Impact of the Zn source on the RSN-type zeolite formation†
Ana
Palčić
 *a,
Bartłomiej M.
Szyja
*a,
Bartłomiej M.
Szyja
 b,
Maja
Mičetić
c,
Tomaž
Čendak
b,
Maja
Mičetić
c,
Tomaž
Čendak
 d,
Mariame
Akouche
e,
Krunoslav
Juraić
d,
Mariame
Akouche
e,
Krunoslav
Juraić
 c,
Marija
Čargonja
c,
Marija
Čargonja
 f,
Darko
Mekterović
f,
Vitomir
Vušak
g and
Valentin
Valtchev
f,
Darko
Mekterović
f,
Vitomir
Vušak
g and
Valentin
Valtchev
 e
e
aDivision of Materials Chemistry, Ruđer Bošković Institute, Bijenička 54, 10000 Zagreb, Croatia. E-mail: ana.palcic@irb.hr
bFaculty of Chemistry, Wrocław University of Science and Technology, Gdańska 7/9, 50-344 Wrocław, Poland
cDivision of Materials Physics, Ruđer Bošković Institute, Bijenička 54, 10000 Zagreb, Croatia
dDepartment of Inorganic Chemistry and Technology, National Institute of Chemistry, Hajdrihova 19, SI-1001 Ljubljana, Slovenia
eNormandie Univ, ENSICAEN, UNICAEN, CNRS, Laboratoire Catalyse et Spectrochimie, 6 Marechal Juin, 14050 Caen, France
fUniversity of Rijeka, Department of Physics, Ulica Radmile Matejčić 2, 51000 Rijeka, Croatia
gPliva Croatia Ltd., Prilaz baruna Filipovića 25, 10000 Zagreb, Croatia
First published on 8th July 2019
Abstract
A typical zeolite synthesis reaction mixture involves a framework atom source, a solvent, a mineraliser, and a structure directing agent. However, these components often have a dual function, and their impact on the nucleation/crystallization process is difficult to establish straightforwardly. The present study deals with the structure directing effect of zinc on the preparation of zincosilicate RUB-17 (RSN-type material). First, an attempt was made to introduce several other heteroatoms (Al, B, Ge, Mg, Li, and Zr) into the RSN framework under the same reaction conditions as those for Zn-RSN. Second, different Zn sources have been employed in order to study their impact on RUB-17 intermediates and the final product properties. The obtained series of samples were analysed by XRD, XRF, GISAXS, SEM, TEM, and TG as well as by Raman, NMR, infrared and solid state UV/VIS spectroscopy. The physicochemical analysis of the obtained solids was complemented by DFT modelling. The results show that a RSN-type material can be solely prepared in the Zn-containing systems, where zinc plays a structure directing role besides its function as a framework cation. Namely, the lov-unit, a basic building unit of the RSN framework, is formed at the very beginning of the crystallization no matter the zinc source employed. Yet, the differences in the crystallization of different systems and relatively long time needed for full transformation of the precursor indicate that there is some other limiting step that accounts for the formation of zeolites.
Introduction
According to the classical definition, zeolites are crystalline tectosilicates with a well-defined system of channels and cages with sizes below 2 nm. The pore size categorizes zeolites as microporous materials by IUPAC classification. Nowadays this definition is extended to all materials possessing structure features typical of zeolites: tetrahedra of oxygen atoms bonded to central T atom (T = Si, Al, P, Ge, Zn, Ga, etc.) connected via vertices thus forming a 3D porous framework.1 Zeolites are widely used materials in various areas of human activities. The most common and the largest volume of application involves their usage as catalysts, ion exchangers, sorbents and separation materials.2–6 The extensive and continuously expanding range of zeolite usage is a direct consequence of their tunable set of properties ensuing from their unique chemical composition and framework structure. For example, introducing T-atoms other than silicon and aluminium (heteroatoms) into the zeolite framework can result in different properties of the materials in terms of hydrophobicity, acidity, ion-exchange capacity, and hydrothermal stability.7 Typical examples are Al-MFI (ZSM-5), Si-MFI (silicalite-1) and Ti-MFI (TS-1) materials. ZSM-5 is a good acid catalyst (xylene isomerization, FCC, MTG, etc.), whereas TS-1 is active in oxidation reactions (epoxidation of alkenes, cyclohexanone ammoxidation, etc.).8,9 In addition, some of the zeolite frameworks have been prepared solely in the presence of a particular heteroatom such as ECR-34 and VPI-9 containing gallium and zinc, respecitvely.7In order to enhance the efficacy and activity of zeolite materials, further studies should be conducted. Preparation and detailed characterization of new materials and improving the properties of “classical” zeolites (materials of desired size, morphology, acidity, ion-exchange capacity, hierarchization, etc.) are the two principal axes of research in zeolite science.10–16 Understanding the mechanisms of formation of a particular material could enable the preparation of materials having the same structure but variable chemical composition and consequently different properties. Herein, the zincosilicate material RUB-17, which is so far the only known RSN-type zeolite material, is investigated.17 Its structure is built of 3-, 4-, 5-, and 6-membered rings forming a tridimensional system of channels 9(3.3 × 4.4 Å) × 9(3.1 × 4.3 Å) × 8(3.4 × 4.1 Å). 3MR frameworks are of particular importance since it has been theoretically predicted that the structures composed of small rings (3MRs and 4MRs) should exhibit lower framework density.7 More open microporous frameworks offer new possibilities to overcome diffusion limitations and process bulkier molecules. Moreover, the 9-membered ring containing materials are expected to have interesting performance in separation processes.
It should be stressed that in total there are 24 3MR containing zeolitic framework types, many of them having only one representative type material. Some of them are not silicates (e.g. gallogermanates) and still there is only one known 3MR aluminosilicate zeolite material (MEI-type).18 Furthermore, heteroatoms such as Be, Zn, Li and Ge were incorporated into the 3MR silicate materials (OSB-1, VPI-9, RUB-29, ITQ-40), where Be and Zn are present in many zeolitic materials, particularly natural zeolites (LOV-, NAB- and VSV-type materials). In addition, most of the Be and Zn zeolites are built of a lov-unit which comprises two 3MRs sharing one vertex (spiro center).18 For this study zincosilicate zeolitic material RUB-17 was chosen due to the availability and non-toxicity of zinc. The results of the investigation of the impact of the employed zinc source on the formation of the RUB-17 material and its properties are presented. The data will provide new findings on the processes affecting the synthesis of the 3MR-containing zincosilicate materials and present a basis for the design of zeolite materials with novel functions.
Experimental part
Synthesis of materials containing various T atoms
In order to study the impact of the presence of T atoms other than zinc, reaction mixtures have been prepared in a procedure analogous to the one reported in ref. 17. The molar oxide composition of the studied reaction mixtures was 1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1TO
0.1TO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH
0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH
0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH
0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O. The framework T atom sources were as follows: aluminium (Al powder, puriss., Kemika) aluminium hydroxide (Al(OH)3, w(Al2O3) = 63–67%, Riedel de Haën), sodium aluminate (NaAlO2, w(Al2O3) = 54%, Riedel de Haën), germanium oxide (GeO2, 99.99%, Sigma Aldrich), lithium hydroxide monohydrate (LiOH·H2O, 99%, Sigma Ultra), boric acid (B(OH)3, p.a., Kemika), boron oxide (B2O3, 99.99%, Ventron), zirconyl chloride octahydrate (ZrOCl2·8H2O, 98%, Aldrich), zirconium(IV) isopropoxide (Zr(OCH(CH3)4·(CH3)2CHOH, 99.9%, Aldrich) and magnesium oxide (MgO, 99.99%, Merck). The reactions were conducted at 180 °C for 11 to 60 days (Table 1). The solids were recovered by filtration and repeatedly washed until the pH value of the supernatant had reached 7.
44H2O. The framework T atom sources were as follows: aluminium (Al powder, puriss., Kemika) aluminium hydroxide (Al(OH)3, w(Al2O3) = 63–67%, Riedel de Haën), sodium aluminate (NaAlO2, w(Al2O3) = 54%, Riedel de Haën), germanium oxide (GeO2, 99.99%, Sigma Aldrich), lithium hydroxide monohydrate (LiOH·H2O, 99%, Sigma Ultra), boric acid (B(OH)3, p.a., Kemika), boron oxide (B2O3, 99.99%, Ventron), zirconyl chloride octahydrate (ZrOCl2·8H2O, 98%, Aldrich), zirconium(IV) isopropoxide (Zr(OCH(CH3)4·(CH3)2CHOH, 99.9%, Aldrich) and magnesium oxide (MgO, 99.99%, Merck). The reactions were conducted at 180 °C for 11 to 60 days (Table 1). The solids were recovered by filtration and repeatedly washed until the pH value of the supernatant had reached 7.
| Molar oxide composition of the starting reaction mixture | Starting chemical | t c (days) | Final product |
|---|---|---|---|
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1ZnO 0.1ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
ZnO | 11 | RUB-17 |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05Al2O3 0.05Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
Powder Al | 12 | Feldspare type aluminosilicates |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05Al2O3 0.05Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
Al(OH)3 | 12 | |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05Al2O3 0.05Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
NaAlO2 | 12 | |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05B2O3 0.05B2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
B2O3 | 20 | No precipitation |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05B2O3 0.05B2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
B(OH)3 | 12 | Boron phyllosilicate |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1GeO2 0.1GeO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
GeO2 | 12 | Mixture of germanium silicates |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1MgO 0.1MgO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
MgO | 12 | Layered material |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1ZrO2 0.1ZrO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
ZrOCl2·8H2O | 25 | Amorphous |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1ZrO2 0.1ZrO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
Zr(IV) isopropoxide | 20 | 3D zirconium silicate |
1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05Li2O 0.05Li2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
LiOH·H2O | 60 | No precipitation |
1.1SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5NaOH 0.5NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5KOH 0.5KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08TEAOH 0.08TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44H2O 44H2O |
TEOS | 30 | No precipitation |
RUB-17 synthesis
Zincosilicate RUB-17 was prepared as previously described.17 The required amounts of sodium hydroxide (pellets, 97%, Acros organics), potassium hydroxide (pellets, 85%, Kemika), tetraethylammonium hydroxide (TEAOH, 35% water solution, Aldrich), a zinc source, and doubly distilled water were mixed. The employed zinc sources were zinc oxide (ZnO, 99.9%, Ventron), zinc chloride (ZnCl2, 98%, Sigma), zinc bromide (ZnBr2, purum p.a., Fluka), zinc nitrate hexahydrate (Zn(NO3)2·6H2O, p.a., Kemika), zinc acetate dihydrate (Zn(OAc)2·2H2O, p.a., Kemika), zinc acetylacetonate monohydrate (Zn(acac)2·H2O, prepared according to the previously published procedure19) and zeolitic imidazolate framework material number 8 (ZIF-8, prepared according to the previously published procedure20). Upon treatment in an ultrasonic bath for 30 minutes, tetraethoxysilane (TEOS, 98%, Aldrich) was added to the reaction mixture and hydrolyzed for 8 h at room temperature. The final molar oxide composition of the starting reaction system was 1 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 ZnO
0.1 ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 NaOH
0.5 NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 KOH
0.5 KOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.08 TEAOH
0.08 TEAOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 H2O. The reaction mixture was transferred into a Teflon lined autoclave and subjected to hydrothermal treatment at 180 °C for 11 days. The final product was recovered by filtration and repeatedly washed until the pH value of the supernatant had reached 7.
44 H2O. The reaction mixture was transferred into a Teflon lined autoclave and subjected to hydrothermal treatment at 180 °C for 11 days. The final product was recovered by filtration and repeatedly washed until the pH value of the supernatant had reached 7.
Characterization of the materials
The phase composition of the prepared solid materials was identified by X-ray diffraction (XRD) analysis of the powder samples collected by using a PHILIPS PW 1840 X-ray diffractometer (Philips Analytical B.V., Almelo, The Netherlands) with CuKα1 (λ = 1.54056 Å) radiation at 40 mA and 40 kV. The scattered intensities were measured with a scintillation counter. The samples were scanned in the range of Bragg angles 2θ = 5–50°. SEM micrographs were acquired by using a high resolution scanning electron microscope JEOL JSM-7000F. For transmission electron microscopy imaging, a small amount of the sample was dispersed in ethanol. After being treated by ultrasonication, one drop of the sample mixture was taken from the ethanol solution and transferred to a copper grid covered by a holey carbon film. A transmission electron microscope JEOL JEM-3010 was used for TEM studies. A Gatan 794 CCD camera was used for recording transmission electron microscopy images.Thermogravimetric analysis of the solid samples was performed with a Setaram Setsys TGA instrument. The heating rate was 5 °C min−1 and the samples were heated up to 800 °C in air flow. FT-IR spectra of the solid samples were recorded on a Bruker TENSOR 37 spectrometer using KBr pellets, in the range from 400 to 4000 cm−1. Raman spectra were acquired by employing a PD-LD LS2 laser source (784.2 nm, 450 mW) with optical fibers. The Raman probe (BWTEK) was coupled with a Maya 2000 Pro spectrometer (Oceanoptics). A Shimadzu UV-3600 UV/Vis/NIR spectrometer was used to obtain solid state UV-Vis spectra of the studied samples mixed with BaSO4 powder supplied by Wako Pure Chemical Industries Ltd. The weight percentage of the samples was 1–5 wt%. The spectra were recorded at 20 °C in the range from 1200 to 200 nm at a resolution of 1 nm. The instrument was linked to an integrating sphere. The background was recorded using pure BaSO4.
The Zn/Si ratio of the solid samples was determined by employing an Energy Dispersive X-Ray Fluorescence (ED-XRF) spectrometer. Powder samples were pressed into pellets with a 7 mm diameter and attached to a carbon tape prior to analysis. A rhodium X-ray tube (X-Ray Optical Systems, model X-Beam) was used for ionisation under 50 kV and 0.04 mA. Each sample was irradiated for 30 min with a beam collimated to a diameter of 2 mm and perpendicular to the sample. Characteristic X-rays were collected with a silicon-drift detector (Amptek X-123SDD, resolution of 145 eV for Fe-Kα line) positioned at 45° with respect to the incoming beam. The measurements were performed in air, with 10 mm of source to sample distance and 25 mm of target to detector distance. The resulting X-ray spectra were fitted using the Quantitative X-ray Analysis System (QXAS) software developed by the International Atomic Energy Agency (IAEA) in order to obtain characteristic line intensities.21 The Si/Zn ratios were calculated from the ratio of these intensities. The detailed description of the calculation procedure is presented in the ESI.†
1H–29Si CPMAS NMR spectra were recorded on a 600 MHz Varian NMR System equipped with a 3.2 mm Varian MAS probehead. Larmor frequency for 29Si nuclei was 119.088 MHz. Chemical shifts of the 29Si nuclei are reported relative to the signal of 29Si in tetramethylsilane. All measurements were performed at a sample rotation frequency of 20 kHz, with a relaxation delay of 2 s and CP contact time of 5 s. For the crystalline RUB-17 samples prepared using ZIF-8 and Zn(NO3)2·6H2O as zinc source chemicals 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scans were accumulated with an acquisition time of 40 ms and 80 kHz XiX decoupling. For the rest of the samples, 28
000 scans were accumulated with an acquisition time of 40 ms and 80 kHz XiX decoupling. For the rest of the samples, 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 scans were accumulated with an acquisition time of 15 ms and 90 kHz XiX decoupling.
000 scans were accumulated with an acquisition time of 15 ms and 90 kHz XiX decoupling.
Structural properties of the materials including the size and arrangement of nanovoids were analysed by grazing incidence small angle X-ray scattering (GISAXS). GISAXS measurements were carried out at Elettra synchrotron Trieste (SAXS beamline), using an X-ray beam with an energy of 8 keV and a 2D Dectris photon detector (Pilatus 1M). The measured data were simulated and analysed using GisaxStudio software.22,23 The powder materials were pressed into a thin tile before the GISAXS measurements.
Model and computational details
All simulations have been carried out using the DFT methodology. The generalized gradient approximation of the PBE functional form24 has been used, as implemented in the CP2k code.25 In this approach, a mixture of the localized atomic orbitals (SZV molopt basis set) to represent the wavefunctions and the plane wave basis set (450 eV cutoff energy) to represent the electronic density have been used.26 The initial models have been optimized to reach forces lower than 0.5 eV A−1. For each of the investigated models, the molecular dynamics simulation has been carried out in the NVT ensemble. The temperature was set to 298 K and controlled by using a Nose thermostat.27 The timestep was set to 0.5 fs and the total simulation time to 25 ps (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps). The occurrence of the reaction was ensured by the gradual change of the distance between the oxygen of the water molecule and one of the T-atoms of interest (Si, Al or Zn). The initial distance was the result of the optimization of the geometry of the system, and the final distance was the average distance between the T-atom and oxygen in the structure (∼1.60 A). The charge of the system has been set accordingly (0, −1 and −2 for Si, Al and Zn, respectively).
000 steps). The occurrence of the reaction was ensured by the gradual change of the distance between the oxygen of the water molecule and one of the T-atoms of interest (Si, Al or Zn). The initial distance was the result of the optimization of the geometry of the system, and the final distance was the average distance between the T-atom and oxygen in the structure (∼1.60 A). The charge of the system has been set accordingly (0, −1 and −2 for Si, Al and Zn, respectively).
In order to obtain a meaningful result, several parallel runs need to be executed to account for random initial velocities. For the needs of this work 20 runs have been carried out for each system; however due to the small differences in the models and similar constraints imposed – all runs have been observed to occur identically, and they led to the same results. The structure representing a computational model has been obtained from the crystal structure recovered from a .cif file available at the International Zeolite Association website.18 It consists of a 3-member ring, with none (pure silica) or one heteroatom (Al, Zn) present. It is basically half of the symmetric lov-unit. The position of the heteroatom is at the vertices of the lov-unit, not in the spiro position. The building unit was embedded in the framework represented by 4 other T sites. In order to maintain the geometry of the cluster constructed this way, the Si atoms of the framework have been saturated with hydrides, and the positions of these hydrides were kept frozen during the simulations. The model is shown in Fig. S1.† Si atoms are represented by yellow spheres, O – smaller red spheres, H – smallest white spheres. The heteroatom is represented by a pink sphere.
The model represents the fully formed building unit, at the end of the process. In other words, the reaction that is carried out in this computational work is reversed and simulates the decomposition of the ring. This approach allows for better control of the process because it always leads to the desired product, i.e. the initial geometry in the MD simulation. At the same time, the transition state structures are the same as if the process was reversed, thus allowing estimation of the energetics of the reaction. This is “de facto” a kinetic approach – under the assumption that the thermodynamics of the process is always the same because from the same reactants (open ring) the same products (closed ring + water) are obtained. The thermodynamic stability of different structures (pure silica and two with heteroatoms) cannot be compared directly because of different compositions of those systems, but for each process, ΔH can be calculated and compared with that of other systems. For each of the systems – pure silica, Al heteroatom, and Zn heteroatom, two kinds of simulations were carried out: one with the water cleaving a bond between the heteroatom and Si atom in the same position or the Si atom in the top vertex of the 3-ring. This approach allowed identifying whether one of these bonds has a different activation barrier – thus whether it is easier to cleave. This approach also allows assessing if the heteroatom is built in a vacancy (“nest”) already formed by Si sites, or actively drives the process towards this particular structure.
Results and discussion
Effect of T atoms
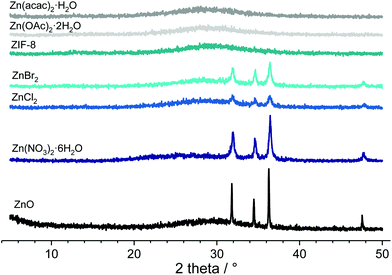
Several different T atoms (Al, B, Ge, Mg, Li, Zr) and the all-silica RSN-type material have been attempted to crystallize. These heteroatoms were selected because they were found to form three-membered ring units in various silicate systems, not always in zeolitic structures. Aluminium is the most common T atom after Si in zeolites and is present in the only 3MRs containing aluminosilicate ZSM-18.18 Germanium and boron are incorporated into three-membered rings within zeolite-type frameworks while lithium and zirconium are present in three-membered ring silicate materials,18,28,29 and magnesium favours the formation of 3MRs in silica glasses.30 The molar oxide compositions of the starting reaction mixtures and the chemicals used are similar to those of the ones employed in the RUB-17 preparation.17 The chemicals employed as the sources of T atoms, the crystallization periods (tC) and the final products are listed in Table 1. Representative X-ray powder diffraction patterns of the obtained solids are displayed in Fig. S2.† The collected data show that the RSN-type material was not obtained in any of the investigated systems. No solid product was found in the all-silica as well as in Li-containing systems even after 60 days heating period. The magnesium-containing system yielded a layered-type material. In the boron system, the precipitation depends on the starting chemical. When boron hydroxide is employed, a highly crystalline phyllosilicate material is obtained, in contrast to the boron oxide system where no solid material was found. The use of two different zirconium sources, zirconyl chloride octahydrate and zirconium(IV) isopropoxide, resulted in the formation of an amorphous material and 3D zirconium silicate, respectively. On the other hand, no matter the Al source employed (metal Al, sodium aluminate and aluminium hydroxide) the system yielded feldspar.Raman spectroscopy is a commonly used method to identify the presence of various structural features within zeolite (silicate) frameworks. It is especially convenient for detecting the small ring building units since three-membered rings exhibit bands in the region between 550 and 620 cm−1, four-membered rings from 470 to 530 cm−1 and five-membered rings from 370 to 430 cm−1. Bands in the range 290–410 cm−1 are attributed to six-membered rings while larger rings have bands below 290 cm−1.31,32 The Raman spectra of the solids prepared by employing various T atom sources are depicted in Fig. S3.† The three-membered rings characteristic of the RSN-type material were not detected in the samples prepared in Al, B and Mg containing systems. Aluminium-containing products exhibit nearly identical spectra with main bands below 550 cm−1. In the spectrum of the B(OH)3 synthesized product, there is a band with a maximum at 555 cm−1, while the MgO product has only one band at 683 cm−1. The ZrOCl2·8H2O product spectrum does not have well defined bands in the framework ring region. There is a band at 602 cm−1 in the spectrum of GeO2-containing systems. This peak could be attributed to the 3MRs in the Ge product which is not a surprise considering a large number of germanium-containing zeolite materials with 3MRs in the structure.18 Nevertheless, this system did not yield an RSN-type material. Furthermore, when Zr(IV) isopropoxide is employed, the end product features a band at 586 cm−1. This kind of material comprises 3MRs as deduced on the grounds of the reported structural data.29 However, in this silicate material, the zirconium has octahedral coordination and cannot be considered as a zeolitic type material.
Clearly, under given conditions only zinc used as a potential zeolite framework T atom together with silicon resulted in the formation of a zeolitic three-membered ring containing a RSN-type material. Thus, our attempt to replace the Zn in the RSN-type structure was not successful, which reveals its important structure-directing effect. In the following sections, we will present the results of the detailed study of the impact of the starting Zn compound on the properties of RUB-17 precursors and the final product.
Effect of the zinc source
The XRD patterns of the solids obtained with various zinc sources are depicted in Fig. 1A. The peak positions in all of the patterns coincide with the RSN framework type material. TG and dTG curves of selected solids all match well with the reference RUB-1717 meaning that the composition of the final product and the water content in the samples are nearly identical (Fig. S4†). Furthermore, the Si/Zn ratios of the materials (Table S1†) vary around the expected value of 3.5 in accordance with the particular site that zinc occupies in the RSN-type framework. The corresponding Raman spectra plotted in Fig. 1B also correlate well with the reference RUB-17 material prepared from ZnO.17 The spikes in the spectra of the solids prepared from ZnO and ZIF-8 are measurement artifacts. In each Raman spectrum of RUB-17, synthesized from various Zn sources, all four types of small rings present in the RSN framework are easily distinguished – 3MRs, 4MRs, 5MRs and 6MRs at 609, 507, 437 and 339 cm−1, respectively. 29Si MAS NMR spectra (Fig. 1C) of the final products are identical. As found previously, there is an isolated peak at −82 ppm assigned to the Si atom surrounded by two Si atoms and two Zn atoms.33 In the RSN-type framework, such an environment can only be attained in the spiro position of the lov-unit. XRD, Raman and 29Si MAS NMR data indicate the formation of a solely RSN-type material in all of the studied systems after 11 days of hydrothermal treatment regardless of the employed Zn source.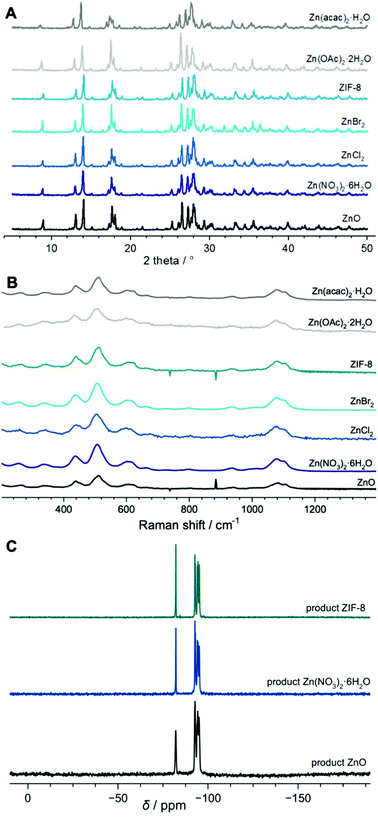 | ||
| Fig. 1 XRD patterns (A), Raman spectra (B) and NMR spectra (C) of the final products obtained after 11 days of hydrothermal treatment using different Zn sources. | ||
In the studied reaction systems, the solid and liquid phases are visibly separated as the solid is precipitated at the bottom of the autoclave. Moreover, the solids are highly aggregated forming a hard monolith. The SEM micrographs of selected representative samples reveal different crystal sizes and morphology depending on the starting Zn chemical (Fig. S5†). In the samples synthesised from ZnO and ZnCl2 the crystals have a wide size distribution ranging between 100 and 1500 nm. Nearly all of the crystals grow together building up complex aggregates of different sizes. In ZnO and ZnCl2 systems, the crystal faces are well-defined, and the edges are sharp. Their morphology is prismatic, ranging from short to long prisms. The crystals in the ZIF-8 containing system differ significantly. There are two types of crystals – very small (∼130 nm) with rounded edges and larger (∼800 nm) having sharper edges and more pronounced crystal faces. These results suggest that there is a difference in the crystal growth processes in the studied series of reaction systems.
Study of the precursors/reaction intermediates
 | ||
| Fig. 2 Powder XRD patterns of the reaction intermediate taken after 8 h of hydrothermal treatment of reaction mixtures prepared from various Zn sources at 180 °C. | ||
The chemical analysis of the solid phases was performed by XRF in order to provide information on zinc distribution in the precursors. The molar Si/Zn ratios of the precursors taken after 8 h of the hydrothermal treatment (Table 2) indicate a higher content of Zn in the solid phases of the systems prepared with ZIF-8, Zn(OAc)2·2H2O and Zn(acac)2·H2O than in the water-soluble Zn salts. This result points out that the Zn from these compounds reacts immediately with the silica species. The precursor in which ZnO was used has a higher amount of Zn as well. In this case ZnO is directly added to the reaction mixture, not precipitated from the solution of Zn salts so less ZnO was dissolved. In the systems containing water soluble zinc salts (Zn(NO3)2·6H2O, ZnCl2 and ZnBr2) there is less Zn in the solid phase in spite of ZnO precipitation. Nevertheless, the yield of the solid phase recovered after 8 h and at the end of the crystallization (Table 2) has a reciprocal trend. The soluble zinc salts yield about 5 wt% of the solid phase while ZnO and complex compounds result in an almost half lower quantity of the obtained material. Clearly, in the studied systems the precipitation of the solid phase is affected by the nature, concentration, and distribution of zinc species in the liquid phase. On the grounds of these data, it can be concluded that Zn bound with organic ligands is more prone to react with Si species. Furthermore, in the complex compound systems, the solubility of zincolilicate species is higher. Finally, it can be conveyed that, similarly to Al, Ge, Zr and Mg, under the studied reaction conditions, Zn promotes the solid phase precipitation and that the final solid phase yield is low in all systems because of the high alkalinity of the system. The fact that no precipitate has been formed in the all silica system corroborates this reasoning.
| Starting chemical | Si/ZnXRF – 8 h | Solid phase yield – 8 h (wt%) | Solid phase yield – 11 d (wt%) | TG release – 8 h (wt%) | dTG minimum – 8 h (°C) | R (Å) | σ R (Å) |
|---|---|---|---|---|---|---|---|
| ZnO | 2.01 | 2.07 | 2.31 | −14.6 | 92.4; 117.3 | 10.2 | 12.5 (2.85) |
| Zn(NO3)2·6H2O | 4.49 | 4.92 | 4.83 | −13.5 | 96.8; 119.6 | 15.6 | 4.1 (7.7) |
| ZnCl2 | 4.31 | 5.12 | 5.55 | −13.9 | 97.3; 121.5 | 8.9 | 1.53 (7.2) |
| ZnBr2 | 3.99 | 5.00 | 4.45 | −13.2 | 96.3; 121.6 | 11.6 | 2.6 (7.01) |
| ZIF-8 | 3.11 | 2.95 | 2.87 | −12.5 | —; 117.0 | 20.5 | 8.65 (6.98) |
| Zn(OAc)2·2H2O | 2.63 | 2.69 | 2.58 | −12.8 | 95.0; 116.7 | 17.6 | 9.85 (5.6) |
| Zn(acac)2·H2O | 2.76 | 2.90 | 2.91 | −12.1 | 96.1; 120.3 | 11.6 | 2.7 (7.05) |
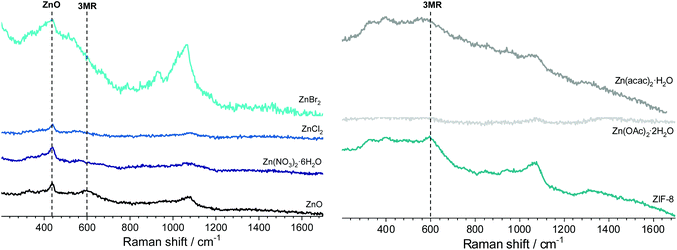 | ||
| Fig. 3 Raman spectra of the 8 h hydrothermally treated solids prepared by employing different Zn sources. | ||
According to the 29Si {1H} CP NMR spectra of the 8 h precursors depicted in Fig. 4 there is a distribution of Si species in the solid phase of the samples. Different zinc sources exhibit an impact on the Si species distribution since the shape of the curves is a function of relative contributions of different species. The spectra range from −69 to −118 ppm which indicates that in all of the samples taken in the initial stage of the reaction there is a certain amount of Si which is located in the spiro position of the lov-unit. Namely, taking into account that the 3MRs have been detected in the Raman spectra of some of the samples and that in a completely crystalline material the Si at the spiro position exhibits a peak at −82 ppm it can be deduced that the three-membered rings connected to build the lov-units contribute to the NMR spectra of the 8 h precursors in the region around −80 ppm.
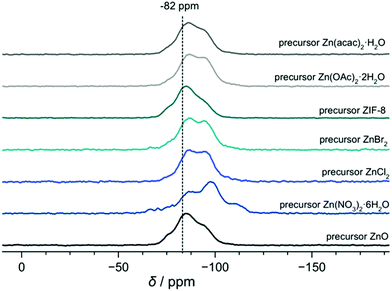 | ||
| Fig. 4 Normalized 29Si {1H} cross-polarization NMR spectra of the samples taken after 8 h of hydrothermal treatment of the reaction mixtures prepared by employing different Zn sources. | ||
Fig. S6† displays the FTIR spectra of the studied RUB-17 precursors and the crystalline RUB-17. The bands from the 1300 to 760 cm−1 region are generally assigned to the asymmetrical O–T–O stretching mode, the symmetrical O–T–O stretching mode and the T–O bending mode of the TO4 tetrahedra.34 The peak positions in the spectra of the precursors coincide with the fully crystalline RUB-17 in this region. However, these peaks are less sharp in the spectra of the precursors. Furthermore, the peaks are shifted towards higher wavenumbers with respect to RUB-17. Clearly, the types of internal tetrahedra T–O chemical bonds are identical in both the crystalline material and its precursors. Still, a significant degree of disorder is present in the precursor materials. Additional structural information (external tetrahedral linkage vibrations) can be obtained from the bands in the region below 750 cm−1 in the infrared spectra. The band around 550 cm−1 is attributed to five membered rings in the structure.34 RUB-17 does exhibit a weak band at 557 cm−1. Moreover, there are three well resolved bands at 520, 445 and 408 cm−1. Any of these bands are present in the spectra of the precursors suggesting once again that there are no well-defined five membered rings, i.e. the parts of the RSN structure larger than the lov-unit have not yet been formed. However, in this region the precursors exhibit broad noisy bands ranging from 540 to 400 cm−1 which indicates that there are intertetrahedral bonds similar to the ones in the crystalline RUB-17 material.
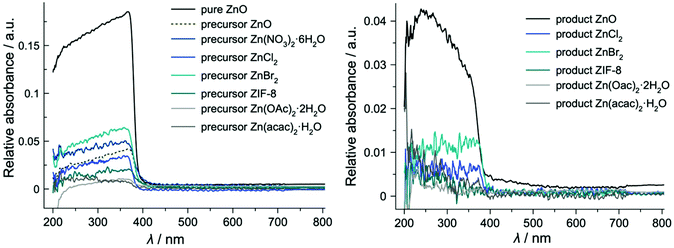
UV/Vis spectral curves of the solid 8 h precursors (Fig. 5) have the same trend as the commercial zinc oxide used as a starting compound in the ZnO-containing system. Likewise, they all exhibit a maximum at 365 nm and have an absorption edge value at 390 nm. However, the intensities of the spectra are significantly lower than those of the pure commercial ZnO. Besides, the spectra of the precursors differ substantially from the spectra of the starting Zn compounds both in the curve shape and intensity (Fig. S7†). RSN-framework materials prepared in this study have an absorption edge position at 390 nm similar to both the precursors and zinc oxide. Although the bands in the spectra of the end products appear in the same wavelength range, there is a difference in their intensity which has been attributed to measurement execution conditions. The ZnO end product exhibits a solid state UV/Vis curve oriented into the opposite direction to the precursor materials with the maximum positioned at 250 nm. These sets of data suggest that the coordination of Zn differs in crystalline RUB-17 from the Zn coordination in the 8 h precursors. The surroundings of Zn in the precursors are more similar to the zinc oxide material than RUB-17. Furthermore, this represents a strong indication that zinc undergoes three states of coordination during RUB-17 synthesis: (1) the coordination in the starting compound; (2) ZnO-like coordination; (3) coordination in the RSN-type framework. It seems that the second coordination is essential for the formation of RUB-17 since it has been detected in all systems. In addition, the infrared and UV/VIS spectroscopy findings suggest that indeed there are similar chemical bonds (building units) in all of the studied 8 h precursors thus substantiating the conclusion that there are lov-units present in these samples.
Thermogravimetric analysis
TG curves of the solid phases taken after 8 h of hydrothermal treatment are plotted in Fig. S8.† They follow the same trend, but the total weight loss differs from one sample to another (column TG release – 8 h (wt%), Table 2). Although the differences are small, the general trend shows higher weight loss in soluble salt precursors which is due to more water present in the samples. Considering a higher water content in precursors comprising precipitated ZnO which have a higher Si content and yield higher quantity of the solid phase, it can be concluded that indeed there are more silanol groups in precursors prepared from ZnBr2, ZnCl2 and Zn(NO3)2·6H2O. Obviously, the solid phase formed by the coprecipitation of Zn coming from dissolved complex compounds and Si species has lower affinity towards water due to the high number of Zn–O–Si chemical bonds. In a fully crystalline RUB-17 material the quantity of water is lower since there are fewer silanol bonds in the crystalline material.17 In the lower temperature regions, the dTG curves exhibit a similar shape. Indeed, it is typical of zeolites and zeolite-like materials to release the water below 200 °C and the position of the dTG minimum provides information on the strength of the water bonding.16 In the studied set of samples the minimums are found at very similar temperatures (Table 2), which indicates that the water in all samples is bonded with approximately the same strength and consequently that there are similarities in the structure of the solids. This supports the spectroscopic data which show the presence of nearly identical types of chemical bonds in all the precursors.TEM microscopy
In order to obtain more insights into the local structure of RSN precursors, a TEM study of the 8 h precursors recovered from the ZnO and Zn(NO3)2·6H2O reaction mixtures was performed (Fig. 6). The gel particles exhibit a porous structure with the spherical voids distributed throughout the particles. The pore size is not uniform, and the dimensions vary from very small to rather large voids. Furthermore, there is a difference between the sizes of the voids in the two samples. Porous precursor gel particles have been found in many aluminosilicate zeolite yielding systems containing high concentrations of alkali cations.35–37 The appearance and development of voids have been identified as an essential stage of the nucleation process and consequently crucial for the zeolite formation. As far as we know, this is the first report on the observation of voids within gel particles in zincosilicate precursors yielding a zeolite-type material.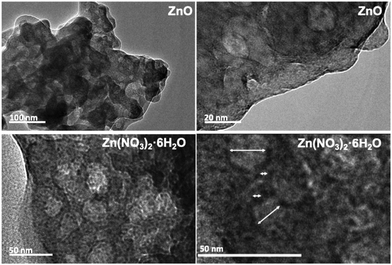 | ||
| Fig. 6 TEM images of the 8 h precursor samples prepared using different Zn sources. Arrows denote the dimensions of the voids. | ||
GISAXS study
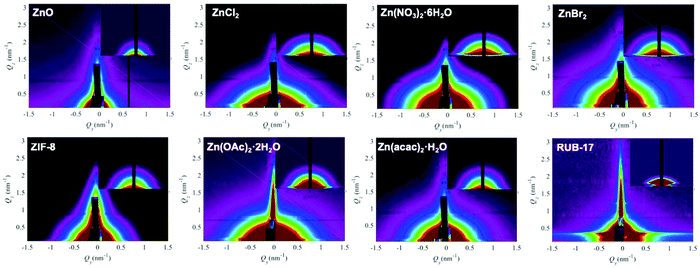
GISAXS was employed to gain a deeper insight into the voids present in the samples. The amorphous precursors exhibit very low micropore (and mesopore) volumes compared to the fully crystalline zeolite.38–40 Melinte et al. performed a very detailed analysis of gel particles containing such voids and found that they contain a shell which is impermeable for N2.35 The majority of the mesopores exhibit a diameter between 3 and 20 nm, with more than 40% of the pores having a diameter between 5 and 7 nm. Consequently, any gas adsorption method could provide reliable information on the inherent porosity of the zeolite precursor samples due to the nonporous zone covering gel particles. Therefore, we employed GISAXS analysis, which provides nanoscale information on the structure of the material. GISAXS maps obtained for the studied samples are displayed in Fig. 7. All maps show semi-circular intensity distribution with different characteristic radii. The maps show no correlation peaks that could be related to the presence of ordered nanostructures in the materials, so a simple model including a spherical shape of the nanovoids was used to fit the experimental data. Such approximation is supported by the TEM findings. The parameters of the fit were the radius of the voids (R) and the size distribution parameter σR representing the standard deviation of the distribution. The gamma distribution of the void sizes was used for the fit. The measured GISAXS range (Qy,z ∼0 to 3 nm−1) is suitable for the analysis of void sizes of a few nanometres. The GISAXS signal of larger voids appears at small Q values where it is superimposed to the surface roughness contribution, which is not taken into account in the present GISAXS analysis. Therefore, the measurement is more sensitive to smaller structures that are visible in the TEM images (Fig. 6), not to the large ones (R > 10 nm). The parameters obtained from the fit to describe the collected GISAXS patterns are listed in Table 2, columns R and σR. In the precursor samples, the radii range from 8.9 Å to 21 Å. This diameter ranging from 2 to 4 nm is comparable with the dimensions of the smallest pores observed in TEM images as well as with the void size observed in the EMT-type aluminosilicate zeolite precursor.35 Voids with a similar size were detected even in the completely crystalline RUB-17. These voids most probably originate from the texture of the agglomerated crystals.In addition, the applied model enabled calculating the pore size distribution for each sample (Fig. 8). The data indicate that the distribution curves differ depending on the employed zinc source. Clearly, the overall chemical composition of the reaction mixture determines the structure of the gel particles.
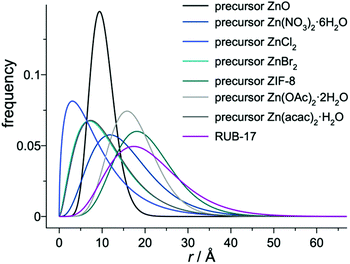 | ||
| Fig. 8 Calculated spherical void radii distribution curves for the 8 h precursors prepared using various zinc sources. | ||
DFT modelling of the formation of the lov-unit
The formation of the lov-unit is a complex process and therefore different possibilities have to be considered. The formation of the T–O–T bridges is the two-step process, with the first being the formation of the T–O bond leading to the 5-coordinated T site, and the other being a water release.41 Depending on the type of the oligomer and its composition, either of the steps can be a limiting one in the reaction. It has to be underlined that for the pure silica system the final configuration was still a 5-coordinated Si atom, and the Si–O bond cleavage was not observed. This means that the thermodynamics of this process could not be correctly evaluated; however as the product of two pathways was always the same – the thermodynamics does not play a role in the determination of the mechanism.Two options have been considered for the formation of the lov-unit by the direct ring closure – one route (route 1) depends on the fully formed ste-unit (defined by the IZA Structure Commission),18 and assumes that the oxygen bridge is already formed between two Si sites in the ste-unit, and ring closure yields the lov-unit. The other route (route 2) assumes the opposite – the lov-unit is already formed, and upon the reaction, it gets embedded on the surface yielding a ste-unit. For the sake of comparison, the pure silica system, with a Si atom in place of the heteroatom, has also been considered and will be discussed first.
The energy profile of the reaction with the 5-coordinated Si atom at the ste (route 2) with the water molecule attached is not shown, yet the formation of this state is via the negligible barrier of 10 kJ mol−1, and the subsequent step of water release is spontaneous and stabilizes the system by 68 kJ mol−1 with respect to the 5-coordinated intermediate. Note that this intermediate is additionally stabilized by the hydrogen bond between the water and the oxygen of the top Si atom. The final product, the 3MR, is thermodynamically stable. Expectedly, it is similar to the route 1 pathway, because both these systems contain 5-coordinated Si atoms, thus are chemically similar and differ only in the constraints. This result leads to the conclusion that once the ste-unit is formed, the ring closure is a relatively easy and feasible process.
The situation is very similar for the Al system; however for pathway 1, the reaction is thermoneutral. There is no significant thermodynamic driving force, and additionally, the kinetic barrier is relatively high, compared to other investigated systems (87 kJ mol−1). This allows us to conclude that the formation of the lov-unit with the Al atom is unlikely. On the other hand, the formation of the ste-unit with the Al heteroatom should be considered an easy process. The process is exothermic by 57 kJ mol−1 and is accompanied by the barrier of only 47 kJ mol−1. The only problem in this process is that it assumes that the lov-unit is already formed in a reaction similar to pathway 1, which is much more difficult. The formation of the 3-membered ring with the Al atom in one T-site is thus the limiting step of the process of Al-containing RSN-type material formation.
The system containing zinc behaves similarly in both investigated pathways as shown in Fig. S9,† and in contrast to all other systems reaction occurs in one concerted step (indeed, the water release in Si and Al systems is very easy, but still it is an activated process). Both pathways are only slightly exothermic (approximately 25 kJ mol−1) with a low activation barrier of 40 kJ mol−1. In addition, in contrast to the Al and Si systems, the 5-coordinated Zn atom has not been observed, and rather the Zn–O bond cleavage occurs easier, leading to the Zn in the planar geometry.
All results suggest that the Zn significantly facilitates the formation of 3MRs. However both routes – either the embedding the 3MR into the growing crystal and 3MR closure at the surface – are equally probable. On the other hand – the small barriers suggest that the preparation process should occur much faster than that observed experimentally. This suggests that there is another rate limiting step during the synthesis.
Discussion
There is large interest to prepare RSN-type zeolite materials due to the presence of nine-membered rings which offer new possibilities in zeolite application. So far, the only representative material having this framework is zincosilicate RUB-17. In the current investigation we have attempted to introduce several possible T-atoms (Al, Ge, B, Mg, Li, Zr) into the framework of the RSN-type zeolite material by employing identical reaction conditions as for the preparation of zincosilicate RUB-17. These elements were selected because they can build three-membered rings in silicate systems and because the three-membered rings were found in most zeolite structures comprising odd-numbered pores. Yet, RSN-type or any other zeolite-type material has not been prepared under studied conditions when before-mentioned T atoms were employed. Moreover, the reaction mixture comprising solely Si did not yield any solid precipitate, let alone the zeolite-type material. On the other hand, no matter which zinc initial chemical was used, the RSN-type zeolite material was always produced indicating the vital role of zinc in the formation of this material. On that account, the 8 h precursors have been isolated and subjected to thorough characterization to provide more data on RSN-type material formation.There is a difference between the 8 h precursors prepared from the soluble zinc salts and complex zinc compounds in terms of their phase composition as revealed by XRD and Raman spectroscopy. Namely, zinc oxide precipitates upon the addition of hydroxide into zinc salt solutions, while in the complex compound systems zinc is coordinated with different ligands which prevent ZnO precipitation. This further reflects in the chemical composition of the solid and liquid phase as well as in the solid phase yield. When ZnO is formed, the silicate precipitate is favoured and consequently the Si/Zn ratio and the quantity of the solid phase are higher. Using complex zinc compounds leads to precipitation of less material of higher zinc content. As the reaction advances, the Si/Zn ratio of the solid changes until it reaches the final value of 3.5 in the crystalline material. However, the amount of the materials recovered at the end of the reaction is very similar to that in the 8 h samples. These data suggest that in the preparation of RUB-17 the properties of the Zn source and the equilibrium between the Zn species in the liquid phase and solid phase play an important role in the overall reaction mechanism. It can be assumed that both Zn species from both the solid and liquid phases react with Si species to form RUB-17, but it seems that the liquid phase mediated processes are the dominating ones.
Based on the spectroscopic measurements (NMR, IR, Raman, UV/Vis) and TG analysis it can be deduced that there are similar chemical bonds in the studied series of samples, yet the distribution of the present species is different. The results suggest that there are three-membered rings connected to form lov-units in all 8 h precursor samples. These data indicate that although lov-units are formed during the initial phase of the zeolite formation (8 h), the subsequent processes of establishing a long range order and assuming needed Zn coordination are rather complex and consequently it takes a longer time for the formation of an entirely crystalline material (11 d).17 A possible explanation could be an early, yet rapid precipitation of the solid phase which has a limited surface area exposed to the liquid phase. Namely, after 8 h of hydrothermal treatment at 180 °C the solid yield is quite low (less than 6 wt% in all of the systems – Table 2) and there are already basic building units present but the reaction is still long. Obviously the frequency of successful chemical reactions in terms of RSN-type framework building is low once the solid phase gets precipitated. This once again leads to the conclusion that in the studied system the solution mediated processes prevail over solid phase rearrangement.
TEM images of the selected samples revealed the presence of spherical voids within the precursor particles. The voids were further probed by GISAXS. Despite not being quantitative, the pore size distribution varies with the employed zinc source thus showing that the chemical composition of the reaction mixture directly affects the gel structure. The core–shell structure of zeolite precursor particles with a porous core was observed in aluminosilicate synthesis systems with a high concentration of alkali cations.35 It was concluded that the mother liquor is entrapped inside the pores due to the impermeable shell and that the onset of zeolite nucleation takes place at the shell part. Consequently, the external surface of the initial gel particles governs the entire crystallization process. If this reasoning is applied to the zincosilicate system studied herein, it can explain the formation of highly intergrown crystals in the end product. Taking into consideration that the reaction is completed after a long period of time, in spite of the structure building units formed in the early stage of the reaction, the external surface area of the precursor particles exposed to the liquid phase should be low or the number of RSN-type nuclei able to grow is small. Finally, the zincosilicate zeolite precursors have a structure similar to aluminosilicate zeolite precursors and obviously the events taking place at the molecular level direct the final product.
The experimental findings in this work are corroborated with the theoretical investigations. Namely, the results of the DFT modelling suggest that the limiting step in the system where Al would be located in one of the T sites is the three-membered ring formation. On the other hand, Zn strongly favours the 3MR formation regardless of the process pathway. Moreover, the low barrier energy values indicate that the overall RUB-17 crystallization should take place more rapidly than it, in fact, does which once again leads to the conclusion that there is some still unknown process which determines the RUB-17 formation. Since a variety of zinc sources has been studied herein in terms of the zinc state and chemical bonds, it can be deduced that the observed effect does not arise due to the differences in the availability of Zn neither various anions present in the system. Nevertheless, considering that there are lov-units in the initial stage of the reaction, yet Zn has ZnO-like coordination, it can be argued that the Zn coordination change is the limiting step here. However, the finding of the exact reason will be pursued in further studies.
Conclusion
The set of complementary experimental techniques employed in this study provided new insights into the formation of the RSN-type material. We were able to prepare the RSN-type material exclusively from Zn containing systems under the studied reaction conditions. Moreover, seven different Zn chemicals having different chemical bond natures were used and RUB-17 was obtained in each reaction system. For comparison, in the “classical” aluminosilicate zeolite synthesis systems the end product and its properties are substantially determined by the T atom source, particularly the silicon source.42,43 Obviously, the ability of zinc to bind silicon in this specific way is so strong that the reaction takes place no matter the stability and solubility of the starting Zn compound. The collected results, both experimental and theoretical, indicate that the Zn notably facilitates the formation of the three-membered rings which are formed in the very early stage of the reaction. Conversely, the whole reaction is rather long suggesting that there are some other processes which impact the RUB-17 crystallization. Indeed, it appears that in this case there is a genuine structure directing effect of zinc atoms.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support from the Croatian Academy of Science is gratefully acknowledged. M. M. thanks Croatian Science Foundation, project number O-2594-2018. B.Sz. would like to acknowledge the support from Polish Ministry of Science and Higher Education for statutory subsidy activity for Faculty of Chemistry at Wrocław University of Science and Technology for the year 2019. D. M. and M. Č. were supported by the University of Rijeka under the project number 13.2.1.3.04. The authors would like to thank Dr Željka Petrović for collecting SEM images.References
- J. B. Nagy, P. Bodart, I. Hannus and I. Kiricsi, Synthesis and use of zeolitic microporous materials, DecaGen, Szeged-Szõreg, 1998 Search PubMed.
- C. J. Rhodes, Annu. Rep. Prog. Chem., Sect. C, 2007, 103, 287–325 CAS.
- M. Reháková, S. Čuvanová, M. Dzivák, J. Rimár and Z. Gaval'ová, Curr. Opin. Solid State Mater. Sci., 2004, 8, 397–404 Search PubMed.
- J. Perić, M. Trgo, N. Medvidović Vukojević and I. Nuić, Sep. Sci. Technol., 2009, 44, 3113–3127 Search PubMed.
- A. Dyer, Introduction to Zeolite Science and Practice, ed. J. Čejka, H. van Bekkum, A. Corma and F. Schüth, Elsevier, 3rd revised edn, 2007, pp. 525–553 Search PubMed.
- B. Bogdanov, D. Georgiev, K. Angelova and Y. Hristov, Proceedings of the International Scientific Conference “Economics and Society development on the Base of Knowledge”, ed. S. Atanasova, G. Kostov, V. Mileva and T. Ivanova, Union of Scientists – Stara Zagora, Stara Zagora, 2009, vol. 4, pp. 1–5 Search PubMed.
- P. A. Wright and M. Lozinska, Zeolites and Ordered Porous Solids: Fundamentals and Applications, ed. C. Martínez and J. Pérez-Pariente, Editorial Universitat Politècnica de València, Valencia, 2011, pp. 1–36 Search PubMed.
- W. Vermeiren and J.-P. Gilson, Top. Catal., 2009, 52, 1131–1161 CAS.
- A. Vasile and A. M. Busuioc-Tomoiaga, Mater. Res. Bull., 2012, 47, 35–41 CAS.
- R. Bai, Q. Sun, N. Wang, Y. Zou, G. Guo, S. Iborra, A. Corma and J. Yu, Chem. Mater., 2016, 28, 6455–6458 CAS.
- P. Chlubná, W. J. Roth, H. F. Greer, W. Zhou, O. Shvets, A. Zukal, J. Čejka and R. E. Morris, Chem. Mater., 2013, 25, 542–547 Search PubMed.
- V. Valtchev, E. Balanzat, V. Mavrodinova, I. Diaz, J. El Fallah and J.-M. Goupil, J. Am. Chem. Soc., 2011, 133, 18950–18956 CAS.
- R. Martínez-Franco, C. Paris, M. E. Martínez-Armero, C. Martínez, M. Moliner and A. Corma, Chem. Sci., 2016, 7, 102–108 Search PubMed.
- D. P. Serrano, J. M. Escola and P. Pizarro, Chem. Soc. Rev., 2013, 42, 4004–4035 CAS.
- Z. Qin, J.-P. Gilson and V. Valtchev, Curr. Opin. Chem. Eng., 2015, 8, 1–6 Search PubMed.
- A. Palčić, B. Subotić, V. Valtchev and J. Bronić, CrystEngComm, 2013, 15, 5784–5791 Search PubMed.
- A. Palčić, F. Zapata Abellán, A. Vicente, C. Fernandez, V. Georgieva, J. Bronić and V. Valtchev, CrystEngComm, 2015, 17, 7063–7069 Search PubMed.
- http://www.iza-online.org (accessed 17.4.2019.).
- J. A. Walmsley and F. Walmsley, Chemical Principles, Properties, and Reactions in the Laboratory, Addison-Wesley, Reading, MA, 1985, 180–182 Search PubMed.
- T. Friščić, I. Halasz, P. J. Beldon, A. M. Belenguer, F. Adams, S. A. J. Kimber, V. Honkimäki and R. E. Dinnebier, Nat. Chem., 2013, 5, 66–73 Search PubMed.
- P. Van Espen, K. Janssens and J. Nobels, Chemom. Intell. Lab. Syst., 1986, 1, 109–114 CAS.
- http://homer.zpr.fer.hr/gisaxstudio/doku.php (accessed 17.4.2019.).
- M. Buljan, N. Radić, S. Bernstorff, G. Dražić, I. Bogdanović-Radović and V. Holý, Acta Crystallogr., Sect. A: Found. Crystallogr., 2012, 68, 124–138 CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CAS.
- http://www.cp2k.org/ (accessed 17.4.2019.).
- J. VandeVondele and J. Hutter, J. Chem. Phys., 2007, 127, 114105 Search PubMed.
- S. Nosé, J. Chem. Phys., 1984, 81, 511–519 Search PubMed.
- S. H. Park, P. Daniels and H. Gies, Microporous Mesoporous Mater., 2000, 37, 129–143 CAS.
- D. M. Poojary, A. I. Bortun, L. N. Bortun and A. Clearfield, Inorg. Chem., 1997, 36, 3072–3079 CAS.
- S. Kohara, J. Akola, H. Morita, K. Suzuya, J. K. R. Weber, M. C. Wilding and C. J. Benmore, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(36), 14780–14785 CAS.
- P. K. Dutta, D. C. Shieh and M. Puri, Zeolites, 1988, 8, 306–309 CAS.
- Y. Yu, G. Xiong, C. Li and F.-S. Xiao, Microporous Mesoporous Mater., 2001, 46, 23–34 CAS.
- M. A. Camblor and M. E. Davis, J. Phys. Chem., 1994, 98, 13151–13156 CAS.
- C. Li and Z. Wu, Handbook of Zeolite Science and Technology, ed. S. M. Auerbach, C. A. Carrado and P. K. Dutta, Marcel Dekker, New York, 2003, pp. 423–515 Search PubMed.
- G. Melinte, V. Georgieva, M.-A. Springuel-Huet, A. Nossov, O. Ersen, F. Guenneau, A. Gedeon, A. Palčić, K. N. Bozhilov, C. Pham-Huu, S. Qiu, S. Mintova and V. Valtchev, Chem. – Eur. J., 2015, 21, 18316–18327 CAS.
- V. Valtchev, S. Rigolet and K. N. Bozhilov, Microporous Mesoporous Mater., 2007, 101, 73–82 CAS.
- V. Valtchev and K. N. Bozhilov, J. Am. Chem. Soc., 2005, 127, 16171–16177 CAS.
- S. Inagaki, K. Thomas, V. Ruaux, G. Clet, T. Wakihara, S. Shinoda, S. Okamura, Y. Kubota and V. Valtchev, ACS Catal., 2014, 4, 2333–2341 CAS.
- L. Itani, K. N. Bozhilov, G. Clet, L. Delmotte and V. Valtchev, Chem. – Eur. J., 2011, 17, 2199–2210 CAS.
- D. P. Serrano, R. van Grieken, M. E. Davis, J. A. Melero, A. Garcia and G. Morales, Chem. – Eur. J., 2002, 8(22), 5153–5160 CAS.
- T. T. Trinh, A. P. J. Jansen and R. A. van Santen, J. Phys. Chem. B, 2006, 110(46), 23099–23106 CAS.
- T. Antonić Jelić, B. Subotić, V. Kaučič and R. W. Thompson, Stud. Surf. Sci. Catal., 1999, 125, 13–20 Search PubMed.
- M. L. Occelli, S. Biz, A. Auroux and G. J. Ray, Microporous Mesoporous Mater., 1998, 26, 193–213 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9qi00433e |
| This journal is © the Partner Organisations 2019 |