High-performance quasi-solid-state asymmetric supercapacitors based on BiMn2O5 nanoparticles and redox-additive electrolytes†
Jai
Bhagwan
,
Bhimanaboina
Ramulu
and
Jae Su
Yu
 *
*
Department of Electronic Engineering, Institute for Wearable Convergence Electronics, Kyung Hee University, 1 Seocheon-dong, Giheung-gu, Yongin-si, Gyeonggi-do 446-701, Republic of Korea. E-mail: jsyu@khu.ac.kr; Fax: +82-31-204-8115; Tel: +82-31-201-3820
First published on 20th June 2019
Abstract
The investigation of nanomaterials with improved energy storage performance is essential in the development of high energy density supercapacitors. Herein, bismuth manganese oxide (BiMn2O5, BMO) nanoparticles were facilely synthesized by a combustion method and used as an electrode material in aqueous and quasi-solid-state asymmetric supercapacitors (ASCs). In a three-electrode system, the BMO electrode exhibited a specific capacitance of 228 F g−1 at a current density of 2 A g−1 in 1 M H2SO4 electrolyte. Furthermore, to enhance the performance of the electrode material, the bare electrolyte was modified by redox-additive potassium iodide (KI), and then an improved specific capacitance of 405 F g−1 at 2 A g−1 was obtained. Moreover, the ASC device was fabricated using a gel electrolyte (PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 = 1
H2SO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a small amount of redox-additive KI, which demonstrated the maximum specific capacitance of 206 F g−1 at 1 A g−1. The energy and power densities of the device were found to be 114 W h kg−1 and 0.99 kW kg−1, respectively, at 1 A g−1. The almost stable specific capacitance of the device was obtained until 2500 cycles, indicating the excellent durability of the ASC. To check the viability of the device, twelve parallel connected red color light-emitting diodes were successfully operated with two series connected ASCs, suggesting its good energy storage performance. Furthermore, an electric motor fan was also powered by ASCs. The BMO nanoparticles and redox-additive electrolytes are expected to be economically favorable strategies to enable the high energy storage performance of supercapacitor devices.
1) with a small amount of redox-additive KI, which demonstrated the maximum specific capacitance of 206 F g−1 at 1 A g−1. The energy and power densities of the device were found to be 114 W h kg−1 and 0.99 kW kg−1, respectively, at 1 A g−1. The almost stable specific capacitance of the device was obtained until 2500 cycles, indicating the excellent durability of the ASC. To check the viability of the device, twelve parallel connected red color light-emitting diodes were successfully operated with two series connected ASCs, suggesting its good energy storage performance. Furthermore, an electric motor fan was also powered by ASCs. The BMO nanoparticles and redox-additive electrolytes are expected to be economically favorable strategies to enable the high energy storage performance of supercapacitor devices.
1. Introduction
Owing to the depletion of fossil fuels, surging oil price, increasing global warming and demand for portable electronics, the development of eco-designed energy storage systems coupled with renewable energy sources is urgently required to meet the ever-growing energy demands. In particular, supercapacitors with high power density, long cycle life, high durability and eco-friendly features have become a big stipulation in energy storage systems.1–4 However, high-end applications of supercapacitors are mainly restricted by their low energy density and weak electrochemical stability of electrode materials.5,6 In parallel, considering the environmental and cost issues, a great deal of attention is being paid to explore environmentally friendly and cost-effective electrode materials.7,8 In regard to this, nanomaterials are of great interest compared to bulk materials in energy storage devices.9 Various nanostructures of different materials such as conducting polymers, carbon nanotubes, graphene and transition metal oxides have been prepared and used to increase the kinetics of ions and transportation of electrons in the electrode material, which may lead to high energy storage performance.10–12 Among them, conducting polymers like polypyrrole, polyaniline and their derivatives show high volumetric and gravimetric capacitance values, but they exhibit limited stability during redox cycling due to their substantial expansion and contraction.13 Furthermore, the low specific capacitance is a major hindrance for carbonaceous material, which is widely used in the application of electric double-layer capacitors (EDLCs).14 There are several binary and ternary transition metal oxide electrode materials which have been used as electrode materials for supercapacitors owing to their variable oxidation states, which leads to high pseudocapacitive behavior. For example, Co3O4,15 NiO,16 NiCo2O4,17 CuCo2O4,18 NiMoO4,19 NiFe2O4,20etc. with different morphologies have been studied to improve the supercapacitive performance of an electrode material due to the variable oxidation states of a transition metal in its crystal framework. Meanwhile, some of the bismuth-based materials are also investigated as electrode materials for supercapacitors due to their high specific capacitance, high electrochemical stability, high redox reversibility, good ionic conductivity and high power density.21–24 Additionally, the main challenge with supercapacitors is to provide high energy density which depends upon the capacitance of the electrode material and potential window of the device.25,26 The specific capacitance of the device may be improved significantly by nanotechnology. As the size of the electrode material approaches the nanoscale, the number of atoms available at the surface of the material becomes more effective. The surface modification of the electrode material improves the accessibility of the electrode material for the electrolyte. In addition, it shows a better adaptability to strain/stress arising from long cyclings, which could effectively enhance the electrochemical durability of the electrode material. Furthermore, the potential window may be improved using non-faradaic EDLC materials as a negative electrode and faradaic pseudocapacitive materials as a positive electrode.27 Also, for safety issues, a gel electrolyte is superior to its aqueous counterpart because robust encapsulation is required to avert the leakage problem of the electrolyte.28 It was observed that the ionic conductivity of the gel electrolyte could be increased with the appropriate amount of redox additive.29 Moreover, a redox additive in a gel electrolyte may give more redox couples compared to a bare gel electrolyte, so improved capacitance may be obtained. Yu et al. used methylene blue as a redox mediator in the PVA-PVP-H2SO4 gel electrolyte and obtained 164% improved capacitance.30 Senthilkumar et al. doped potassium iodide (KI) as a redox additive in H2SO4 and nearly two-fold improvement in the capacitance was achieved.31 Shanmugavani et al. added the redox additive of K4[Fe(CN)6] in a H2SO4 electrolyte and owing to the influence of Fe(CN)64−/Fe(CN)63− redox ion pairs, improved capacitance was obtained.32 Hence, a redox additive provides more redox reactions for the electrochemical performance and as a result, the capacitance of the electrode material may be enhanced greatly.In this study, BiMn2O5 (BMO) nanoparticles were fabricated by a simple, adaptable and cost-effective combustion method. The synthesized nanomaterials are used as an electrode material for supercapacitors and characterized using various electrochemical measurements. Moreover, the effect of the redox additive (i.e., KI) on the supercapacitive performance of the electrode material was observed. BMO shows improved specific capacitance in the redox-additive electrolyte (i.e., 1 M H2SO4 + 0.04 M KI) compared to the bare electrolyte. For practical application, quasi-solid-state asymmetric supercapacitors (ASCs) were fabricated using a gel-type H2SO4-PVA electrolyte with a redox additive and the obtained results were discussed in detail.
2. Experimental section
2.1. Materials
Analytical grade manganese nitrate tetrahydrate (Mn(NO3)2·4H2O) and citric acid (C6H8O7) were obtained from Sigma Aldrich, South Korea. Bi(NO3)3·5H2O, N-methyl-2-pyrrolidone (NMP, C5H9NO), polyvinylidene difluoride (PVDF, –(C2H2F2)n–), polyvinyl alcohol (PVA, (C2H4O)x–), nitric acid (HNO3) and KI were supplied by Daejung Chemicals Ltd, South Korea. All the chemicals were used as received without further purification.2.2. Material synthesis
The BMO nanostructures were synthesized by a simple, less time-consuming and cost-effective combustion method. In a typical synthesis, 0.97 g of Bi(NO3)3·5H2O was dissolved in 3 ml of HNO3 under magnetic stirring. In another beaker, 0.5 g of Mn(NO3)2·4H2O was dissolved in 1.5 ml of distilled (DI) water. Thereafter, the aqueous solution of Mn(NO3)2·4H2O was slowly added to the acidic solution of Bi(NO3)3·5H2O under magnetic stirring at 80 °C. Subsequently, 2.3 g of citric acid was added to this solution mixture and it was continuously stirred for 10 min. Thereafter, this mixture was kept in a preheated muffle furnace at 300 °C. Within 15 min, smoke evolved, leading to the nanomaterials. The as-obtained materials were crushed using a mortar pestle and heated at 800 °C for 4 h with a ramping rate of 5 °C min−1. The schematic representation of the material synthesis is shown in Fig. 1. | ||
| Fig. 1 Schematic representation of the synthesis process of BMO nanoparticles by a combustion method. | ||
2.3. Material characterization
The structures and phase purity of the synthesized materials were determined using an X-ray diffractometer (XRD; M18XHF-SRA, Mac Science) equipped with Cu-Kα radiation (λ = 1.541 Å). The morphology of BMO was analyzed using a field-emission scanning electron microscope (FESEM; Carl Zeiss, LEO SUPRA) and a transmission electron microscope (TEM; JEM-2100F, JEOL, 200 kV). The surface chemical composition and chemical states of the elements in BMO were determined by X-ray photoelectron spectroscopy (XPS; Thermo Electron MultiLab 2000). The Fourier transform infrared (FTIR) spectrum was recorded on a JASCO FT-IR 470 Plus spectrometer. The information regarding the vibrational modes of the BMO was obtained by high-resolution Raman (HR-Raman) spectroscopy.2.4. Electrode fabrication for electrochemical characterization
The active material (BMO), conducting carbon (super P) and PVDF were taken in the weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and the NMP was used as a milling medium to prepare a slurry. All the materials were mixed and stirred for 12 h, and then the prepared homogeneous slurry was coated on a graphite sheet (1 cm2) to make a working electrode. The working electrode was aged at 80 °C for 12 h to ensure good soakage of the active material. The loading mass of the active material was 1 mg cm−2. The electrochemical measurements were completed in 1 M H2SO4, where Ag/AgCl and Pt wire were used as the reference and counter electrodes, respectively. The electrochemical performance of BMO was determined by cyclic voltammetry (CV), galvanostatic charging–discharging (GCD) and electrochemical impedance spectroscopy (EIS) measurements using an IVIUMSTAT electrochemical interface under normal atmospheric conditions.
10, and the NMP was used as a milling medium to prepare a slurry. All the materials were mixed and stirred for 12 h, and then the prepared homogeneous slurry was coated on a graphite sheet (1 cm2) to make a working electrode. The working electrode was aged at 80 °C for 12 h to ensure good soakage of the active material. The loading mass of the active material was 1 mg cm−2. The electrochemical measurements were completed in 1 M H2SO4, where Ag/AgCl and Pt wire were used as the reference and counter electrodes, respectively. The electrochemical performance of BMO was determined by cyclic voltammetry (CV), galvanostatic charging–discharging (GCD) and electrochemical impedance spectroscopy (EIS) measurements using an IVIUMSTAT electrochemical interface under normal atmospheric conditions.
3. Results and discussion
3.1. Material characterization
XRD analysis of the prepared material is shown in Fig. 2a. No impurity phase was observed in the XRD pattern, implying that the as-prepared sample was pure in phase. The diffraction pattern of the prepared material is completely indexed based on the orthorhombic space group Pbam (55) (JCPDS file: 01-074-1096) and assigned to BiMn2O5 with cell parameters of a = 7.547 (±0.001) Å, b = 8.533 (±0.001) Å and c = 5.765 (±0.001) Å, which is found to be in good agreement with the literature.33 The sharp and intense diffraction peaks of the spectrum indicate that the prepared material was well in the crystalline phase. The average crystallite size was obtained from the XRD pattern and found to be 58.96 nm which was determined from Scherrer's formula: t = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where β is the full width at half maximum of the diffraction peak that corresponds to the (211) plane, θ is the corresponding diffraction angle for Bragg's diffraction, t is the mean crystallite size and λ denotes the wavelength of the X-ray (0.154 nm). The crystal structure of the BMO is shown in Fig. 2b. In the structure, one Bi atom is coordinated to six O atoms to form distorted octahedra, while Mn atoms occupy two different crystallographic sites of 4f and 4 h. The trivalent Mn1 atom is connected to six O atoms with an octahedral geometry (green color), and the tetravalent Mn2 atom is linked with five O atoms (yellow color) in a quadrangular pyramid, which is in good agreement with the literature.34Fig. 2c shows the FTIR spectrum of BMO nanoparticles with 7 IR active modes in the range of 400 to 1000 cm−1. According to the group theory analysis, 48 Raman and 36 IR active modes should be observed in BMO.35,36 However, the number of observed modes is always lower than group theoretical predictions. Modes between 425 and 471 cm−1 could be attributed to the Mn–O bending vibration of Mn3+O5. The modes observed between 547 and 608 cm−1 could be linked to the Mn–O stretching vibration within Mn4+O6.36Fig. 2d shows the Raman spectrum of BMO, where 5 Raman active bands are clearly observed, and Raman data are well matched with the literature.37 It could be considered that the experimental parameters (such as the synthesis process and temperature) may play an important role in the frequency shift and band broadening of the Raman performance.
θ, where β is the full width at half maximum of the diffraction peak that corresponds to the (211) plane, θ is the corresponding diffraction angle for Bragg's diffraction, t is the mean crystallite size and λ denotes the wavelength of the X-ray (0.154 nm). The crystal structure of the BMO is shown in Fig. 2b. In the structure, one Bi atom is coordinated to six O atoms to form distorted octahedra, while Mn atoms occupy two different crystallographic sites of 4f and 4 h. The trivalent Mn1 atom is connected to six O atoms with an octahedral geometry (green color), and the tetravalent Mn2 atom is linked with five O atoms (yellow color) in a quadrangular pyramid, which is in good agreement with the literature.34Fig. 2c shows the FTIR spectrum of BMO nanoparticles with 7 IR active modes in the range of 400 to 1000 cm−1. According to the group theory analysis, 48 Raman and 36 IR active modes should be observed in BMO.35,36 However, the number of observed modes is always lower than group theoretical predictions. Modes between 425 and 471 cm−1 could be attributed to the Mn–O bending vibration of Mn3+O5. The modes observed between 547 and 608 cm−1 could be linked to the Mn–O stretching vibration within Mn4+O6.36Fig. 2d shows the Raman spectrum of BMO, where 5 Raman active bands are clearly observed, and Raman data are well matched with the literature.37 It could be considered that the experimental parameters (such as the synthesis process and temperature) may play an important role in the frequency shift and band broadening of the Raman performance.
The FESEM image of the BMO is shown in Fig. 2e, which indicates the spherical shape of the BMO nanostructure with an average particle size of 100–150 nm. The interconnected arrangement of BMO nanoparticles could be expected to enhance the energy storage performance. The TEM image also reveals the spherical morphology of the prepared material as depicted in Fig. 2f. To gain information about elemental dispersion, elemental mapping analysis was carried out using an energy dispersive X-ray (EDX) analyzer and the elemental mapping images are shown in Fig. 2g(i)–(iii). The homogeneous dispersion of Bi, Mn and O elements in the prepared material evidences the successful formation of BMO without any impurities. The EDX spectrum of the BMO is also shown in Fig. 2h, which again indicates the presence of Bi, Mn and O elements in the final product.
The information about the surface chemical composition of the prepared samples was obtained from the XPS peak fitting. The survey spectra of BMO demonstrated the presence of Bi, Mn and O (Fig. 3a). No other contaminant species were detected except for a small amount of absorbed carbon that was used for internal energy calibration. The Bi 4f7/2 and Bi 4f5/2 peaks were observed at 157.08 and 162.48 eV, respectively (Fig. 3b). The observed binding energy difference between these peaks was calculated to be 5.4. This observed value was matched with the reported value in the literature, which could be assigned to the Bi3+.33 The Mn 2p spectrum displayed the binding energy peaks of Mn 2p3/2 and Mn 2p1/2 at 640.47 and 652.17 eV, respectively, and may be assigned to the Mn3+ ions (Fig. 3c). The peaks at 642.69 and 654.41 eV may be linked to the Mn4+ ions.38 Meanwhile, the O 1s spectrum of BMO shows two major peaks, as presented in Fig. 3d. One peak is found to be at 529.72 eV corresponding to the metal–oxygen bond, while the other peak at 528.06 eV is attributed to the defect sites lacking oxygen coordination adsorbed on the sample surface of BMO. The weak peak at 531.48 eV may be associated with a variety of physi/chemisorbed water (H–O–H) at the surface.39,40
 | ||
| Fig. 3 (a) Total survey scan XPS spectrum of BMO nanomaterials. High-resolution XPS spectra for (b) Bi 4f, (c) Mn 2p and (d) O 1s. | ||
3.2. Electrochemical studies
To investigate the supercapacitive performance of BMO, electrochemical characterization methods such as CV, GCD and EIS were performed in aqueous 1 M H2SO4 electrolyte in the potential window of 0.0 to 1.0 V. Fig. 4a shows the typical CV curves at different scan rates of 10–50 mV s−1. It can be seen that CV curves remain in quasi-rectangular shape with increasing the scan rate, indicating the high charge storage performance in the total range of the potential window. The GCD analysis of BMO was carried out at different current densities and the corresponding charge–discharge curves are shown in Fig. 4b. The specific capacitance (Cs) of the BMO was calculated from the discharging time of the GCD curves according to the below equation:41,42 | (1) |
| 3I− ↔ I3− + 2e− | (2) |
| 2I− ↔ I2 + 2e− | (3) |
| 2I3− ↔ 3I2 + 2e− | (4) |
| I2 + 6H2O ↔ 2IO3− + 12H+ + 10e−. | (5) |
The GCD measurement was also carried out in the redox additive electrolyte and the obtained results are shown in Fig. 4e. Noticeably, the BMO shows approximately 2 fold higher specific capacitance in the redox electrolyte compared to the bare electrolyte. The Cs values of 405, 336 and 300 F g−1 were obtained at the current densities of 2, 3 and 4 A g−1, respectively. In addition, the redox additive in the bare electrolyte provides extra foreign ions for electrochemical reactions, and thus an improved Cs value from 228 to 405 F g−1 was obtained. The cyclability of the electrode material was determined at a current density of 4 A g−1, as shown in Fig. 4f. The electrode material showed the capacitance retention of 98% until 2000 cycles, suggesting the excellent stability of the electrode material. Specific capacitance as a function of current density in the redox additive electrolyte is shown in the inset of Fig. 4f, indicating the good rate capability of the BMO. Clearly, the effect of the redox additive in the bare electrolyte can be seen from the comparative CV and GCD curves as shown in Fig. 4g and h, respectively. Such high capacitance is further attributed to the effect of the redox additive as well as the nano-sized materials, which brings numerous merits like more active sites, high surface area, short diffusion path length and easy access to the electrolyte. The EIS is the most powerful technique to recognize the charge kinetics and electrolyte diffusion behavior of the prepared electrode materials. The EIS measurement of the BMO electrode material was carried out using both the electrolytes in the frequency range of 100 kHz to 0.01 Hz with a bias potential of 5 mV and the corresponding results are shown in Fig. 4i. The intercept of the semi-circle in the high-frequency region represents the solution resistance (Rs). The diameter of the semicircle indicates the charge transfer resistance (Rct) which is attributed to the faradaic charge transfer resistance. The low-frequency straight line inclining at ∼45° with the x-axis shows Warburg impedance (W). The solution resistance (Rs) and charge transfer resistance (Rct) of the electrode were found to be 4 Ω and 0.37 Ω, respectively, in 1 M H2SO4, while in 1 M H2SO4 + 0.04 M KI electrolyte, Rs and Rct were found to be 50 mΩ and 2.0 Ω, respectively.
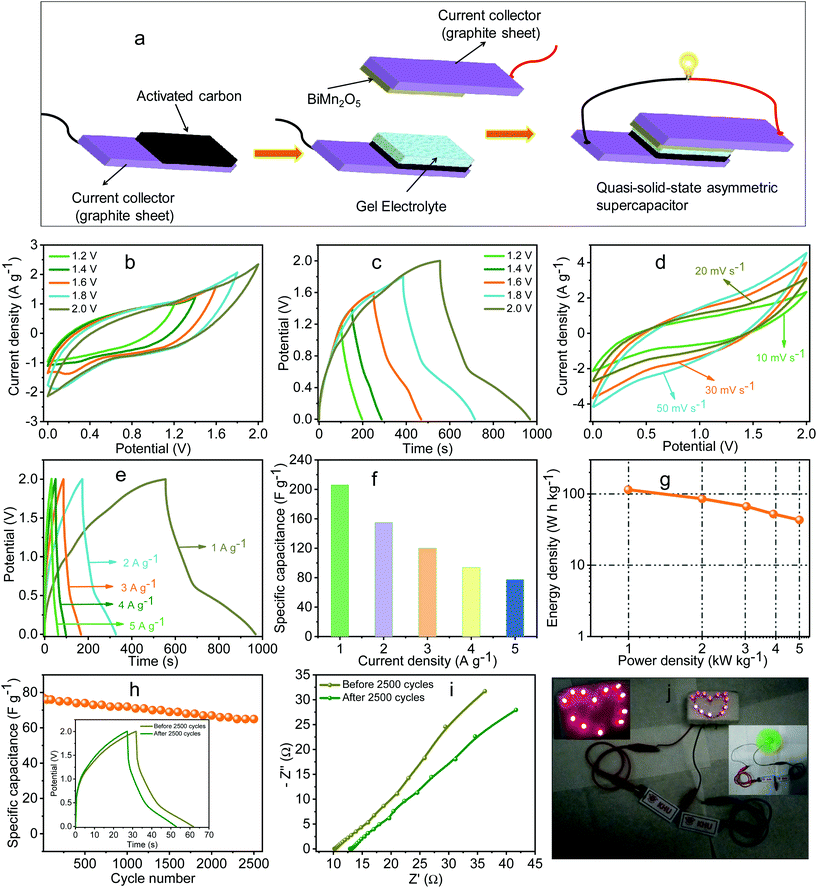
For practical application of BMO, quasi-solid-state ASC was fabricated using BMO as a positive electrode and activated carbon (AC) as a negative electrode, and the detail is given in the ESI.† For a gel electrolyte, 18 ml of 10% PVA solution was prepared using DI in which an equal amount of H2SO4 (PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 = 1
H2SO4 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used. A small amount of KI (0.13 g) was dissolved in 2 ml of DI water and mixed with the gel electrolyte. The prepared gel electrolyte was sandwiched between the positive and negative electrodes (1 × 1 cm2). The schematic representation of the quasi-solid-state ASC is shown in Fig. 5a. The potential window of the fabricated device was checked using CV analysis in different potential ranges. From the CV curves in Fig. 5b, it can be seen that the potential of the device may be extended up to 2.0 V. The potential window of the device was further confirmed by GCD analysis, as shown in Fig. 5c. With this potential range, CV curves at different scan rates were obtained (Fig. 5d) and no change in the shape of CV curves was observed, suggesting the good storage performance of the electrode material. To investigate the capacitive performance and rate capability of the device, the GCD curves at different current densities were observed, as shown in Fig. 5e. The specific capacitance of the device was calculated using eqn (1), where m represents the total mass (g) of both the electrode materials. The Cs values of the ASC were found to be 206, 154 and 120 F g−1 at 1, 2 and 3 A g−1, respectively. This high capacitance of the device is attributed to the high electrochemical activity of the electrode materials and the effect of the redox additive in the electrolyte. Moreover, the discharge curves are made up of mainly two regions: the initial part (straight) may be attributed to the double layer charge storage process and the lower part (diffusion part) may be ascribed mainly to the redox process. Hence, both the redox reaction and EDLC contribute to the storage properties in the device. The specific capacitance as a function of current density is shown in Fig. 5f.
1) was used. A small amount of KI (0.13 g) was dissolved in 2 ml of DI water and mixed with the gel electrolyte. The prepared gel electrolyte was sandwiched between the positive and negative electrodes (1 × 1 cm2). The schematic representation of the quasi-solid-state ASC is shown in Fig. 5a. The potential window of the fabricated device was checked using CV analysis in different potential ranges. From the CV curves in Fig. 5b, it can be seen that the potential of the device may be extended up to 2.0 V. The potential window of the device was further confirmed by GCD analysis, as shown in Fig. 5c. With this potential range, CV curves at different scan rates were obtained (Fig. 5d) and no change in the shape of CV curves was observed, suggesting the good storage performance of the electrode material. To investigate the capacitive performance and rate capability of the device, the GCD curves at different current densities were observed, as shown in Fig. 5e. The specific capacitance of the device was calculated using eqn (1), where m represents the total mass (g) of both the electrode materials. The Cs values of the ASC were found to be 206, 154 and 120 F g−1 at 1, 2 and 3 A g−1, respectively. This high capacitance of the device is attributed to the high electrochemical activity of the electrode materials and the effect of the redox additive in the electrolyte. Moreover, the discharge curves are made up of mainly two regions: the initial part (straight) may be attributed to the double layer charge storage process and the lower part (diffusion part) may be ascribed mainly to the redox process. Hence, both the redox reaction and EDLC contribute to the storage properties in the device. The specific capacitance as a function of current density is shown in Fig. 5f.
The energy and power densities of the device are important parameters to determine the feasibility of the devices. At the current densities of 1, 2 and 3 A g−1, the device exhibited the maximum energy densities of 114.4, 85.5 and 66.6 W h kg−1 with the power densities of 0.99, 1.99 and 3.03 kW kg−1, respectively. The Ragone plot (curves between the energy and power densities) was used to evaluate the device performance (Fig. 5g). The energy density of the device was found to be higher as compared to the literature. For example, Ahuja et al. reported the energy and power densities of 37 W h kg−1 and 30 kW kg−1, respectively, at 1.25 A g−1 using ZnMn2O4/GN//ZnMn2O4/GNR supercapacitors.46 Gao et al. achieved the energy and power densities of 60.9 W h kg−1 and 11.36 kW kg−1, respectively, using NiCo2O4/carbon cloth as a positive electrode and porous graphene paper as a negative electrode.47 Kong et al. synthesized three-dimensional NiCo2O4@polypyrrole coaxial nanowire arrays on carbon textiles and obtained the energy and power densities of 58.8 W h kg−1 and 365 W kg−1, respectively, from a flexible asymmetric solid-state supercapacitor.48 Liu et al. synthesized a BiMn2O5/MWCNT composite and reported an energy density of 9 W h kg−1 from the BiMn2O5/MWCNT//AC device.49 To justify the stability of the electrode material, the cycling performance of the device was further measured at a current density of 5 A g−1 as can be seen in Fig. 5h. No significant loss in capacitance was observed up to 2500 cycles, showing the good stability of the electrode material. GCD curves of 1st and 2500th cycles at 5 A g−1 are shown in the inset of Fig. 5h. The EIS plots of the device are also obtained to complement the findings of CV and GCD studies. The Nyquist plots of the device before and after 2500 cycles are illustrated in Fig. 5i. The solution resistances of the device before and after 2500 cycles were found to be 10 and 12.8 Ω, respectively. This increment in the resistance may be linked to solidify the gel electrolyte and thereby, after 2500 cycles, loss in the capacitance was obtained as discussed above. Furthermore, to test the practical applicability of the device, twelve red color light-emitting diodes (LEDs) (parallel connected) were lit from two series connected quasi-solid-state ASCs as shown in Fig. 5j. An electric motor fan was also rotated by two series connected devices (inset of Fig. 5j (right)). Considering the excellent energy storage performance along with the redox-additive electrolyte, the facile synthesis method for the fabrication of BMO-based ASCs paves a new avenue for the development of high-performance supercapacitors.
4. Conclusion
In summary, combustion-routed BMO nanostructures were facilely prepared and used as electrode materials in supercapacitors. The electrochemical performance of the BMO was studied in redox additive-based aqueous and gel electrolytes. Compared to the pristine H2SO4 electrolyte, the BMO showed higher specific capacitance in the redox-additive electrolyte. Specifically, the specific capacitance of 405 F g−1 at a current density of 2 A g−1 was two times higher than that in the pristine electrolyte (Cs of 228 F g−1) at the same current density. Moreover, the ASCs were fabricated using a redox additive in PVA-H2SO4, which showed a high specific capacitance of 206 F g−1, an energy density of 114.4 W h kg−1 and a power density of 0.99 kW kg−1 at the current density of 1 A g−1. This energy density was found to be higher than that of a lead acid battery and comparable to the Ni-metal hydride battery. Furthermore, a small difference in capacitance was observed after 2500 cycles, suggesting the good stability of the electrode material. For practical applications, twelve parallel connected red-emitting LEDs and a motor fan were powered through two series connected ASCs, confirming the high energy storage performance. This work demonstrates a facile, cost-effective, pragmatic, and scalable approach toward obtaining high energy and power densities by the BMO electrode material and may provide a significant step forward to bringing BMO-based nanomaterials to diverse applications in lithium-ion batteries, biosensors, supercapacitors, solar cells and other electronic systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (2017R1A2B4011998 and 2018R1A6A1A03025708).References
- A. M. Abioye, F. N. Ani and F. Nasir, Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review, Renewable Sustainable Energy Rev., 2015, 52, 1282–1293 CrossRef CAS.
- A. González, E. Goikolea, J. A. Barrena and R. Mysyk, Review on supercapacitors: Technologies and materials, Renewable Sustainable Energy Rev., 2016, 58, 1189–1206 CrossRef.
- S. Zheng, H. Xue and H. Pang, Supercapacitors based on metal coordination materials, Coord. Chem. Rev., 2017, 373, 2–21 CrossRef.
- S. Faraji and F. N. Ani, The development supercapacitor from activated carbon by electroless plating - A review, Renewable Sustainable Energy Rev., 2015, 42, 823–834 CrossRef CAS.
- S. Dhibar, P. Bhattacharya, D. Ghosh, G. Hatui and C. K. Das, Graphene-single-walled carbon nanotubes−poly (3-methylthiophene) ternary nanocomposite for supercapacitor electrode materials, Ind. Eng. Chem. Res., 2014, 53, 13030–13045 CrossRef CAS.
- R. B. Rakhi, W. Chen, M. N. Hedhili, D. Cha and H. N. Alshareef, Enhanced rate performance of mesoporous Co3O4 nanosheet supercapacitor electrodes by hydrous RuO2 nanoparticle decoration, ACS Appl. Mater. Interfaces, 2014, 6, 4196–4206 CrossRef CAS PubMed.
- J. Bhagwan, A. Sahoo, K. L. Yadav and Y. Sharma, Nanofibers of spinel-CdMn2O4: A new and high performance material for supercapacitor and Li-ion batteries, J. Alloys Compd., 2017, 703, 86–95 CrossRef CAS.
- B. Yang, C. Hao, F. Wen, B. Wang, C. Mu, J. Xiang, L. Li, B. Xu, Z. Zhao, Z. Liu and Y. Tian, Flexible black-phosphorus nanoflake/carbon nanotube composite paper for high-performance all-solid-state supercapacitors, ACS Appl. Mater. Interfaces, 2017, 9, 44478–44484 CrossRef CAS PubMed.
- J. S. Chen, C. Guan, Y. Gui and D. J. Blackwood, Rational design of self-supported Ni3S2 nanosheets array for advanced asymmetric supercapacitor with a superior energy density, ACS Appl. Mater. Interfaces, 2017, 9, 496–504 CrossRef CAS.
- S. E. Moosavifard, J. Shamsi, S. Fani and S. Kadkhodazade, 3D ordered nanoporous NiMoO4 for high performance supercapacitor electrode materials, RSC Adv., 2014, 4, 52555–52561 RSC.
- Z.-S. Wu, D.-W. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H.-M. Cheng, Anchoring hydrous RuO2 on graphene sheets for high-performance electrochemical capacitors, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- T. M. Higgins and J. N. Coleman, Avoiding resistance limitations in high-performance transparent supercapacitor electrodes based on large-area, high-conductivity PEDOT:PSS films, ACS Appl. Mater. Interfaces, 2015, 7, 16495–16506 CrossRef CAS PubMed.
- M.-C. Liu, L. Kong, C. Lu, X. Li, Y. Luo and L. Kang, A sol−gel process for fabrication of NiO/NiCo2O4/Co3O4 composite with improved electrochemical behavior for electrochemical capacitors, ACS Appl. Mater. Interfaces, 2012, 4, 4631–4636 CrossRef CAS PubMed.
- D. P. Dubal, P. Gomez-Romero, B. R. Sankapal and R. Holze, Nickel cobaltite as an emerging material for supercapacitors: An overview, Nano Energy, 2015, 11, 377–399 CrossRef CAS.
- X. Hu, H. Huang, J. Zhang, J. Shi, S. Zhu and N. Su, Controllable hydrothermal-assisted synthesis of mesoporous Co3O4 nanosheets, RSC Adv., 2015, 5, 99899–99906 RSC.
- K. K. Purushothaman, I. M. Babu, B. Sethuraman and G. Muralidharan, Nanosheet-assembled NiO microstructures for high-performance supercapacitors, ACS Appl. Mater. Interfaces, 2013, 5, 10767–10773 CrossRef CAS PubMed.
- Y. Zhang, M. Ma, J. Yang, H. Su, W. Huang and X. Dong, Selective synthesis of hierarchical mesoporous spinel NiCo2O4 for high-performance supercapacitors, Nanoscale, 2014, 6, 4303–4308 RSC.
- A. Pendashteh, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, Facile synthesis of nanostructured CuCo2O4 as a novel electrode material for high-rate supercapacitors, Chem. Commun., 2014, 50, 1972–1975 RSC.
- D. Cai, D. Wang, B. Liu, Y. Wang, Y. Liu, L. Wang, H. Li, H. Huang, Q. Li and T. Wang, Comparison of the electrochemical performance of NiMoO4 nanorods and hierarchical nanospheres for supercapacitor applications, ACS Appl. Mater. Interfaces, 2013, 5, 12905–12910 CrossRef CAS PubMed.
- Z.-Y. Yu, L.-F. Chen and S.-H. Yu, Growth of NiFe2O4 nanoparticles on carbon cloth for high performance flexible supercapacitors, J. Mater. Chem. A, 2014, 2, 10889–10894 RSC.
- Y. Qiu, H. Fan, X. Chang, H. Dang, Q. Luo and Z. Cheng, Novel ultrathin Bi2O3 nanowires for supercapacitor electrode materials with high performance, Appl. Surf. Sci., 2018, 434, 16–20 CrossRef CAS.
- R. C. Ambare, P. Shinde, U. T. Nakate, B. J. Lokhande and R. S. Mane, Sprayed bismuth oxide interconnected nanoplates supercapacitor electrode materials, Appl. Surf. Sci., 2018, 453, 214–219 CrossRef CAS.
- S. X. Wang, C. C. Jin and W. J. Qian, Bi2O3 with activated carbon composite as a supercapacitor electrode, J. Alloys Compd., 2014, 615, 12–17 CrossRef CAS.
- E. Miniach and G. Gryglewicz, Solvent-controlled morphology of bismuth sulfide for supercapacitor applications, J. Mater. Sci., 2018, 53, 16511–16523 CrossRef CAS.
- K. S. Kumar, N. Choudhary, Y. Jung and J. Thomas, Recent advances in two-dimensional nanomaterials for supercapacitor electrode applications, ACS Energy Lett., 2018, 3, 482–495 CrossRef CAS.
- C. Zhu, P. Yang, D. Chao, X. Wang, X. Zhang, S. Chen, B. K. Tay, H. Huang, H. Zhang, W. Mai and H. J. Fan, All metal nitrides solid-state asymmetric supercapacitors, Adv. Mater., 2015, 27, 4566–4571 CrossRef CAS PubMed.
- T. Chen, Y. Tang, Y. Qiao, Z. Liu, W. Guo, J. Song, S. Mu, S. Yu, Y. Zhao and F. Gao, All-solid-state high performance asymmetric supercapacitors based on novel MnS nanocrystal and activated carbon materials, Sci. Rep., 2016, 6, 23289–23297 CrossRef CAS PubMed.
- L. Yuan, X. H. Lu, X. Xiao, T. Zhai, J. Dai, F. Zhang, B. Hu, X. Wang, L. Gong, J. Chen, C. Hu, Y. Tong, J. Zhou and Z. L. Wang, Flexible solid-state supercapacitors based on carbon nanoparticles/MnO2 nanorods hybrid structure, ACS Nano, 2012, 6, 656–661 CrossRef CAS PubMed.
- H. Yu, J. Wu, L. Fan, K. Xu, X. Zhong, Y. Lin and J. Lin, Improvement of the performance for quasi-solid-state supercapacitor by using PVA-KOH-KI polymer gel electrolyte, Electrochim. Acta, 2011, 56, 6881–6886 CrossRef CAS.
- F. Yu, M. Huang, J. Wu, Z. Qiu, L. Fan, J. Lin and Y. Lin, A redox-mediator-doped gel polymer electrolyte applied in quasi-solid-state supercapacitors, J. Appl. Polym. Sci., 2014, 131, 1–7 Search PubMed.
- S. T. Senthilkumar, R. K. Selvan, Y. S. Lee and J. S. Melo, Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte, J. Mater. Chem. A, 2013, 1, 1086–1095 RSC.
- A. Shanmugavani, S. Kaviselvi, K. V. Sankar and R. K. Selvan, Enhanced electrochemical performances of PANI using redox additive of K4[Fe(CN)6] in aqueous electrolyte for symmetric supercapacitors, Mater. Res. Bull., 2015, 62, 161–167 CrossRef CAS.
- R. J. Jia, J. T. Han, X. J. Wu, C. L. Wu, Y. H. Huang and W. Huang, Controllable synthesis and magnetic property of BiMn2O5 crystals, Mater. Res. Bull., 2008, 43, 1702–1708 CrossRef CAS.
- Z. Song, H. Zhang, K. Feng, H. Wang, X. Li and H. Zhang, Bi2Mn4O10: A new mullite-type anode material for lithium-ion batteries, Dalton Trans., 2018, 47, 7739–7746 RSC.
- F. M. S. Júnior, C. W. A. Paschoal, R. M. Almeida, R. L. Moreira, W. Paraguassu, M. C. C. Junior, A. P. Ayala, Z. R. Kann and M. W. Lufaso, Room-temperature vibrational properties of the BiMn2O5 mullite, Vib. Spectrosc., 2013, 66, 43–49 CrossRef.
- V. M. Gaikwad, S. Goyal, P. Yanda, A. Sundaresan, S. Chakraverty and A. K. Ganguli, Influence of Fe substitution on structural and magnetic features of BiMn2O5 nanostructures, J. Magn. Magn. Mater., 2018, 452, 120–128 CrossRef CAS.
- D. K. Shukla, R. Kumar, S. K. Sharma, P. Thakur, R. J. Choudhary, S. Mollah, N. B. Brookes, M. Knobel, K. H. Chae and W. K. Choi, Thin film growth of multiferroic BiMn2O5 using pulsed laser ablation and its characterization, J. Phys. D: Appl. Phys., 2009, 42, 125304–125309 CrossRef.
- J. Zhan and Y. Long, Synthesis of Bi2Mn4O10 nanoparticles and its anode properties for LIB, Ceram. Int., 2018, 44, 14891–14895 CrossRef CAS.
- R. Chen, Y. Hu, Z. Shen, Y. Chen, X. He, X. Zhang and Y. Zhang, Controlled synthesis of carbon nanofibers anchored with Znx,Co3–xO4 nanocubes as binder-free anode materials for lithium-ion batteries, ACS Appl. Mater. Interfaces, 2016, 8, 2591–2599 CrossRef CAS PubMed.
- N. Yu, H. Yin, W. Zhang, Y. Liu, Z. Tang and M. Q. Zhu, High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics, Adv. Energy Mater., 2016, 6, 1–9 Search PubMed.
- Y. Wang, Y. Song and Y. Xia, Electrochemical capacitors: mechanism, materials, systems, characterization and applications, Chem. Soc. Rev., 2016, 45, 5925–5950 RSC.
- Q. Shao, J. Tang, Y. Lin, F. Zhang, J. Yuan, H. Zhang, N. Shinya and L.-C. Qin, Synthesis and characterization of graphene hollow spheres for application in supercapacitors, J. Mater. Chem. A, 2013, 1, 15423–15428 RSC.
- S. T. Senthilkumar, R. K. Selvan and J. S. Melo, Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors, J. Mater. Chem. A, 2013, 1, 12386–12394 RSC.
- G. Lota and G. Milczarek, The effect of lignosulfonates as electrolyte additives on the electrochemical performance of supercapacitors, Electrochem. Commun., 2011, 13, 470–473 CrossRef CAS.
- A. Singh and A. Chandra, Enhancing specific energy and power in asymmetric supercapacitors–A synergetic strategy based on the use of redox additive electrolytes, Sci. Rep., 2016, 6, 1–13 CrossRef PubMed.
- P. Ahuja, R. K. Sharma and G. Singh, Solid-state, high-performance supercapacitor using graphene nanoribbons embedded with zinc manganite, J. Mater. Chem. A, 2015, 3, 4931–4937 RSC.
- Z. Gao, W. Yang, J. Wang, N. Song and X. Li, Flexible all-solid-state hierarchical NiCo2O4/porous graphene paper asymmetric supercapacitors with an exceptional combination of electrochemical properties, Nano Energy, 2015, 13, 306–317 CrossRef CAS.
- D. Kong, W. Ren, C. Cheng, Y. Wang, Z. Huang and H. Y. Yang, Three-dimensional NiCo2O4 polypyrrole coaxial nanowire arrays on carbon textiles for high-performance flexible asymmetric solid-state supercapacitor, ACS Appl. Mater. Interfaces, 2015, 7, 21334–21346 CrossRef CAS PubMed.
- Y. Liu and I. Zhitomirsky, Electrochemical supercapacitor based on multiferroic BiMn2O5, J. Power Sources, 2015, 284, 377–382 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qi00613c |
| This journal is © the Partner Organisations 2019 |