and pyrene-fused PDIs show similar trends, that is, relative to the in-plane lamellar packing intensity of J71:ITIC, J71:ITIC:pyrene-fused PDI-NFAs show slight diminution, while J71:ITIC:PDI-NFAs exhibit distinct diminution (Fig. 6e and f). Taking O-PDI and O-PYPDI as examples, J71:ITIC:O-PYPDI have slightly lower (200) peak compared to J71:ITIC, suggesting that the addition of O-PYPDI suppresses the crystallinity of J71:ITIC as well as weakens charge transport. More obvious diminution of the (200) peak at a similar q value was observed in the J71:ITIC:O-PDI film, revealing its weaker crystallinity than that of J71:ITIC:O-PYPDI. The deteriorating molecular arrangement and crystallinity of J71:ITIC:O-PYPDI and J71:ITIC:O-PDI films can be further confirmed by their declined JSC in ternary OSCs.
0.1) ternary films, each set of parent PDIs and pyrene-fused PDIs show similar trends, that is, relative to the in-plane lamellar packing intensity of J71:ITIC, J71:ITIC:pyrene-fused PDI-NFAs show slight diminution, while J71:ITIC:PDI-NFAs exhibit distinct diminution (Fig. 6e and f). Taking O-PDI and O-PYPDI as examples, J71:ITIC:O-PYPDI have slightly lower (200) peak compared to J71:ITIC, suggesting that the addition of O-PYPDI suppresses the crystallinity of J71:ITIC as well as weakens charge transport. More obvious diminution of the (200) peak at a similar q value was observed in the J71:ITIC:O-PDI film, revealing its weaker crystallinity than that of J71:ITIC:O-PYPDI. The deteriorating molecular arrangement and crystallinity of J71:ITIC:O-PYPDI and J71:ITIC:O-PDI films can be further confirmed by their declined JSC in ternary OSCs.
Thermal stability
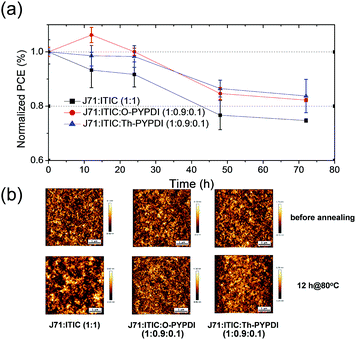
Generally, the stability of OSC devices includes the photochemical and thermal stability, which is significantly important for practical applications.17 Most studies have focused on the photochemical stability, while investigations on the thermal stability are rarely reported, particularly for those small molecule based OSC devices. Accordingly, thanks to the excellent thermal stability of six NFAs and different morphology of 10% content NFA based ternary films compared to the J71:ITIC binary film, we attempted to explore the thermal stability of ternary active layers with the incorporation of 10% content of NFAs. O-PYPDI and Th-PYPDI based ternary films and host binary films were chosen as the research objects for their good device performance. After spin-coating HTL (PEDOT:PSS) and active layers on the ITO-coated glass substrates in the same way, all substrates were put on a hot plate at 80 °C for different annealing times (12 hours, 24 hours, 48 hours and 72 hours). Then, the PCE test was performed after spin-coating the ETL (PDINO) and vacuum evaporating Al. As shown in Fig. 7a, after annealing for 72 h, the Th-PYPDI and O-PYPDI based devices retained 84% and 82% of their initial PCE values, respectively, while the PCE of the J71:ITIC binary device declined to 75%. The results suggested that both the incorporation of 10% content of Th-PYPDI and O-PYPDI improved the thermal stability of ternary films. In this work, due to the difficulty of measuring the thermal stability of the electron transport layer (PDINO), we investigated the thermal annealing process of the active layer under an inert gas atmosphere. On one hand, the effect of an electrode on the active layer could be excluded, because the electrode had not been heated at 80 °C for 12 h. On the other hand, the effect of oxygen and vapor could be excluded due to the inert gas atmosphere. Therefore, the morphology stability should be the reason for the improved thermal stability of O-PYPDI and Th-PYPDI based ternary films. Thus, to understand the enhanced thermal stability of O-PYPDI and Th-PYPDI based ternary films, AFM measurements of films upon annealing for 12 h at 80 °C were conducted. As shown in Fig. 7b, before annealing, all of the three films display regular and uniform profiles. After annealing, the J71:ITIC binary film exhibited distinct deterioration with a large domain size appearing, and the PCE declined to 93% of the initial value. However, no apparent deterioration was observed in the O-PYPDI and Th-PYPDI based ternary films under the same annealing conditions. The enhanced thermal stability of O-PYPDI and Th-PYPDI based ternary films may be derived from their better morphology stability, in comparison with the J71:ITIC binary film undergoing the same annealing process.Conclusions
A class of novel pyrene-fused PDI-NFAs were designed to fabricate ternary OSC devices blended with a host J71:ITIC system, which exhibited much better device performances than those of parent PDI counterparts with the same incorporation content. The superior device performance of pyrene-fused PDI-NFA based ternary OSCs should be ascribed to preferable photovoltaic properties, better miscibility and crystallization of the ternary films. The O-PYPDI based ternary device exhibited the best PCE of 10.96% and a good thermal stability with the PCE remaining at 82% of the initial value after thermal annealing at 80 °C for 72 h, demonstrating that pyrene-fused PDI-NFAs are good candidates for fabrication of ternary OSC devices to improve the absorption in the short wavelength region, VOC and thermal stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (no. 51573140, 21734007, 21702154, and 51773157), Hubei Province (2017CFA002), and Special funds for basic scientific research services in central colleges and Universities (2042017kf0247). This work was performed in part on the SAXS/WAXS beamline at the Australian Synchrotron, part of ANSTO.Notes and references
- (a) L. X. Meng, Y. M. Zhang, X. J. Wan, C. X. Li, X. Zhang, Y. B. Wang, X. Ke, Z. Xiao, L. M. Ding, R. X. Xia, H. L. Yip, Y. Cao and Y. S. Chen, Science, 2018, 361, 1094 CrossRef PubMed; (b) P. Cheng, G. Li, X. W. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131–142 CrossRef; (c) J. Q. Zhang, H. S. Tan, X. G. Guo, A. Facchetti and H. Yan, Nat. Energy, 2018, 3, 720–731 CrossRef; (d) Y. Z. Lin, Y. F. Li and X. W. Zhan, Chem. Soc. Rev., 2012, 41, 4245 RSC; (e) Y. Z. Lin and X. W. Zhan, Mater. Horiz., 2014, 1, 470 RSC; (f) A. Gupta, A. Rananaware, P. S. Rao, D. D. La, A. Bilic, W. C. Xiang, J. L. Li, R. A. Evans, S. V. Bhosalec and S. V. Bhosale, Mater. Chem. Front., 2017, 1, 1600 RSC; (g) A. Rananaware, A. Gupta, G. Kadam, D. D. La, A. Bilic, W. C. Xiang, R. A. Evansf and S. V. Bhosale, Mater. Chem. Front., 2017, 1, 2511 RSC; (h) F. Yang, C. Li, W. B. Lai, A. D. Zhang, H. Huang and W. W. Li, Mater. Chem. Front., 2017, 1, 1389 RSC; (i) J. C. Jia, N. N. Zheng, Z. F. Wang, Y. P. Huang, C. H. Duan, F. Huang and Y. Cao, Sci. China: Chem., 2017, 60, 1458 CrossRef; (j) H. T. Zhang, Y. T. Liu, Y. N. Sun, M. M. Li, W. Ni, Q. Zhang, X. J. Wan and Y. S. Chen, Sci. China: Chem., 2017, 60, 366 CrossRef; (k) J. Q. Xu, W. Q. Liu, S. Y. Liu, J. Ling, J. Q. Mai, X. H. Lu, C. Z. Li, A. K.-Y. Jen and H. Z. Chen, Sci. China: Chem., 2017, 60, 561 CrossRef; (l) J. H. Hou, O. Inganäs, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119 CrossRef PubMed.
- (a) C. Q. Yan, S. Barlow, Z. H. Wang, H. Yan, A. K.-Y. Jen, S. R. Marder and X. W. Zhan, Nat. Rev. Mater., 2018, 3, 18003 CrossRef; (b) Z. Y. Li, S. X. Dai, J. M. Xin, L. Zhang, Y. Wu, J. Rech, F. W. Zhao, T. F. Li, K. Liu, Q. Liu, W. Ma, W. You, C. R. Wang and X. W. Zhan, Mater. Chem. Front., 2018, 2, 537 RSC; (c) L. Feng, C. Wang, X. Y. Deng, X. C. Miao, J. Q. Wang, Y. Y. Wang and Z. Li, Mater. Chem. Front., 2018, 2, 264 RSC; (d) C. Zhang, T. Liu, W. X. Zeng, D. J. Xie, Z. H. Luo, Y. M. Sun and C. L. Yang, Mater. Chem. Front., 2017, 1, 749 RSC; (e) W. Y. Liu, Z. C. Zhou, T. Vergote, S. J. Xu and X. Z. Zhu, Mater. Chem. Front., 2017, 1, 2349 RSC; (f) X. J. Long, Z. C. Ding, C. D. Dou, J. Liu and L. X. Wang, Mater. Chem. Front., 2017, 1, 852 RSC; (g) L. Lv, X. F. Wang, T. Dong, X. L. Wang, X. X. Wu, L. Yang and H. Huang, Mater. Chem. Front., 2017, 1, 1317 RSC.
- (a) N. S. Saricifci, L. Saricifci, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474 CrossRef PubMed; (b) J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876 CrossRef PubMed; (c) Y. He and Y. Li, Phys. Chem. Chem. Phys., 2011, 13, 1970 RSC.
- (a) A. a. F. Eftaiha, J.-P. Sun, I. G. Hill and G. C. Welch, J. Mater. Chem. A, 2014, 2, 1201 RSC; (b) P. Sonar, J. P. Fong Lim and K. L. Chan, Energy Environ. Sci., 2011, 4, 1558 RSC.
- (a) G. Y. Zhang, J. B. Zhao, P. C. Y. Chow, K. Jiang, J. Q. Zhang, Z. L. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447 CrossRef PubMed; (b) W. Q. Chen and Q. C. Zhang, J. Mater. Chem. C, 2017, 5, 1275 RSC; (c) N. N. Liang, W. Jiang, J. H. Hou and Z. H. Wang, Mater. Chem. Front., 2017, 1, 1291 RSC; (d) S. X. Dai, S. M. Zhang, Q. D. Ling and X. W. Zhan, Chin. J. Polym. Sci., 2017, 35, 230 CrossRef; (e) A. Islama, Z. Y. Liu, R. X. Peng, W. G. Jiang, T. Lei, W. Li, L. Zhang, R. J. Yang, Q. Guan and Z. Y. Ge, Chin. J. Polym. Sci., 2017, 35, 171 CrossRef.
- (a) G. P. Gao, N. N. Liang, H. Geng, W. Jiang, H. T. Fu, J. J. Feng, J. H. Hou, X. L. Feng and Z. H. Wang, J. Am. Chem. Soc., 2017, 139, 15914 CrossRef PubMed; (b) Y. W. Duan, X. Q. Xu, H. Yan, W. L. Wu, Z. J. Li and Q. Peng, Adv. Mater., 2017, 29, 1605115 CrossRef PubMed; (c) D. Meng, H. T. Fu, C. Y. Xiao, X. Y. Meng, T. Winands, W. Ma, W. Wei, B. B. Fan, L. J. Huo, N. L. Doltsinis, Y. Li, Y. M. Sun and Z. H. Wang, J. Am. Chem. Soc., 2016, 138, 10184 CrossRef PubMed; (d) J. Liu, S. S. Chen, D. P. Qian, B. Gautam, G. F. Yang, J. B. Zhao, J. Bergqvist, F. L. Zhang, W. Ma, H. Ade, O. Inganäs, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 16089 CrossRef; (e) L. Yang, W. X. Gu, L. Lv, Y. S. Chen, Y. F. Yang, P. Ye, J. F. Wu, L. Hong, A. D. Peng and H. Huang, Angew. Chem., Int. Ed., 2018, 57, 1096 CrossRef PubMed; (f) T. J. Sisto, Y. Zhong, B. Y. Zhang, M. T. Trinh, K. Miyata, X. J. Zhong, X. Y. Zhu, M. L. Steigerwald, F. Ng and C. Nuckolls, J. Am. Chem. Soc., 2017, 139, 5648 CrossRef PubMed; (g) H. Chang, Z. M. Chen, X. Y. Yang, Q. W. Yin, J. Zhang, L. Ying, X. F. Jiang, B. M. Xu, F. Huang and Y. Cao, Org. Electron., 2017, 45, 227 CrossRef.
- J. Q. Zhang, Y. K. Li, J. C. Huang, H. W. Hu, G. Y. Zhang, T. X. Ma, P. C. Y. Chow, H. Ade, D. Pan and H. Yan, J. Am. Chem. Soc., 2017, 139, 16092 CrossRef PubMed.
- X. J. Zhan, W. T. Xiong, Y. B. Gong, T. Liu, Y. J. Xie, Q. Peng, Y. M. Sun and Z. Li, Sol. RRL, 2017, 1, 1700123 CrossRef.
- (a) X. Y. Liu, Y. J. Yan, Y. Yao and Z. Q. Liang, Adv. Funct. Mater., 2018, 28, 1802004 CrossRef; (b) M. Zhang, W. Gao, F. J. Zhang, Y. Mi, W. B. Wang, Q. S. An, J. Wang, X. L. Ma, J. L. Miao, Z. H. Hu, X. F. Liu, J. Zhang and C. L. Yang, Energy Environ. Sci., 2018, 11, 841 RSC; (c) Z. Xiao, X. Jia and L. M. Ding, Sci. Bull., 2017, 62, 1562 CrossRef; (d) L. Y. Lu, W. Chen, T. Xu and L. P. Yu, Nat. Commun., 2015, 6, 7327 CrossRef PubMed; (e) H. Li, Z. Xiao, L. M. Ding and J. Z. Wang, Sci. Bull., 2018, 63, 340 CrossRef; (f) H. Lu, J. C. Zhang, J. Y. Chen, Q. Liu, X. Gong, S. Y. Feng, X. J. Xu, W. Ma and Z. S. Bo, Adv. Mater., 2016, 28, 9559 CrossRef CAS PubMed; (g) L. Zhang and W. Ma, Chin. J. Polym. Sci., 2017, 35, 184 CrossRef CAS.
- (a) Y. S. Chen, P. Ye, Z. G. Zhu, X. L. Wang, L. Yang, X. Z. Xu, X. X. Wu, T. Dong, H. Zhang, J. H. Hou, F. Liu and H. Huang, Adv. Mater., 2017, 29, 1603154 CrossRef PubMed; (b) T. Liu, Y. Guo, Y. P. Yi, L. J. Huo, X. N. Xue, X. B. Sun, H. T. Fu, W. T. Xiong, D. Meng, Z. H. Wang, F. Liu, T. P. Russell and Y. M. Sun, Adv. Mater., 2016, 28, 10008 CrossRef CAS PubMed; (c) K. Jiang, G. Y. Zhang, G. F. Yang, J. Q. Zhang, Z. K. Li, T. X. Ma, H. W. Hu, W. Ma, H. Ade and H. Yan, Adv. Energy Mater., 2018, 8, 1701370 CrossRef.
- (a) X. L. Ma, W. Gao, J. S. Yu, Q. S. An, M. Zhang, Z. H. Hu, J. X. Wang, W. H. Tang, C. L. Yang and F. J. Zhang, Energy Environ. Sci., 2018, 11, 2134 RSC; (b) Y. P. Xie, F. Yang, Y. X. Li, M. A. Uddin, P. Q. Bi, B. B. Fan, Y. H. Cai, X. T. Hao, H. Y. Woo, W. W. Li, F. Liu and Y. M. Sun, Adv. Mater., 2018, 1803045 CrossRef PubMed.
- (a) Y. B. Gong, P. Zhang, Y. R. Gu, J. Q. Wang, M. M. Han, C. Chen, X. J. Zhan, Z. L. Xie, B. Zou, Q. Peng, Z. G. Chi and Z. Li, Adv. Opt. Mater., 2018, 6, 1800198 CrossRef; (b) X. J. Zhan, J. Zhang, Y. B. Gong, S. Tang, J. Tu, Y. Xie, Q. Peng, G. Yu and Z. Li, Mater. Chem. Front., 2017, 1, 2341 RSC; (c) Y. B. Gong, X. J. Zhan, Q. Q. Li and Z. Li, Sci. China: Chem., 2016, 59, 1623 CrossRef CAS; (d) X. Zhan, J. Zhang, S. Tang, Y. X. Lin, M. Zhao, J. Yang, H. L. Zhang, Q. Peng, G. Yu and Z. Li, Chem. Commun., 2015, 51, 7156 RSC.
- Y. Z. Lin, J. Y. Wang, Z. G. Zhang, H. T. Bai, Y. F. Li, D. B. Zhu and X. W. Zhan, Adv. Mater., 2015, 27, 1170 CrossRef CAS PubMed.
- H. J. Bin, L. Gao, Z. G. Zhang, Y. K. Yang, Y. D. Zhang, C. F. Zhang, S. S. Chen, L. W. Xue, C. D. Yang, M. Xiao and Y. F. Li, Nat. Commun., 2016, 7, 13651 CrossRef CAS PubMed.
- (a) P. P. Khlyabich, A. E. Rudenko, R. A. Street and B. C. Thompson, ACS Appl. Mater. Interfaces, 2014, 6, 9913 CrossRef CAS PubMed; (b) N. S. Gobalasingham, S. T. Noh, J. B. Howard and B. C. Thompson, ACS Appl. Mater. Interfaces, 2016, 8, 27931 CrossRef CAS PubMed.
- S. Wu, J. Polym. Sci., Part C: Polym. Lett., 1971, 34, 19 CrossRef.
- (a) K. Zhang, R. X. Xia, B. B. Fan, X. Liu, Z. F. Wang, S. Dong, H. L. Yip, L. Ying, F. Huang and Y. Cao, Adv. Mater., 2018, 1803166 CrossRef PubMed; (b) D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Röhr, S. Holliday, A. Wadsworth, S. Lockett, M. Neophytou, C. J. M. Emmott, J. Nelson, C. J. Brabec, A. Amassian, A. Salleo, T. Kirchartz, J. R. Durrant and I. McCulloch, Nat. Mater., 2017, 16, 4797 CrossRef PubMed; (c) D. Landerer, A. Mertens, D. Freis, R. Droll, T. Leonhard, A. D. Schulz, D. Bahro and A. Colsmann, npj Flexible Electron., 2017, 11, 1 Search PubMed; (d) Z. Wang, H. Y. Jiang, L. J. Zhang, X. C. Liu, J. M. Sun, Y. Cao and J. W. Chen, Org. Electron., 2018, 61, 359 CrossRef CAS; (e) X. Y. Du, T. Heumueller, W. Gruber, A. Classen, T. Unruh, N. Li and C. J. Brabec, Joule DOI:10.1016/j.joule.2018.09.001.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00486b |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2019 |