Open Access Article
Open Access ArticleNovel monomers in radical ring-opening polymerisation for biodegradable and pH responsive nanoparticles†
Jenny
Folini
 a,
Chao-Hung
Huang
b,
James C.
Anderson
a,
Chao-Hung
Huang
b,
James C.
Anderson
 b,
Wolfgang P.
Meier
b,
Wolfgang P.
Meier
 a and
Jens
Gaitzsch
a and
Jens
Gaitzsch
 *ac
*ac
aDepartement Chemie, Universität Basel, Mattenstrasse 24a – BPR 1096, 4058 Basel, Switzerland
bDepartment of Chemistry, University College London, 20 Gower Street, London WC1H 0AJ, UK
cLeibniz-Institut für Polymerforschung Dresden e.V., Hohe Strasse 6, 01069 Dresden, Germany. E-mail: gaitzsch@ipfdd.de
First published on 24th September 2019
Abstract
Responsive and biodegradable nanoparticles are essential for functional drug delivery systems. We herein report the first pH sensitive polyester from radical ring-opening polymerisation of novel amine-bearing cyclic ketene acetals (CKAs). The CKAs were synthesised via an intermediate carbonate and the resulting polyesters showed a pKa around pH 6. Together with an initial application in biodegradable nanoparticles, they open the pathway for a new generation of functional polyesters.
Amphiphilic block-copolymers are the basis of modern research into nanoparticles (NPs) such as micelles or vesicles that can act as nanoreactors and drug delivery systems.1–4 Materials originating from radical polymerisation (RP) include a number of pH sensitive hydrophobic blocks. For drug delivery systems, pH sensitivity towards protonation in the pH range 4–6 is desired in the final NPs. The range is beneficial, because during endosomal uptake into cells, the pH around the NPs drops from 7.4 in the cytosol to below 4 in lysosomes.5–7 Such pH sensitivity originates almost exclusively from tertiary amines in the side chain of the hydrophobic block of the amphiphilic block-copolymers. The tertiary amines are protonated upon acidification and turn the hydrophobic block into a hydrophilic one. This causes the NPs to disassemble and release any encapsulated content.1,4,8,9 Polymers from RP bear an all-carbon main chain, rendering them non-biodegradable, which is a disadvantage. Ring-opening polymerisation (ROP) yields biodegradable polymers such as polyamides or polyesters,10–13 but does not tolerate amines. A chain-growth polymerisation that delivers both pH sensitivity and biodegradability is required if NPs for drug delivery are to have both these desirable properties. Poly(β-aminoesters) do combine both properties, but are only available from polycondensation, which strongly limits their use in block-copolymers.14 Radical ring-opening polymerisation (RROP) from cyclic ketene acetals (CKAs)15–17 is a chain-growth polymerisation with the potential to yield the desired polymers. In RROP, a radical opens the cyclic monomer and the original acetal functionality is transformed into a biodegradable polyester.18–24 CKAs have been transformed into homopolymers and statistical copolymers with vinyl acetates to introduce biodegradable bonds into the vinylic or methacrylic main chain.25–27 In earlier work from our group, polyesters from RROP-homopolymerisation were introduced into amphiphilic block-copolymers to yield biodegradable NPs.28 RROP is currently only known to form non-pH responsive polyesters, giving it no decisive advantage over ROP.29 However, due to its radical nature, RROP has the potential to tolerate amines and give pH sensitive biodegradable NPs (Fig. 1).
In order to unlock the full potential of RROP, we aimed to synthesise amine-bearing CKAs and to transform them into pH sensitive polyesters that are inaccessible by RP or ROP. Starting from two diols (1a and 1b), we synthesised two previously unknown CKAs with tertiary amines, 6-iso-propyl-4,8-dimethyl-2-methylene-1,3,6-dioxazocane (i-DMMAC, 2a, Fig. 1) and 6-iso-propyl-2-methylene-1,3,6-dioxazocane (i-MAC, 2b, Fig. 1). Both were polymerised into pH sensitive biodegradable polyesters for an initial study of biodegradable and pH-sensitive NPs (Fig. 1).
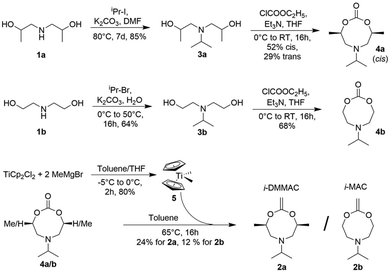
To ensure that the amine could not induce instability, or be separated from the polymer by elimination or substitution, we decided that the tertiary amine should be in the main chain of the polyester. This required a CKA bearing a tertiary amine within its ring and in turn required the starting diols 1a and 1b to have a secondary amine in their main chain. Protonated poly(diisopropylaminoethyl methacrylate) (PDPA) has a pKa of about 6–6.5 in water, and is widely used in NPs for drug delivery.30–32 To mimic this physical property, we synthesised the N-isopropyl derivatives 3a and 3b.
Alkylation of bis(2-hydroxypropyl)amine (1a) with 2-iodopropane gave the tertiary amine 3a in 85% yield. Multiple attempts at the traditional ring-closure to form CKAs via an intermediate haloacetal17,23,28 failed. Our recently published route via an intermediate cyclic carbonate28 proved to be successful. Formation of the cyclic carbonate with ethyl chloroformate gave 4a as a crude mixture of cis- and trans-diastereomers. To investigate any difference in reactivity of the corresponding diastereomeric CKAs in the polymerisation, the individual major cis-(52%) and minor trans-(29%) diastereomers were separated by standard silica gel chromatography. Olefination28,33 of the major cis-4a with freshly prepared Petasis reagent gave CKA 2a as a liquid in 24% yield (i-DMMAC, Fig. 2). Attempted olefination of trans-4a under identical conditions gave an undefined complex mixture, in which the CKA could not be detected by NMR. The non-methylated analogue amine-bearing CKA 2b was synthesised to investigate the influence of the methyl groups on the polymerisation (see mechanism in section 3b of the ESI†). The synthesis of 2b followed a similar pathway to that for 2a. The reaction conditions for the N-alkylation had to be optimised and used 2-bromopropane in water, giving the tertiary amine 3b in 64% yield. Conversion of 3b into the achiral cyclic carbonate 4b (68%) and subsequent treatment with freshly prepared Petasis reagent (5) gave i-MAC 2b, as a liquid in 12% yield (Fig. 2). The low yields for both CKAs reflected their lower stability compared to CKAs without amines, which also resulted in a lower shelf-life (typically 2 weeks at −20 °C under argon).
Both novel CKAs 2a and 2b were then subject to RROP polymerisation. Instead of the intended ring opening, CKAs can remain closed and the acetal-radical can propagate the polymerisation. The percentage of ring-opening in all polymers was assessed by 13C-NMR spectroscopy (reported to the nearest 5%; see section 3b of the ESI†) and despite investigating several conditions (Fig. 3), i-DMMAC 2a did not polymerise in the presence of different radical initiators and solvents. With azo-bis-isobutyronitrile (AIBN, 6) as the initiator, small amounts of solvent (50 μL for 200 μL of CKA) had to be added to dissolve AIBN. Ethyl acetate, toluene and chlorobenzene as solvents gave no polymerisation at various temperatures. All 1H-NMR spectra of the post-reaction mixtures contained the prominent methylene peak of the CKA (section 2i of the ESI†). Changing the reaction conditions to photo-initiated RROP34 with benzoin methyl ether (7, BME, Fig. 3) as initiator, yielded a solid material and GPC verified the formation of an oligomer (see section 3a of the ESI†). Although the material exhibited pH sensitive behaviour around pH 5 (section 4b of the ESI†), the suggested structure of the polyester could not be proven by 1H-NMR and 13C-NMR. Furthermore, BME-initiated photo-polymerisation did not allow the use of a PEG macroinitiator, hindering the production of amphiphilic block-copolymers for NPs. The second amine-bearing CKA, i-MAC 2b, did polymerise using AIBN 6 as an initiator with small amounts of toluene as solvent (50 μL, Fig. 3). Classic pH titration of poly(i-MAC) proved the pH sensitivity of the polyester and showed a pKa of 6.0 (inflection point in Fig. 4a), which is similar to the pKa of PDPA.7 The titration also confirmed the precipitation of the polymer upon deprotonation as it switched from hydrophilic (blue in Fig. 4a) to hydrophobic (red in Fig. 4a). A ring-opening content of 70% (by 13C NMR; see sections 2l and 3b of the ESI† for structure and calculations) now allowed for the synthesis of amphiphilic block-copolymers for biodegradable and pH-sensitive NPs.
Poly(ethylene glycol) (PEG12) was the set hydrophilic block as it could be transformed into a macroinitiator based on AIBN for free radical polymerisation.28 It was combined with two different hydrophobic blocks; one was a pure polymerised i-MAC (P(i-MAC)) and one was a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of i-MAC 2b with 4,7-dimethyl-2-methylene-1,3-dioxepane (8) (DMMDO, Fig. 3). DMMDO was already a known CKA for RROP and was added to compare the degradation behaviour of pure P(i-MAC) in the hydrophobic block to a statistical copolymer with P(DMMDO). GPC of both block co-polymers (PEG-P(i-MAC), 70% ring-opening) and (PEG-P(DMMDO-stat-i-MAC), 100% ring-opening) revealed a short hydrophobic block, confirming a successful reaction (Fig. 3). Short hydrophobic blocks were expected due to the high termination rate, which is typical for RROP.15,28 Impurities of homopolymers, especially from PEG, were still present. This was not necessarily detrimental as PEG homopolymers are known to help in nanoparticle formation in some cases.35,36 The two amphiphilic block-copolymers allowed for an initial study of NP formation and their biodegradation.
2 mixture of i-MAC 2b with 4,7-dimethyl-2-methylene-1,3-dioxepane (8) (DMMDO, Fig. 3). DMMDO was already a known CKA for RROP and was added to compare the degradation behaviour of pure P(i-MAC) in the hydrophobic block to a statistical copolymer with P(DMMDO). GPC of both block co-polymers (PEG-P(i-MAC), 70% ring-opening) and (PEG-P(DMMDO-stat-i-MAC), 100% ring-opening) revealed a short hydrophobic block, confirming a successful reaction (Fig. 3). Short hydrophobic blocks were expected due to the high termination rate, which is typical for RROP.15,28 Impurities of homopolymers, especially from PEG, were still present. This was not necessarily detrimental as PEG homopolymers are known to help in nanoparticle formation in some cases.35,36 The two amphiphilic block-copolymers allowed for an initial study of NP formation and their biodegradation.
The NPs were formed by basifying (with 0.1 M NaOH) a homogeneous acidic (pH 5.0) solution of each polymer. The addition of acid (0.1 M HCl) triggered a pH dependent disassembly of the NPs, proving their pH-sensitivity. The disassembly process was monitored by the count rate of dynamic light scattering (DLS) as a function of pH. PEG-P(i-MAC) showed a continuous decay starting from pH 7, indicating that the disassembly started around the pKa value of P(i-MAC) (see section 4c of the ESI†). NPs from PEG-P(DMMDO-stat-i-MAC) disassembled slightly below pH 4 (Fig. 4). Since only a third of the hydrophobic block is composed of the pH sensitive P(i-MAC), a greater acidity was needed to achieve the same overall charge required to trigger a disassembly of the NPs. DLS traces, especially of intermediate stages, were multimodal (Table S1 in the ESI†), suggesting a diverse degradation process, which will be addressed in detail in future studies.
With pH sensitivity demonstrated, biodegradability was investigated by adding 2 wt% esterase (porcine liver) to the NPs in phosphate buffer saline (PBS). DLS of NPs of both block-copolymers showed a brief increase in scattering intensity before decaying rapidly (Fig. 4). This has been reported for a similar system.28 When the esterase started digesting the block-copolymers, we postulate that the first ester bond attacked was the one between PEG and the polyester. The exposed hydrophobic polymers then agglomerated and led to a brief period where larger NPs scattered the light with a greater intensity. NPs from PEG-P(i-MAC) degraded considerably more slowly than ones from the copolymer with DMMDO (10 h instead of 90 minutes; see section 4d of the ESI†). Adding DMMDO was thus beneficial for the esterase-based degradation process. The partially multimodal DLS traces (Table S1 of the ESI†) suggested that the NPs had large size distribution, which was consistent with the broadly dispersed polymer from the free radical polymerisation. A more controlled polymerisation needs to be developed in order to obtain NPs with a narrower size distribution, which are required for a biomedical or pharmaceutical application as a drug-delivery system. These initial results show the formation of the first pH sensitive and biodegradable NPs from a polymer formed from RROP.
Conclusions
Two new CKAs 2a,b containing tertiary amines were synthesised via our recently reported carbonate route.28 The intermediate carbonates were transformed into the CKAs using the Petasis reagent. Both CKAs mark a major addition to the library of CKAs since they unlock pH responsive behaviour for polyesters from CKAs. RROP proved to be feasible with these monomers, transforming them into pH sensitive materials using photo-initiated radical polymerisation or free radical polymerisation. The consecutive formation of amphiphilic block-copolymers enabled the formation of NPs with a new combination of properties. All NPs formed showed the intended pH responsiveness originating from the tertiary amines as well as biodegradability resulting from the ester units. This combination of properties was previously unknown in polymers from RROP and from other chain-growth polymerisations. The results represent an important milestone for the development of biodegradable polymers from RROP as it can now produce polymers inaccessible by any other polymerisation. The combination of new monomers and new functional polymers form the basis for a large new class of materials to be used in nanoparticle research.Conflicts of interest
There are no conflicts to declare for all authors.Acknowledgements
Financial support of the Novartis–University of Basel Fellowship for Excellence in Life Sciences for J. G. and the Swiss National Science Foundation (SNSF) National Centre for Competence in Research for Molecular Systems Engineering (NCCR-MSE) is gratefully acknowledged.References
- J. Gaitzsch, X. Huang and B. Voit, Chem. Rev., 2016, 116, 1053–1093 CrossRef CAS PubMed
.
- M. Garni, R. Wehr, S. Y. Avsar, C. John, C. Palivan and W. Meier, Eur. Polym. J., 2019, 112, 346–364 CrossRef CAS
.
- E. Konishcheva, D. Daubian, J. Gaitzsch and W. Meier, Helv. Chim. Acta, 2018, 101, e1700287 CrossRef
.
- L. Messager, J. Gaitzsch, L. Chierico and G. Battaglia, Curr. Opin. Pharmacol., 2014, 18, 104–111 CrossRef CAS PubMed
.
- R. T. Pearson, N. J. Warren, A. L. Lewis, S. P. Armes and G. Battaglia, Macromolecules, 2013, 46, 1400–1407 CrossRef CAS
.
- S. J. Lin, J. J. Shang and P. Theato, Polym. Chem., 2017, 8, 2619–2629 RSC
.
- I. Canton and G. Battaglia, Chem. Soc. Rev., 2012, 41, 2718–2739 RSC
.
- R. P. Brinkhuis, F. P. J. T. Rutjes and J. C. M. van Hest, Polym. Chem., 2011, 2, 1449–1462 RSC
.
- H. Gumz, T. H. Lai, B. Voit and D. Appelhans, Polym. Chem., 2017, 8, 2904–2908 RSC
.
- E. V. Konishcheva, U. E. Zhumaev and W. P. Meier, Macromolecules, 2017, 50, 1512–1520 CrossRef CAS
.
- C. Fetsch, J. Gaitzsch, L. Messager, G. Battaglia and R. Luxenhofer, Sci. Rep., 2016, 6, 33491 CrossRef PubMed
.
- M. A. Petersen, L. Yin, E. Kokkoli and M. A. Hillmyer, Polym. Chem., 2010, 1, 1281–1290 RSC
.
- A. Birke, D. Huesmann, A. Kelsch, M. Weilbacher, J. Xie, M. Bros, T. Bopp, C. Becker, K. Landfester and M. Barz, Biomacromolecules, 2014, 15, 548–557 CrossRef CAS PubMed
.
- Y. Liu, Y. Li, D. Keskin and L. Shi, Adv. Healthcare Mater., 2019, 8, 1801359 Search PubMed
.
- A. Tardy, J. Nicolas, D. Gigmes, C. Lefay and Y. Guillaneuf, Chem. Rev., 2017, 117, 1319–1406 CrossRef CAS PubMed
.
- S. Agarwal, Polym. Chem., 2010, 1, 953–964 RSC
.
- W. J. Bailey, Z. Ni and S. R. Wu, J. Polym. Sci., Part A: Polym. Chem., 1982, 20, 3021–3030 CAS
.
- G. G. Hedir, C. A. Bell, N. S. Ieong, E. Chapman, I. R. Collins, R. K. O'Reilly and A. P. Dove, Macromolecules, 2014, 47, 2847–2852 CrossRef CAS
.
- C. A. Bell, G. G. Hedir, R. K. O'Reilly and A. P. Dove, Polym. Chem., 2015, 6, 7447–7454 RSC
.
- M. R. Hill, E. Guégain, J. Tran, C. A. Figg, A. C. Turner, J. Nicolas and B. S. Sumerlin, ACS Macro Lett., 2017, 6, 1071–1077 CrossRef CAS
.
- A. Tardy, V. Delplace, D. Siri, C. Lefay, S. Harrisson, B. D. A. Pereira, L. Charles, D. Gigmes, J. Nicolas and Y. Guillaneuf, Polym. Chem., 2013, 4, 4776–4787 RSC
.
- V. Delplace, S. Harrisson, A. Tardy, D. Gigmes, Y. Guillaneuf and J. Nicolas, Macromol. Rapid Commun., 2014, 35, 484–491 CrossRef CAS PubMed
.
- A. Tardy, J. C. Honore, D. Siri, D. Siri, D. Gigmes, C. Lefay and Y. Guillaneuf, Polym. Chem., 2017, 8, 5139–5147 RSC
.
- M. R. Hill, T. Kubo, S. L. Goodrich, C. A. Figg and B. S. Sumerlin, Macromolecules, 2018, 51, 5079–5084 CrossRef CAS
.
- S. Ganda, Y. Jiang, D. S. Thomas, J. Eliezar and M. H. Stenzel, Macromolecules, 2016, 49, 4136–4146 CrossRef CAS
.
- E. Guégain, C. Zhu, E. Giovanardi and J. Nicolas, Macromolecules, 2019, 52, 3612–3624 CrossRef
.
- G. G. Hedir, C. A. Bell, R. K. O'Reilly and A. P. Dove, Biomacromolecules, 2015, 16, 2049–2058 CrossRef CAS PubMed
.
- J. Gaitzsch, P. C. Welsch, J. Folini, C.-A. Schoenenberger, J. C. Anderson and W. P. Meier, Eur. Polym. J., 2018, 101, 113–119 CrossRef CAS
.
- Y. Zhu, A. Poma, L. Rizzello, V. M. Gouveia, L. Ruiz-Perez, G. Battaglia and C. K. Williams, Angew. Chem., 2019, 131, 4629–4634 CrossRef
.
- J. Gaitzsch, S. Hirschi, S. Freimann, D. Fotiadis and W. Meier, Nano Lett., 2019, 19, 2503–2508 CrossRef CAS PubMed
.
- C. Pegoraro, D. Cecchin, L. S. Gracia, N. Warren, J. Madsen, S. P. Armes, A. Lewis, S. MacNeil and G. Battaglia, Cancer Lett., 2013, 334, 328–337 CrossRef CAS PubMed
.
- J. Qian and C. Berkland, Polym. Chem., 2015, 6, 3472–3479 RSC
.
- N. A. Petasis and E. I. Bzowej, J. Am. Chem. Soc., 1990, 112, 6392–6394 CrossRef CAS
.
- I. Cho, B. G. Kim, Y. C. Park, C. B. Kim and M. S. Gong, Makromol. Chem., Rapid Commun., 1991, 12, 141–146 CrossRef CAS
.
- R. Bleul, R. Thiermann and M. Maskos, Macromolecules, 2015, 48, 7396–7409 CrossRef CAS
.
- X. Sui, P. Kujala, G.-J. Janssen, E. de Jong, I. S. Zuhorn and J. C. M. van Hest, Polym. Chem., 2015, 6, 691–696 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional graphs and calculations. See DOI: 10.1039/c9py01103j |
| This journal is © The Royal Society of Chemistry 2019 |