Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and supramolecular assembly of fluorinated biogenic amine recognition host polymers†
Ervin
Kovács
 a,
János
Deme
a,
Gábor
Turczel
a,
Tibor
Nagy
a,
János
Deme
a,
Gábor
Turczel
a,
Tibor
Nagy
 a,
Vajk
Farkas
a,
Vajk
Farkas
 a,
László
Trif
a,
László
Trif
 a,
Sándor
Kéki
a,
Sándor
Kéki
 b,
Péter
Huszthy
b,
Péter
Huszthy
 c and
Robert
Tuba
c and
Robert
Tuba
 *a
*a
aInstitute of Materials and Environmental Chemistry, Research Centre for Natural Sciences, Hungarian Academy of Sciences, Magyar tudósok körútja 2, P.O. Box 286, H-1519 Budapest, Hungary. E-mail: tuba.robert@ttk.mta.hu
bDepartment of Applied Chemistry, University of Debrecen, Egyetem tér 1, H-4032 Debrecen, Hungary
cDepartment of Organic Chemistry and Technology, Budapest University of Technology and Economics, Szent Gellért tér 4, H-1111 Budapest, Hungary
First published on 27th September 2019
Abstract
Copolymers containing hydroxyl (i.e. vinyl alcohol, VA) or fluorine functionalities are synthetic macromolecules having prominent biomedical applications. The concentration of hydroxyl groups along the polymer chain controls the polymer polarity. Moreover, the introduction of perfluorinated organic moieties via the OH functionalities may lead to macromolecules having potential magnetic resonance imaging (MRI) active properties. The ring-opening metathesis polymerization (ROMP) reaction using well-defined ruthenium-catalyzed systems is one of the most promising synthetic tools to fabricate such polymers. Co-polymerization of norbornene grafted pyridino-18-crown-6 ether (7) with fluorine-functionalized norbornenes (10 and 11) results in polymers bearing host molecular moieties. It has been demonstrated that the complexation of these host copolymers with biogenic amines including dopamine hydrochloride (12) and L-alanyl-L-lysine dipeptide hydrochloride (13) is straightforward. Based on the 1H NMR investigation of the 7 and 12 complexation, an equilibrium constant of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.3 ± 0.6 could be calculated. The in situ1H NMR investigations have revealed that the complex formation of 13 with monomer 7 and perfluorinated copolymer cp-7-10 takes place via both the lysine –NH3+ and the alanine –NH3+ moieties. However, in the case of homopolymer poly-7, the lysine–NH3+ group coordination was observed exclusively. According to theoretical calculations, molecular switching of the crown ether structure of both the 7 monomer and its cp-7-10 copolymer were observed from 90 degrees bent to planar structure upon –NH3+ ion coordination.
K = 4.3 ± 0.6 could be calculated. The in situ1H NMR investigations have revealed that the complex formation of 13 with monomer 7 and perfluorinated copolymer cp-7-10 takes place via both the lysine –NH3+ and the alanine –NH3+ moieties. However, in the case of homopolymer poly-7, the lysine–NH3+ group coordination was observed exclusively. According to theoretical calculations, molecular switching of the crown ether structure of both the 7 monomer and its cp-7-10 copolymer were observed from 90 degrees bent to planar structure upon –NH3+ ion coordination.
Introduction
Supramolecular chemistry utilizes reversible non-covalent bonding for the assembly of molecular architectures.1 Crown ethers (CEs) are well-known host molecules in supramolecular chemistry. Due to their excellent selectivity, crown ether-based molecular recognition motifs are widely used to synthesize polymers with unique properties.2,3 They can be involved either in the main chain or attached covalently to a polymer backbone as a side-chain.4 The synthesis of such host polymers can be carried out not only by classical polymerization methods but also via ring opening metathesis polymerization (ROMP) reactions.5,6 Crown ethers can form stable complexes not only with metal cations but also with protonated amines.7 This possibility has prompted many studies of CEs with peptides and proteins.8,9 The utilization of CEs in medical applications is emerging.10,11 For example, amino-crown ethers have been utilized for the delivery of 5-fluorouracil (5-FU), an anticancer agent used for the treatment of metastatic carcinomas of pancreas and breast.12 Moreover, several 18-crown-6 ethers have shown antitumor activity and reversal effect on multidrug resistance.13We have recently reported the synthesis of vinyl alcohol (VA) copolymers having fine tunable polarities, which are considered as emerging nanocomposite materials for drug delivery applications.14a
Fine tuning of the polarity of drug delivery polymers may enable preprogrammed and sustained drug release.14b Moreover, it is envisioned that the fine tuning of the polarity of the CEs containing host polymers should make possible the transport of highly hydrophilic biogenic amines in a non-polar environment using a well-adjusted lipophilic polymer/nanocomposite matrix. For example, it is tentatively supposed that tagging of dopamine via non-covalent reversible bonds to semi- or non-polar host polymers may enable its direct transport through the blood–brain barrier, leading to alternative treatment of Parkinson's disease.15,16 Furthermore, the introduction of perfluorinated organic moieties may open the way to magnetic resonance imaging (MRI) active properties of the potential drug carrier macromolecules.17 Here, we report the synthesis and supramolecular assembly of hydroxyl and fluorine functionalized biogenic amine recognition host polymers.
Results and discussion
Norbornene-functionalized pyridino-18-crown-6 ether (7) synthesis
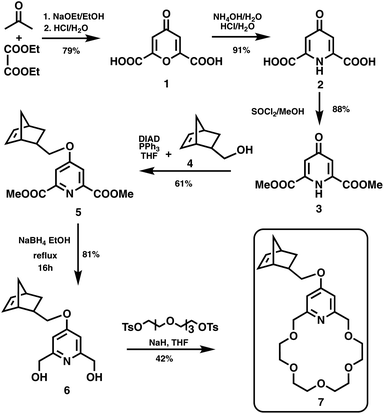
As shown in Scheme 1, norbornene-functionalized pyridino-18-crown-6 ether 7 was synthesized via chelidonic acid (1) in 15% overall yield. 1 was prepared from diethyl oxalate and acetone in the presence of sodium ethoxide according to literature sources.18 Treatment of 1 with aqueous concentrated ammonium hydroxide resulted in chelidamic acid (2).19 Esterification was carried out with thionyl chloride and methanol leading to chelidamic acid dimethyl ester (3).20 Following the Mitsunobu reaction of 3 with the endo norbornene derivative 4, the ether 5 could be obtained by column chromatography. The reduction of 5 to diol 6 followed by macrocyclization gave the crown ether 7 in moderate yield (42%). When the mixture of endo/exo isomers of 5 was used without separation, the product 7 contained endo and exo stereoisomers in 74% and 26% ratios, respectively.Complexation of pyridino-18-crown-6 ether (7) with biogenic amines
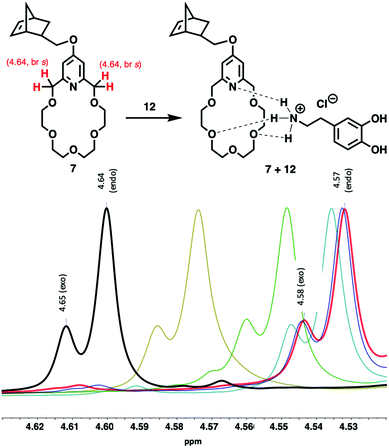
The complexation of crown ether (7) with dopamine hydrochloride (12) (Fig. 1, 2 and Fig. S47†) and L-alanyl-L-lysine dipeptide hydrochloride (13) (Fig. 2 and Fig. S48†) has been investigated. Upon mixing 7 with 12, the C(py)-CH2-O proton signals of 7 shifted upfield significantly from 4.64 to 4.57 ppm (Fig. 1). This is aligned with the literature data reported for the complexation of organic ammonium salts and similar pyridino-18-crown-6 ethers.21–24 The titration of 7 with 12 indicated a gradually increasing upfield shift of C(py)-CH2-O signals of 7 (4.64 ppm) until reaching the stoichiometric ratio. However, as the stoichiometric ratio has been achieved there was no further significant shifts observed for the C(py)-CH2-O signal in 1H NMR spectra (Fig. 1). Based on the chemical shift changes, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.3 ± 0.6 could be calculated,25 which is consistent with the reported data for similar complexes shown in Fig. S54.† Izatt et al. found a slightly lower log
K = 4.3 ± 0.6 could be calculated,25 which is consistent with the reported data for similar complexes shown in Fig. S54.† Izatt et al. found a slightly lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K value of 3.62 for the complexation of a bulkier isoalkyl-ammonium ion, (R)-1-phenylethylamine with a less flexible dimethylated pyridino-18-crown-6 ether molecule (see details of calculation in the ESI†).25 The K values for complexation of crown ethers using ammonium salts are in general high, and the equilibrium is shifted toward the complex formation side.21–24 Reproduction of the complexation using dipeptide 13 resulted in a similar supramolecular complex formation (see also ESI Fig. S48†).
K value of 3.62 for the complexation of a bulkier isoalkyl-ammonium ion, (R)-1-phenylethylamine with a less flexible dimethylated pyridino-18-crown-6 ether molecule (see details of calculation in the ESI†).25 The K values for complexation of crown ethers using ammonium salts are in general high, and the equilibrium is shifted toward the complex formation side.21–24 Reproduction of the complexation using dipeptide 13 resulted in a similar supramolecular complex formation (see also ESI Fig. S48†).
Electronic structure calculations using Gaussian 09 program package26 were carried out to determine the lowest-energy conformers of 7 and its complexes formed with dopamine·HCl (12) and L-alanyl-L-lysine dipeptide·HCl (13) at the M06-2X/cc-pVDZ level of density functional theory (DFT)27,28 using the SMD implicit solvent model.29 Coordinates of optimized geometries are listed in the ESI.† Molecular dynamics (MD) studies with explicit solvent molecules and accurately parameterized solvent–solute interaction are needed to account for such a complex environment.30 Implicit solvent models do not allow the realistic treatment of solvent mixtures, especially in the case of different solvent types (e.g., protic vs. aprotic, highly polar vs. slightly polar/apolar).
The recent MD study of Benay and Wipff investigated solvation and complexation of alkali cations (picrate salts) by 18-crown-6 in a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 chloroform/methanol mixture and found that the ions and the highly polar moieties are surrounded mainly by the polar and protic methanol molecules.30 Accordingly, they found that the calculated free energy change of complexation in the solvent mixture was very much like in pure methanol as intense interaction energies of polar and ionic groups dominated the energy change of the process. While specific MD studies are beyond the scope of the present work, these findings allow a realistic treatment of the complexation in methanol/dichloromethane and methanol/chloroform 50/50 solvent mixtures even with an implicit solvent model if pure methanol solvent is assumed.
10 chloroform/methanol mixture and found that the ions and the highly polar moieties are surrounded mainly by the polar and protic methanol molecules.30 Accordingly, they found that the calculated free energy change of complexation in the solvent mixture was very much like in pure methanol as intense interaction energies of polar and ionic groups dominated the energy change of the process. While specific MD studies are beyond the scope of the present work, these findings allow a realistic treatment of the complexation in methanol/dichloromethane and methanol/chloroform 50/50 solvent mixtures even with an implicit solvent model if pure methanol solvent is assumed.
Consequently, all electronic structure calculations presented here were carried out using methanol as an implicit solvent. A systematic (though probably non-exhaustive) DFT exploration of the high-dimensional conformational space of the crown ether ring identified several low-energy conformers for 7.
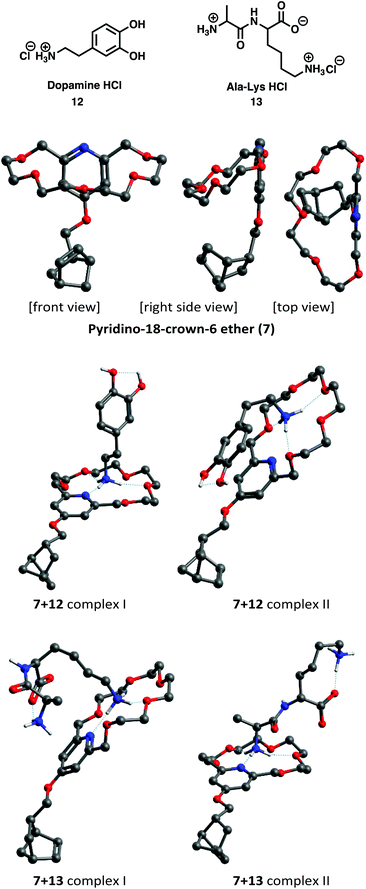
The one with the lowest energy is shown in three perpendicular directions in Fig. 2 (top three 3D structures). The minimum energy structure of the pyridino-18-crown-6 ether ring is largely different from the regular chair-type S6-symmetric structure expected for 18-crown-6 ethers. Driven by secondary interactions, the crown shrinks and folds back over the pyridine ring. Complexation of 7 with dopamine and L-alanyl-L-lysine dipeptide hydrochloride (12 and 13) was investigated in methanol as an implicit solvent without explicit consideration of the chloride ion and by assuming a zwitterionic form for the dipeptide (Fig. 2).
During complexation, the crown ether folds out, and the cavity of the macrocycle expands and takes on a much more regular shape that can host the primary ammonium cations. Complexation of 12 proceeds by forming three H-bonds (distributed at every ∼120°) with the crown ether by two different ways: either involving the nitrogen atom of the pyridine ring (7+12 complex I) or without it (7+12 complex II) (Fig. 2 middle two 3D structures). The former is energetically favored when no other interactions of the ligand with the crown ether are considered. However, when the nitrogen atom is not involved in the complexation, the conformational motion of the dopamine allows the aromatic ring of the dopamine to fold back over the pyridine ring (Fig. 2 middle right) and this structure is stabilized by π–π interactions.25 The protonated dipeptide cation can form a complex with the crown ether via either its lysine side chain (7+13 complex I) or its N-terminal (7+13 complex II) (Fig. 2 bottom two 3D structures). The formation of the intramolecular H-bond on the one hand significantly lowers the energy of the latter structure, and on the other hand, it hinders its conformational motion. The crown ether (7) and all complexes of 7+12 and 7+13 turned out to be very flexible as they have several low-energy very low-frequency vibrational modes (∼20 cm−1). Thus, to assess their relative stability through their standard free energy differences, full conformational sampling using MD simulations30 would be required. This, however, is beyond the scope of this study. Nevertheless, one can conclude that in proteins, which are the main targets of the proposed application, complexation through the lysine side chain will be dominant statistically as the number of lysine residues in them is usually much higher than one.
Polymer synthesis
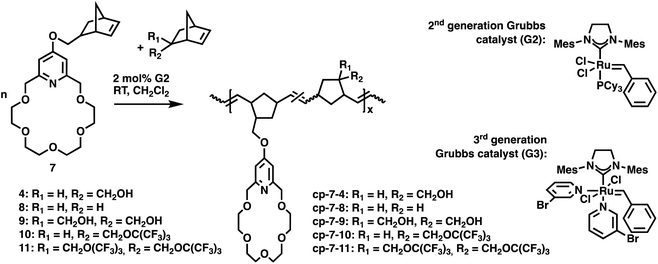
Cycloolefin-functionalized crown ethers are expected to participate in polymerization and co-polymerization reactions such as ruthenium-catalyzed ring-opening metathesis polymerization (ROMP).31,32 Indeed, it was found that the norbornene-functionalized pyridino-18-crown-6 ether can readily be polymerized using commercially available ruthenium metathesis catalysts G2 (Grubbs 2nd generation catalyst) and G3 (Grubbs 3rd generation catalyst). Although G2 provides polynorbornene having a moderate polydispersity (Đ > 1.5) – especially at relatively high catalyst loading – it has a higher functional group tolerance compared to the G3 metathesis catalysts.33 The ROMP reactions of norbornenes, in general, are relatively straightforward reactions, and they can be carried out at a low catalyst loading in any common organic solvent giving insoluble high molecular weight polymers.34In these tests, however, a relatively high (2 mol%) catalyst loading was applied to obtain relatively short polymers (Mw (mass-average molar mass) < 20 kDa) with reasonable THF solubility for GPC analysis. The ROMP of 7 was carried out at room temperature in dichloromethane using the G2 catalyst giving the homopolymer poly-7 in 99% isolated yield (Table 1). The formed polymer was sparingly soluble in CDCl3, CD2Cl2 and CD2Cl2/MeOD mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); however, it was poorly soluble in THF, which did not allow its GPC analysis. When the polymerization was carried out using the G3 catalyst, the product showed increased THF solubility (Mw: 1.53 × 103 Da, Đ (dispersity): 1.17). This observation can be explained by the significantly faster initiation and living polymerization character of the G3 catalyst leading to polymers with lower Mw values and narrow dispersity (Đ).33 MALDI-TOF MS measurements showed molecular weights corresponding to the oligomers containing 7 units (m/z: 419, Fig. S15†). Preliminary co-polymerizations of 7 have been carried out with norbornene (8) at 1
1); however, it was poorly soluble in THF, which did not allow its GPC analysis. When the polymerization was carried out using the G3 catalyst, the product showed increased THF solubility (Mw: 1.53 × 103 Da, Đ (dispersity): 1.17). This observation can be explained by the significantly faster initiation and living polymerization character of the G3 catalyst leading to polymers with lower Mw values and narrow dispersity (Đ).33 MALDI-TOF MS measurements showed molecular weights corresponding to the oligomers containing 7 units (m/z: 419, Fig. S15†). Preliminary co-polymerizations of 7 have been carried out with norbornene (8) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratios using the G3 catalyst.33 The Mw of the copolymers were 4.49 × 103 Da (Đ: 1.68) at 7
5 molar ratios using the G3 catalyst.33 The Mw of the copolymers were 4.49 × 103 Da (Đ: 1.68) at 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 = 1
8 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 7.19 × 103 Da, (Đ: 1.87) at 7
1 and 7.19 × 103 Da, (Đ: 1.87) at 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 = 1
8 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratio, respectively. Co-polymerization carried out at the 7
5 molar ratio, respectively. Co-polymerization carried out at the 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 = 1
8 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with the G2 catalyst resulted in a non-THF soluble polymer (98% yield) (Scheme 2). However, the co-polymerization of 7 and 8 at the 1
1 molar ratio with the G2 catalyst resulted in a non-THF soluble polymer (98% yield) (Scheme 2). However, the co-polymerization of 7 and 8 at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar monomer ratio led to a THF soluble polymer in 97% yield with a comparable molecular weight (Mw: 5.97 × 103 Da) and higher polydispersity (Đ: 1.80). The MALDI-TOF MS measurements indicate that the oligomers are comprised of both 7 (m/z: 419) and 8 (m/z: 94) monomer units (Fig. S23†), as expected. Considering the observed molecular weights for cp-7-8 polymers (Table 1), it can be concluded that these data are consistent with the Mw value of polynorbornene synthesized under the similar condition and a slightly lower catalyst loading (0.6 mol%).35
5 molar monomer ratio led to a THF soluble polymer in 97% yield with a comparable molecular weight (Mw: 5.97 × 103 Da) and higher polydispersity (Đ: 1.80). The MALDI-TOF MS measurements indicate that the oligomers are comprised of both 7 (m/z: 419) and 8 (m/z: 94) monomer units (Fig. S23†), as expected. Considering the observed molecular weights for cp-7-8 polymers (Table 1), it can be concluded that these data are consistent with the Mw value of polynorbornene synthesized under the similar condition and a slightly lower catalyst loading (0.6 mol%).35
| Entry | Polymer | Yield (%) | Cp–M–M′ M/M′ | Catalysts | THF solubility | M w (Da) × 103 | Đ | T g (°C) | T d (°C) |
|---|---|---|---|---|---|---|---|---|---|
| a Crystalline structure, no Tg data were obtained. Cp–M–M′: copolymer of monomer 1 (M) and monomer 2 (M′). M/M′: theoretical monomer 1 and monomer 2 ratio in the copolymer. | |||||||||
| 1 | poly-7 | 99 | NA | G2 | Poor | — | — | −36 | 347 |
| 2 | poly-7 | 93 | NA | G3 | Sparing | 1.53 | 1.17 | — | — |
| 3 | cp-7-8 | 98 | 1/1 | G2 | Poor | — | — | 14.9 | 329 |
| 4 | cp-7-8 | 95 | 1/1 | G3 | Moderate | 4.49 | 1.68 | — | — |
| 5 | cp-7-8 | 91 | 1/5 | G3 | Moderate | 7.19 | 1.87 | — | — |
| 6 | cp-7-8 | 97 | 1/5 | G2 | Moderate | 5.97 | 1.80 | 19.7 | 248 |
| 7 | cp-7-10 | 92 | 1/1 | G2 | Poor | — | — | −16 | 295 |
| 8 | cp-7-10 | 98 | 1/5 | G2 | Sparing | 9.85 | 1.43 | 307 | |
| 9 | cp-7-11 | 98 | 1/5 | G2 | Moderate | 5.59 | 2.16 | 403 | |
Following the preliminary co-polymerization tests with 8, the incorporation of hydroxyl and perfluoro-tert-butyl groups into the host polymer chain was investigated. Perfluorinated moieties containing monomer 10 was synthesized by the reaction of the tosyl ester derivative of 4 (Tos-4)36 with the sodium salt of nonafluoro-tert-butyl alcohol37 in reasonable yield (60%). Meanwhile, the bis-perfluorinated moieties containing monomer 11 has been synthesized by the Mitsunobu reaction38via the reaction of 9 and nonafluoro-tert-butyl alcohol in moderate yield (27%). The co-polymerization of crown ether 7 with 4 and 9 and perfluoro-tert-butyl-functionalized norbornenes 10 and 11 gave copolymers having randomly distributed dyads.
The co-polymerization of 7 with 4 in the stoichiometric ratio resulted in the cp-7-4 copolymer in quantitative yield. The polymer does not render any THF solubility. However, it is sparingly soluble in the methylene chloride–methanol mixture. The co-polymerization of 7 with 9 led to the formation of entirely insoluble copolymer cp-7-9. These observations are consistent with literature data reporting that poly-4 and poly-9 have minimal solubility in common organic solvents.39 However, the introduction of perfluoro-tert-butyl units has significantly improved the copolymer solubility even in tetrahydrofuran. The co-polymerization of 7 has been carried out with 10 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 molar ratios using the G2 catalyst (cp-7-10 (1
5 molar ratios using the G2 catalyst (cp-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 92% and cp-7-10 (1
1): 92% and cp-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5): 98% yield) in dichloromethane solution. cp-7-10 (1
5): 98% yield) in dichloromethane solution. cp-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was only sparingly soluble in THF; however, cp-7-10 (1
1) was only sparingly soluble in THF; however, cp-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) rendered reasonable THF solubility. The GPC tests of cp-7-10 (1
5) rendered reasonable THF solubility. The GPC tests of cp-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) revealed a reasonable Mw = 9.85 × 103 Da (Đ: 1.43) value (Table 1). As it was expected, the MALDI-TOF MS investigations have clearly indicated that the copolymers are comprised of both 7 (m/z: 419) and 10 (m/z: 342) monomer units as well (Fig. 3). Polymer cp-7-11 containing the bis-perfluoro-tert-butyl moieties in 1
5) revealed a reasonable Mw = 9.85 × 103 Da (Đ: 1.43) value (Table 1). As it was expected, the MALDI-TOF MS investigations have clearly indicated that the copolymers are comprised of both 7 (m/z: 419) and 10 (m/z: 342) monomer units as well (Fig. 3). Polymer cp-7-11 containing the bis-perfluoro-tert-butyl moieties in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 = 7
5 = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 ratio has been synthesized in high yield (98%). The polymer rendered a similar molecular weight and dispersity (5.59 × 103 Da, Đ: 2.16) to copolymers cp-7-8 and cp-7-10 synthesized under similar reaction conditions (Table 1). Based on the DSC measurements (Table 1), it can be concluded that the inclusion of side groups increases the Td values (as seen at poly-7: 347 °C and cp-7-11 (1
11 ratio has been synthesized in high yield (98%). The polymer rendered a similar molecular weight and dispersity (5.59 × 103 Da, Đ: 2.16) to copolymers cp-7-8 and cp-7-10 synthesized under similar reaction conditions (Table 1). Based on the DSC measurements (Table 1), it can be concluded that the inclusion of side groups increases the Td values (as seen at poly-7: 347 °C and cp-7-11 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5): 403 °C vs.cp-7-8 (1
5): 403 °C vs.cp-7-8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1): 329 °C; cp-7-8 (1
1): 329 °C; cp-7-8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5): 248 °C). The co-polymerization of 7 with 8 at the 1
5): 248 °C). The co-polymerization of 7 with 8 at the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 monomer ratio significantly increased the Tg value (poly-7 = −36 °C; poly-7-8 (1
1 monomer ratio significantly increased the Tg value (poly-7 = −36 °C; poly-7-8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) = 14.9 °C). However, increasing the 7
1) = 14.9 °C). However, increasing the 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 monomer ratio up to 1
8 monomer ratio up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 resulted in only a slight increase of the Tg value (poly-7-8 (1
5 resulted in only a slight increase of the Tg value (poly-7-8 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) = 19.7 °C).
5) = 19.7 °C).
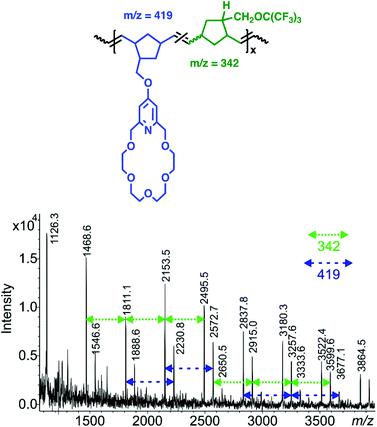 | ||
| Fig. 3 MALDI TOF MS spectrum of copolymer cp-7-10, 7 (m/z: 419), 10 (m/z: 342) (7/10 = 1/5) (linear mode, DHB/NaTFA). | ||
Complexation of pyridino-18-crown-6 ether (7)-functionalized polymers with biogenic amines
The complexation of poly-7 with 12 (Fig. 4 and Fig. S49†) and 13 (Fig. S50†) have been investigated by 1H NMR spectroscopy in the MeOD–CD2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solvent mixture at 30 °C. As expected, upon complexation the poly-7 broad aromatic proton signals shifted upfield from 6.83 to 6.79 ppm; meanwhile, the aromatic protons of 12 have also shown significant shifts from 6.80 (d), 6.76 (d) and 6.62 (dd) ppm to 6.76, 6.71 and 6.55 ppm, respectively (Fig. 4).
1 solvent mixture at 30 °C. As expected, upon complexation the poly-7 broad aromatic proton signals shifted upfield from 6.83 to 6.79 ppm; meanwhile, the aromatic protons of 12 have also shown significant shifts from 6.80 (d), 6.76 (d) and 6.62 (dd) ppm to 6.76, 6.71 and 6.55 ppm, respectively (Fig. 4).
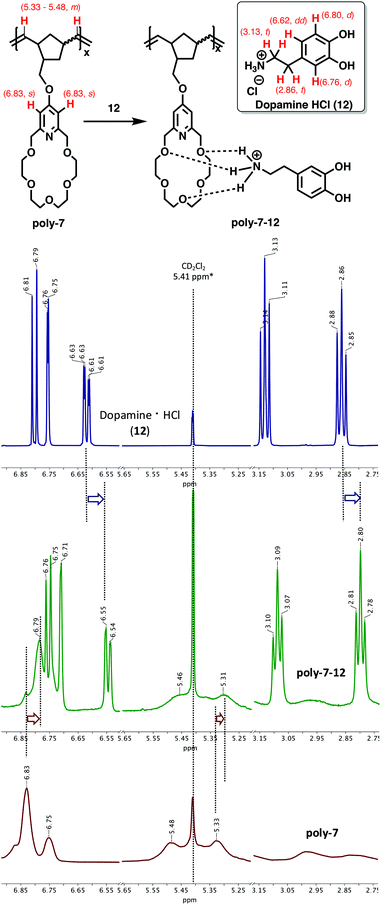 | ||
Fig. 4 Complexation of poly-7 (bottom) with Dopamine·HCl, 12 (top), and poly-7-12 complex (middle) (MeOD–CD2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, 30 °C, [poly-7] = [12] = 0.01 mmol mL−1). 1 mixture, 30 °C, [poly-7] = [12] = 0.01 mmol mL−1). | ||
These observations are in accordance with the literature data reported for the supramolecular complex formation of pyridino-18-crown-6 ethers with protonated primary amines.21 Similar chemical shift could be clearly observed in the aliphatic proton region as well. The aliphatic CH2 proton signals of 12 shifted from 3.13 (t) and 2.86 (t) ppm to 3.09 and 2.80 ppm, respectively (Fig. 4). As was expected, the proton signals of 12 significantly broadened upon complexation with the crown ether-functionalized polymer.
The complexation of poly-7 with 13 also revealed significant changes of the chemical shifts of the reacting molecules. The aromatic proton signals of poly-7 shifted upfield from 6.83 ppm to 6.79 ppm (Fig. S50†). Unlike the complexation of 7 with 12, it can be seen that upon complexation of poly-7 with 13 the most characteristic peaks CH (t) (4.33 ppm) and CH2 (t) (2.95 ppm) of 13 have revealed significant chemical shifts to 4.21 ppm and 2.80 ppm; meanwhile, the CH (q) peak at 3.97 ppm did not show any significant changes (Fig. 5, bottom). This observation indicates that the complexation between poly-7 and 13 most probably occurs exclusively via the lysine –NH3+ group rather than the alanine –NH3+ moiety, unlike the complexation between 7 and 13 where all the three peaks revealed significant upshift indicating that it takes place by both –NH3+ moieties (Fig. 5, top).
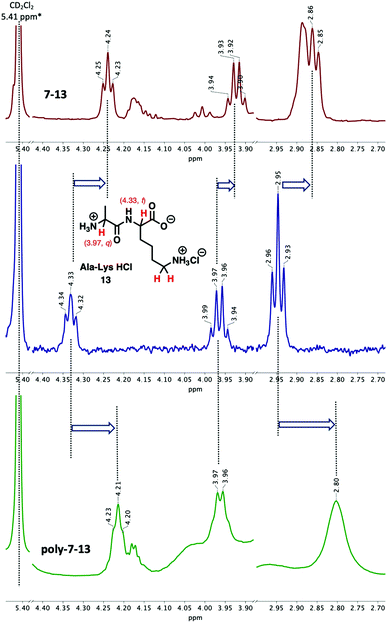 | ||
Fig. 5 Complexation of 13 (middle) with pyridino-18-crown-6 ether, 7-13 (top) and polymer, poly-7-13 (bottom) (MeOD–CD2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, 30 °C, [7] = [13] = 0.01 mmol mL−1). 1 mixture, 30 °C, [7] = [13] = 0.01 mmol mL−1). | ||
Following the investigation of the complexation of homopolymer poly-7 with 12, the complexation of perfluoro tert-butyl group-functionalized copolymer cp-7-10 has been investigated (Fig. 6).
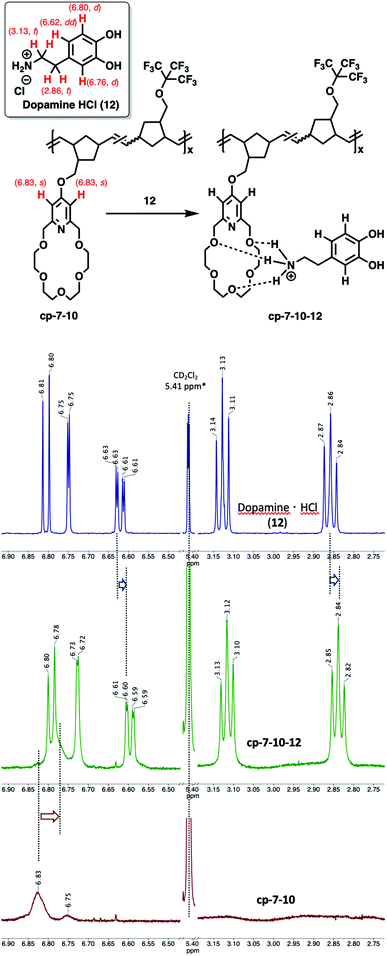 | ||
Fig. 6 Complexation of poly-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, bottom) with Dopamine·HCl, 12 (top), and poly-7-10-12 complex (middle) (MeOD–CD2Cl2 1 1, bottom) with Dopamine·HCl, 12 (top), and poly-7-10-12 complex (middle) (MeOD–CD2Cl2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, 30 °C, [cp-7-10] = [12] = 0.01 mmol mL−1). 1 mixture, 30 °C, [cp-7-10] = [12] = 0.01 mmol mL−1). | ||
As expected, upon addition of 12 to a MeOD–CD2Cl2 solution of cp-7-10 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the aromatic proton signals of 12 (6.81 (d), 6.75 (d) and 6.62 (dd) ppm) significantly shifted to 6.79 (d), 6.73 (d) and 6.60 (dd) ppm, respectively. Meanwhile, broadening of the proton peaks of 12 was observed.
1), the aromatic proton signals of 12 (6.81 (d), 6.75 (d) and 6.62 (dd) ppm) significantly shifted to 6.79 (d), 6.73 (d) and 6.60 (dd) ppm, respectively. Meanwhile, broadening of the proton peaks of 12 was observed.
The aliphatic proton signals of 12 have also shifted upfield from 3.13 (t) and 2.86 (t) ppm to 3.12 (t) and 2.84 (t) ppm, respectively (Fig. 6 and Fig. S51†). Meanwhile, the broad aromatic CH proton peaks of cp-7-10 were also shifted upfield from 6.83 ppm to 6.77 ppm. The observed simultaneous upfield shifts and peak broadenings of 12 clearly indicate an interaction between the polymer (cp-7-10) and Dopamine·HCl (12). The complexation of cp-7-10 with Ala-Lys·HCl (13) led to similar shifts of the pyridino-18-crown-6 ether aromatic protons (from 6.83 (br) to 6.78 (br) ppm) and lysine protons (from 4.33 (t) to 4.26 (br) ppm and from 2.95 (t) to 2.91 (br) ppm) (Fig. S52†). Just as in the case of complexation of cp-7-10 with 12, the proton signal broadening of 13 were unambiguous, indicating the specific intermolecular interaction between cp-7-10 and Ala-Lys·HCl (13). It should be noted that based on the 1H NMR spectra, there was significant upshift for the quartet signal of the Ala unit from 3.97 ppm to 3.91 ppm (CH-CH3), indicating that most probably the complexation occurs not only via the protonated lysine side chain but also on the protonated N-terminal alanine dyad (Fig. S52†).
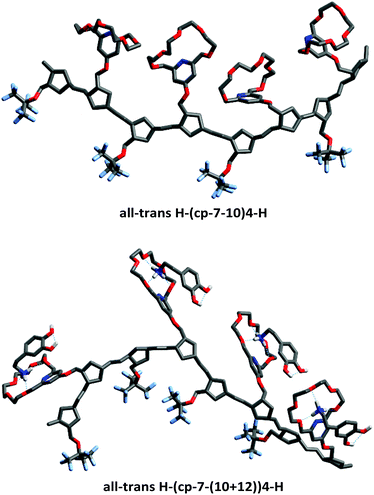
The formed copolymers are not only non-periodic, but also highly flexible; thus, they do not exhibit a well-defined geometry. The all-trans H–(poly-7-10)4–H alternating cooligomer can be considered as the most abundant possible dyad40 of the formed host polymers. A local optimum structure of its complex formed with 12 was determined in an implicit methanol solvent using the PM6 semiempirical theory and is shown as an illustration in Fig. 7 to help the reader to visualize them.41
Coordinates of optimized geometries are listed in the ESI.† If no complexation takes place, the strong attraction between the tilted and folded crown ether units aligns them and bends the copolymer chain toward the side groups. In the complex state, the side groups are positively charged due to the presence of the primary ammonium ions. The strong electrostatic repulsion is only partially ameliorated by the methanol solvent (and the scattered, solvated chloride ions). Thus, the side groups tilt away from each other and the polymer chain becomes distorted.
Conclusions
In summary, a wide range of copolymers having host (pyridino-18-crown-6 ether), OH and perfluoro tert-butyl functionalities have been synthetized via ruthenium-catalyzed ring opening metathesis polymerization.The complexation of poly-7 and its perfluoro-tert-butyl group containing copolymers (poly-7-10) with biogenic amines including dopamine (12) and L-alanyl-L-lysine dipeptide (13) has led to the formation of the supramolecular complexes indicated by 1H NMR spectroscopy. Upon complexation, significant upfield shift of the proton signals (0.02–0.06 ppm) of both reactants and peak broadening of the biogenic amines were observed. The investigations have revealed that the complex formation of 13 with monomer 7 and copolymer cp-7-10 may take place via either the lysine or alanine –NH3+ moieties. However, in the case of poly-7, the lysine amine group coordination was observed exclusively.
The experimental data have been supported by theoretical calculations, also indicating that the complexation of a biogenic amine to the presented host polymer is straightforward.
It is envisioned that these polymers may have potential to serve as “biogenic amine carriers” opening new alternatives for drug delivery applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by grants provided by the National Competitiveness and Excellence Program, Hungary (NVKP-16-1-2016-0007), and by the BIONANO-GINOP-2.3.2-15-2016-00017 project. The authors are grateful to Materia, Inc., for providing G2. Tibor Nagy and Péter Huszthy are grateful to the Hungarian Academy of Sciences and National Research, Development and Innovation Office (NKFIH) for financial support under Grant No. PD 120776 and K128473, for János Bolyai Research Fellowship BO/00279/16/7 and for the Hungarian NIIF HPC infrastructure. Ervin Kovács is grateful to the National Research, Development and Innovation Office (NKFIH) for Postdoctoral Excellence Award (PD 128612). Attila Domján is acknowledged for solid NMR analysis. The authors thank Prof. John A. Gladysz (TAMU) for the revision and the constructive comments.Notes and references
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2012, 101, 4071–4097 CrossRef.
- C. R. South, M. N. Higley, K. C. F. Leung, D. Lanari, A. Nelson, R. H. Grubbs, J. F. Stoddart and M. Weck, Chem. – Eur. J., 2006, 12, 3789–3797 CrossRef CAS PubMed.
- C. R. South, K. C. F. Leung, D. Lanari, J. F. Stoddart and M. Weck, Macromolecules, 2006, 39, 3738–3744 CrossRef CAS.
- U. Tunca and Y. Yagci, Prog. Polym. Sci., 1994, 19, 233–286 CrossRef CAS.
- R. Ji, C. G. Chao, Y. C. Huang, Y. K. Lan, C. L. Lu and T. Y. Luh, Macromolecules, 2010, 43, 8813–8820 CrossRef CAS.
- E. Eder, P. Preishuber-Pflügl and F. Stelzer, J. Mol. Catal. A: Chem., 2000, 160, 63–69 CrossRef CAS.
- V. Rüdiger, H. J. Schneider, V. P. Solov'ev, V. P. Kazachenko and O. A. Raevsky, Eur. J. Org. Chem., 1999, 1847–1856 CrossRef.
- S. W. Lee, H. N. Lee, H. S. Kim and J. L. Beauchamp, J. Am. Chem. Soc., 1998, 120, 5800–5805 CrossRef CAS.
- R. R. Julian and J. L. Beauchamp, J. Am. Soc. Mass Spectrom., 2002, 13, 493–498 CrossRef CAS PubMed.
- X. Liu, S. Zhu, S. Wu, P. Wang and G. Han, Colloids Surf., A, 2013, 417, 140–145 CrossRef CAS.
- I. A. Darwish and I. F. Uchegbu, Int. J. Pharm., 1997, 159, 207–213 CrossRef CAS.
- R. Muzzalupo, F. P. Nicoletta, S. Trombino, R. Cassano, F. Iemma and N. Picci, Colloids Surf., B, 2007, 58, 197–202 CrossRef CAS PubMed.
- I. Guberović, M. Marjanović, M. Mioč, K. Ester, I. Martin-kleiner, T. Š. Ramljak, K. Mlinarić-majerski and M. Kralj, Sci. Rep., 2018, 8, 14467 CrossRef PubMed.
- (a) T. Feczkó, G. Merza, G. Babos, B. Varga, E. Gyetvai, L. Trif, E. Kovács and R. Tuba, Int. J. Pharm., 2019, 562, 333–341 CrossRef PubMed; (b) S. Miyazaki, S. Takeuchi, W. Hou, N. Hashiguchi, C. Yokouchi, M. Takada, M. Hosokawa, Y. Koga and H. Kobayashi, Chem. Pharm. Bull., 1985, 33, 2490–2498 CrossRef CAS PubMed.
- C. Saraiva, C. Praça, R. Ferreira, T. Santos, L. Ferreira and L. Bernardino, J. Controlled Release, 2016, 235, 34–47 CrossRef CAS PubMed.
- A. M. Grabrucker, B. Ruozi, D. Belletti, F. Pederzoli, F. Forni, M. A. Vandelli and G. Tosi, Tissue Barriers, 2016, 4, e1153568 CrossRef PubMed.
- S. Decato, T. Bemis, E. Madsen and S. Mecozzi, Polym. Chem., 2014, 5, 6461–6471 RSC.
- E. R. Riegel and F. Zwilgmeyer, Org. Synth., 1937, 2, 126–128 Search PubMed.
- E. R. Riegel and M. C. Reinhard, J. Am. Chem. Soc., 1926, 48, 1334–1345 CrossRef CAS.
- J. S. Bradshaw, P. Huszthy, T. Wang, C. Zhu, A. Y. Nazarenko and R. M. Izatt, Supramol. Chem., 1993, 1, 267–275 CrossRef CAS.
- T.-M. Wang, J. S. Bradshaw, J. C. Curtis, P. Huszthy and R. M. Izatt, J. Inclusion Phenom. Mol. Recognit. Chem., 1993, 16, 113–122 CrossRef CAS.
- T.-M. Wang, J. S. Bradshaw and R. M. Izatt, J. Heterocycl. Chem., 1994, 31, 1097–1114 CrossRef CAS.
- C.-Y. Zhu, J. S. Bradshaw, J. L. Oscarson and R. M. Izatt, J. Inclusion Phenom. Mol. Recognit. Chem., 1992, 12, 275–289 CrossRef CAS.
- (a) J. S. Bradshaw, P. Huszthy, C. W. McDaniel, C.-Y. Zhu, N. K. Dalley, R. M. Izatt and S. Lifson, J. Org. Chem., 1990, 55, 3129–3137 CrossRef CAS; (b) S. V. Brignell and D. K. Smith, New J. Chem., 2007, 31, 1243–1249 RSC.
- R. M. Izatt, T. Wang, J. K. Hathaway, X. X. Zhang, J. C. Curtis, J. S. Bradshaw, C. Y. Zhu and P. Huszthy, J. Inclusion Phenom. Mol. Recognit. Chem., 1994, 17, 157–175 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian Inc., Wallingford, CT, 2009.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- T. H. Dunning, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- G. Benay and G. Wipff, New J. Chem., 2016, 40, 4662–4671 RSC.
- C. W. Bielawski and R. H. Grubbs, in Controlled and Living Polymerizations, 2009, pp. 297–342 Search PubMed.
- C. W. Bielawski and R. H. Grubbs, Prog. Polym. Sci., 2007, 32, 1–29 CrossRef CAS.
- T. Choi and R. H. Grubbs, Angew. Chem., Int. Ed., 2003, 42, 1743–1746 CrossRef CAS PubMed.
- J. Suriboot, Y. Hu, T. J. Malinski, H. S. Bazzi and D. E. Bergbreiter, ACS Omega, 2016, 1, 714–721 CrossRef CAS PubMed.
- R. Tuba, R. Corrêa Da Costa, H. S. Bazzi and J. A. Gladysz, ACS Catal., 2012, 2, 155–162 CrossRef CAS.
- V. De Matteis, F. L. Van Delft, J. Tiebes and F. P. Rutjes, Eur. J. Org. Chem., 2006, 1166–1176 CrossRef CAS.
- D. Szabó, J. Mohl, A. M. Bálint, A. Bodor and J. Rábai, J. Fluor. Chem., 2006, 127, 1496–1504 CrossRef.
- K. C. Kumara Swamy, N. N. Bhuvan Kumar, E. Balaraman and K. V. P. Pavan Kumar, Chem. Rev., 2009, 109, 2551–2651 CrossRef PubMed.
- U. Frenzel, T. Weskamp, F. J. Kohl, W. C. Schattenmann, O. Nuyken and W. A. Herrmann, J. Organomet. Chem., 1999, 586, 263–265 CrossRef CAS.
- R. Tuba, M. Al-Hashimi, H. Bazzi and R. Grubbs, Macromolecules, 2014, 47, 8190–8195 CrossRef CAS.
- J. J. P. Stewart, J. Mol. Model., 2007, 13, 1173–1213 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9py00929a |
| This journal is © The Royal Society of Chemistry 2019 |