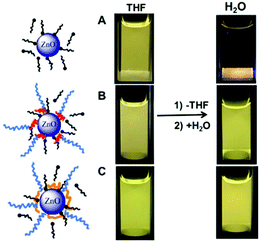
Luminescent zinc oxide nanoparticles: from stabilization to slow digestion depending on the nature of polymer coating†
Zhiqin
Zheng‡
ab,
Margaux
Mounsamy
a,
Nancy
Lauth-de Viguerie
a,
Yannick
Coppel
 b,
Simon
Harrisson
b,
Simon
Harrisson
 a,
Mathias
Destarac
a,
Mathias
Destarac
 a,
Christophe
Mingotaud
a,
Christophe
Mingotaud
 a,
Myrtil L.
Kahn
a,
Myrtil L.
Kahn
 *b and
Jean-Daniel
Marty
*b and
Jean-Daniel
Marty
 *a
*a
aLaboratoire des IMRCP CNRS UMR 5623, University of Toulouse, 118, route de Narbonne 31062, Toulouse Cedex 9, France. E-mail: marty@chimie.ups-tlse.fr
bLaboratoire de Chimie de Coordination CNRS UPR 8241, University of Toulouse, 205, route de Narbonne, 31062 Toulouse Cedex 9, France. E-mail: myrtil.kahn@lcc-toulouse.fr
First published on 15th November 2018
Abstract
Poly(ethylene glycol)-poly(vinylphosphonic acid) and poly (ethylene glycol)-poly(acrylic acid) block copolymers (PEG-b-PVPA and PEG-b-PAA) were synthesized by RAFT/MADIX polymerization. The interaction of PAA or PVPA blocks with preformed ZnO NPs and the presence of a highly soluble PEG block enable a stable colloidal solution of ZnO NPs to be obtained in both organic solvent and water. While in THF a significant enhancement of luminescent properties is observed in both cases, a slow decrease of these properties is observed in water, which is due to the slow digestion of the ZnO NPs. Lastly, compared to the PAA block, the PVPA block favors both the interaction with ZnO NPs and their digestion.
1. Introduction
The synthesis of luminescent inorganic nanoparticles (NPs), better known as quantum dots, with narrow size distribution and high luminescent efficiency has attracted intensive interest due to their applications in biological fluorescence labelling.1,2 Compared to traditionally used CdSe or CdTe quantum dots, ZnO is an environmentally friendly and inexpensive luminescent semiconductor, which makes it very attractive for practical biological applications.3,4 Many studies have been performed to control the size and shape of the ZnO inorganic core and/or to choose the chemical structure of the stabilizing ligand shell. Nevertheless, the methods of functionalization generally used suffer from a lack of flexibility. The control of core size or shape is often obtained through restrictive growth conditions that require a specific combination of solvent and reducing and/or functionalizing agent.While nanosized ZnO is often prepared via the sol–gel route,5,6 synthesis via an organometallic approach enables better shape control, higher crystallinity and more tunable luminescent properties but requires the use of organic solvent.7 Therefore different strategies have been developed to transfer the ZnO NPs into water.1,8–12 The first involves in situ synthesis of ZnO NPs in organic solvent in the presence of water-compatible stabilizing agents that strongly interact with both the zinc precursor and the ZnO NPs.1,12 For instance, amino-terminated oligo(ethylene glycol) has been used to obtain ZnO NPs in THF that are also dispersible in water.12
Ligand exchange on the surface of preformed hydrophobic ZnO NPs is the most commonly used method to obtain water-compatible particles with controlled functionality. Understanding to what extent ligand exchange occurs and what factors affect it is important for the optimization of this critical procedure. Previously developed strategies leading to stable ZnO NPs in water have involved the use of molecular stabilizing agents with weak affinity to the surface of NPs, such as oleic acid or amine compounds.8,10,12 As a result, free ligands are always present in solution which is a severe drawback for biological applications. In this context, the use of polymers to promote ligand exchange and get rid more easily of remaining free ligands is of particular interest due to their multiple interactions and kinetic hindrance. Hence different families of polymers with different architectures (branched, block…) and compositions have been shown to favor ligand exchange on preformed ZnO NPs.9,10b,13 These polymers comprise a poly(ethylene glycol) (PEG) segment which is of moderate cost, approved by regulatory agencies, and water soluble at body temperature,14 as well as a hydrophobic polysiloxane10 or polymethacrylate block9 to ensure interaction with pristine hydrophobic ZnO NPs.
In this work, our main objectives are to evaluate and compare the stabilizing properties for ZnO NPs of double hydrophilic block copolymer stabilizers comprising a PEG stabilizing block and a block bearing interacting groups with high affinity for the surface of nanoparticles. The nature of the anchoring group is of paramount importance in order to promote a strong attachment of the ligand to the ZnO NPs surface. Nevertheless surface modifications can significantly impact the luminescence properties of ZnO QDs.10 Thus it is necessary to carefully control the surface chemistry in order to obtain water-stable dispersions while maintaining the emission properties. Amine and carboxy groups promote targeted luminescent properties while ensuring good anchoring to the surface of ZnO NPs. We chose here poly(acrylic acid) (PAA) as a first interacting block to promote the replacement of amine ligands present on the surface of ZnO NPs. As phosphonic acid groups also demonstrate high affinity with zinc15 and oxide-based NPS like iron oxide NPs,16 a second type of diblock copolymer based on poly(vinylphosphonic acid) (PVPA) as an interacting block was also studied.16a We therefore describe here the synthesis of poly(ethylene glycol)-poly(acrylic acid) (PEG-b-PAA) and poly(ethylene glycol)-poly(vinylphosphonic acid) (PEG-b-PVPA) double hydrophilic block copolymers of low molar mass by RAFT/MADIX polymerization. These two polymers were used to modify the surface of preformed ZnO NPs covered by amine ligands. The ligand exchange mechanism was studied through NMR studies, dynamic light scattering (DLS) and luminescent properties. The ability of the two block copolymers to ensure efficient colloidal stability and long-term luminescent properties of the obtained hybrid materials was studied both in THF and in water. The advantages and drawbacks of phosphonic acid groups over carboxylic acid groups are discussed, and a slow digestion mechanism is suggested in the case of ZnO NPs covered with PVPA polymer which could be of great interest for biological applications.
2. Results and discussion
A. Synthesis of diblock copolymers
Double hydrophilic block copolymers containing a neutral PEG segment and an ionizable segment of PVPA or PAA were synthesized by RAFT/MADIX (reversible addition–fragmentation chain transfer/macromolecular design by interchange of xanthates) polymerization from a xanthate-functionalized PEG macroRAFT agent (Scheme 1) as previously described.16a The PEG macroRAFT agent with a number-average molecular weight (Mn) of 2000 g mol−1 was prepared in a two-step synthesis from ω-hydroxy-functional PEG.17 Chain extension was then carried out by polymerizing AA or VPA in water, targeting Mn of 1000 g mol−1. This corresponds to a number-average degree of polymerization (DPn) of 13 and 9 respectively. Conditions for the VPA polymerization were adapted from those reported by Layrac et al.18a for the synthesis of polyacrylamide-b-PVPA. In all cases, the polymerizations reached approximately 50% conversion after 8 h of reaction at 65 °C; unreacted VPA monomer was then removed by dialysis (MWCO = 1000 g mol−1).18bThe block copolymers were characterized by NMR (1H and 31P) and size exclusion chromatography (SEC). Molecular weight distributions were narrow (Đ < 1.2), and good agreement was observed between the theoretical Mn (Mn,th) and the apparent Mn obtained from either NMR or SEC (Table 1). In addition, a shift to lower elution volumes was observed in the SEC trace of the block copolymer relative to the PEG substrate, indicating complete transformation of the macroRAFT agent into block copolymer (Fig. S1 in ESI†).
M
n,th![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (g mol−1) a (g mol−1) |
M
n NMR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (g mol−1) b (g mol−1) |
M
n SEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (g mol−1) c (g mol−1) |
Đ | |
|---|---|---|---|---|
| a M n,th = ([monomer]0/[X]0) × Conv. × M(monomer) + M(X). MVPA = 108 g mol−1, MAA = 72 g mol−1, MPEG2k-X = 2188 g mol−1. b Determined by 1H NMR taking the molar mass of the PEG block into account. c Measured by SEC-RI-MALS. d PEG2k is a commercially available product (PEG-OH). | ||||
PEG2k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
2188 | nd | 2000 | 1.09 |
| PAA1k | 1000 | 1050 | 1000 | 1.18 |
| PVPA1k | 944 | 960 | 2900 | 1.20 |
| PEG2k-b-PAA1k | 2998 | 3130 | 2900 | 1.03 |
| PEG2k-b-PVPA1k | 3820 | 3530 | 3500 | 1.17 |
An analogous PEG2k-b-PAA1k copolymer was prepared by substituting AA for VPA in the method above. As for the PEG-b-PVPA copolymers, SEC analysis revealed narrowly distributed polymer with good agreement between theoretical and observed molecular weights and a clear shift to lower elution volume relative to the macroRAFT agent. All block copolymers prepared in this work are listed in Table 1. Additionally, homopolymers of PAA and PVPA were prepared by RAFT from the carboxy-xanthate RAFT agent X1 (Scheme 1) for comparison with the block copolymers. The differences in Mn values determined by NMR and SEC-RI-MALS for PVPA1K are well understood and have been explained in a recent publication.18b Their characteristics are also shown in Table 1.
B. Synthesis of zinc oxide nanoparticles and stabilization in THF
Formation of ZnO nanoparticles was performed via a one-step hydrolysis of dicyclohexyl zinc ([Zn(Cy)2]) precursor in THF in the presence of n-octylamine (OA) (Scheme 2). | ||
| Scheme 2 Isotropic ZnO NPs synthesized by n-octylamine (OA) and the schematic representation (deduced from NMR results as depicted below) for the coated process by two polymers respectively. | ||
An equimolar ratio of OA to [Zn(Cy)2] was used. The solution containing [Zn(Cy)2] and OA was prepared in a glove box under argon, then a defined quantity of water was added leading after hydrolysis to the formation of ZnO isotropic NPs. This method involves the use of long alkyl chain amine ligands as stabilizers and takes advantage of the exothermic hydrolysis of organometallic complexes in air. Using [Zn(Cy)2] as a precursor provides a safer synthetic method than other organometallic complexes such as diethyl zinc, as its hydrolysis is more easily controllable.19,20 Control over the kinetics of ZnO formation was ensured by the interactions occurring between amine ligands and [Zn(Cy)2] or ZnO NPs, which have been reported to affect the size and shape of the synthesized ZnO NPs21a as well as their optical properties.21b In order to ensure a good control over the ZnO NPs size and morphology, we opted for a ligand exchange process between block copolymer and OA-protected ZnO NPs (Scheme 2) instead of the direct synthesis of the NPs in the presence of the block copolymer.
Block copolymers PEG2k-b-PVPA1k and PEG2k-b-PAA1k were added to the preformed ZnO NPs in THF (Fig. S2 in ESI†). An equimolar ratio of acidic function of copolymer to zinc precursor was used. For these polymers, the presence of several acid functions (either acrylic acid or phosphonic acid groups) should guarantee interactions with ZnO NPs. Moreover, the PEG chains not only act as an external polar layer providing good solubility in organic solvents and in water, but also improve the biocompatibility of the hybrid.
The polymer-modified ZnO NPs were first analyzed by TEM. The diameter of the NPs were 6 ± 4 nm and 5 ± 3 nm respectively for PEG2k-b-PAA1k (Fig. 1A) and PEG2k-b-PVPA1k 48 h after modifications (see Fig. S3C in ESI†). Therefore, as expected, no significant change in NP size was observed compared to the ZnO NPs before modification. As depicted in Fig. 1B, a 2D-plot of the different samples is characteristic of isotropic NPs (cloud point on the diagonal).22
In solution, the intensity-averaged and number-averaged hydrodynamic diameters of these modified ZnO NPs was 20 ± 5 nm for both polymers (Fig. S4 in ESI† and Fig. 1D). Pristine NPs present a number-averaged hydrodynamic diameter of around 200 nm meaning that they are mostly present as aggregates of NPs in THF solutions. Therefore, modification of the surface of NPs by adsorption of the polymer enables isolated (or nearly isolated) ZnO NPs to be obtained in solution. This behavior is corroborated by TEM images showing more dispersed NPs in the case of polymer-modified NPs (see Fig. S3 in ESI†).
ZnO possesses intriguing luminescent properties in the visible range. We studied the photoluminescent properties of ZnO NPs modified by the two polymers. Both solutions were luminescent under a UV lamp (at 365 nm) after redispersion in THF (Fig. S5 in ESI†). All samples display similar absorption spectra, typically a strong absorption is observed up to around 355 nm (≈ 3.49 eV) followed by a sharp decrease (Fig. S6 in ESI†). This is characteristic of nanometric zinc oxide with a band-gap at approximately 365 nm (≈ 3.4 eV). The absorption and emission spectra of polymer-stabilized ZnO NPs are displayed in Fig. 1C and Fig. S7 in ESI.† A broad emission band in the visible region was observed for all samples. This yellow emission was centered at 575 nm for an excitation wavelength between 300 and 360 nm (≈ 4.13 to 3.44 eV) and originates from ZnO surface defects such as oxygen vacancies.23
We then studied the evolution of these photoluminescence properties over a one week period as depicted in Fig. 2 and in Fig. S5–S7 in ESI.† The evolution of luminescence depends strongly on whether polymers are present: luminescence remains constant for pristine ZnO NPs (without polymer), but a strong increase of luminescence (of around 100%) is observed for ZnO NPs modified by the polymers. These modifications may be induced by changes in the ZnO surface induced by polymer adsorption and displacement of OA. No significant change in absorbance was observed for any of the solutions tested, indicating that stable colloidal solutions were obtained in all cases. This is confirmed by TEM and DLS analyses that show no measurable change after one week.
 | ||
| Fig. 2 Evolution through time of (A) normalized absorbance and (B) normalized emission (λexc = 340 nm) of THF dispersions of ZnO NPs coated by n-octylamine or by OA/PEG2k-b-PAA1k or OA/PEG2k-b-PVPA1k (B). Corresponding luminescence of these solutions (samples are irradiated at 365 nm). Normalized absorbance and emission were obtained from Fig. S6 and S7, see ESI† for details. | ||
NMR experiments were performed to obtain further insights on the changes occurring when the polymer is added to ZnO NPs. In THF-d8 (Fig. S8 in ESI†), the 1H NMR lineshapes are sharp, no transfer NOE (tr-NOE) signals are detected for OA H atoms and only one diffusion coefficient is observed with a value of 2.1 × 10−9 m2 s−1 by DOSY NMR, contrary to what has been observed in toluene-d8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 where at least three different modes of interaction of the amines at the surface of the NPs have been observed in thermodynamic equilibrium with the free amines. Thus, in THF, the equilibrium seems to be displaced toward a high population of free amine molecules probably because of competition with THF molecules that can also bind to the ZnO surface.
24 where at least three different modes of interaction of the amines at the surface of the NPs have been observed in thermodynamic equilibrium with the free amines. Thus, in THF, the equilibrium seems to be displaced toward a high population of free amine molecules probably because of competition with THF molecules that can also bind to the ZnO surface.
In THF-d8 and in the presence of PEG2k-b-PAA1k polymer, the OA signals become broader, especially that of CH2(NH2) at 2.6 ppm (Fig. S9 in ESI†). For the PEG2k-b-PAA1k polymer, only the signals of PEG functions between 3.3 and 3.7 ppm are clearly observed, suggesting interaction between the polymer and the nanoparticles surface through AA functions. A decrease in the average diffusion coefficient associated with the PEG moieties is observed in the presence of the nanoparticles (from 3.5 to 0.2 × 10−10 m2 s−1). OA shows a slight decrease of its diffusion coefficient (from 2.1 to 1.5 × 10−9 m2 s−1). Strong tr-NOE signals are observed between intramolecular H atoms of OA and also between H atoms of OA and PEG.25 These results indicate a weak interaction between OA and the PEG2k-b-PAA1k. This interaction explains the notable broadening of the OA 1H resonances, probably due to rapid exchange between free neutral OA molecules and protonated OA molecules interacting with the PEG2k-b-PAA1k polymer through acid–base reaction. Similar results for OA and PEG2k-b-PAA1k are observed when they are mixed in the absence of the ZnO NPs: (i) diffusion coefficients decrease for the PEG moieties (from 3.5 to 0.4 × 10−10 m2 s−1) and for the OA molecules (from 2.1 to 1.5 × 10−9 m2 s−1) and (ii) tr-NOE signals are observed between intramolecular OA H atoms and between OA and PEG H atoms. However, the decrease of the PEG diffusion coefficient and the tr-NOE signal intensities are significantly weaker in the absence of NPs giving evidence for an interaction between the PEG2k-b-PAA1k polymer and the ZnO NPs. OA molecules probably play mostly a role of mobile counter ion (ammonium form) of carboxylate groups in the polymer, in fast exchange with a much higher population of free neutral OA molecules. These results are in agreement with previous observations of ZnO/OA nanoparticles in the presence of long chain alkyl carboxylic acid ligands.26
Solid state NMR spectra performed on ZnO/OA/PEG2k-b-PAA1k gel sample gives additional evidence of direct interactions between the polymer and the surface of the nanoparticle (Fig. 3A and Fig. S10 in ESI†): (i) broad signals associated with the PAA functions are observed in the 13C CPMAS experiment in the presence of ZnO NPs while no signals are detected in the absence of NPs; (ii) the reverse is observed in the 13C MAS experiment where sharp PAA signals are detected only in the absence of nanoparticles. These results indicate a strong rigidification of the PAA groups in presence of the ZnO NPs that lead to: (i) a broadening of PAA resonances due to structural heterogeneity; (ii) an increase of 1H–13C dipolar coupling strength resulting in the appearance of the 13C CPMAS signals and (iii) an increase of the 13C relaxation times resulting in a strong decrease in the signal intensity of the 13C MAS spectrum. These results are certainly due to the interaction of the PAA moieties through the carboxylic functions with the nanoparticle surface.
When PEG2k-b-PVPA1k is used instead of PEG2k-b-PAA1k, the interaction between PVPA moieties and OA is slightly different. DOSY and NOESY experiments showed that the population of OA that interacts weakly with the PVPA polymer is smaller than in the case of the PAA polymer (Fig. S11 in ESI†). However, diffusion filtered 1H NMR (Fig. S12 in ESI†) indicated that a small portion of OA interacts strongly with the PEG2k-b-PVPA1k polymer while for the PEG2k-b-PAA1k polymer a weaker interaction is observed. Broad PVPA signals are detected in the presence of ZnO NPs (Fig. 3B and S13A in ESI†). 31P CPMAS experiments showed that the POO(H) of PVPA becomes more rigid in the presence of OA molecules (Fig. S13B in ESI†). However 13C (CP)MAS NMR experiments were less clear due to the remoteness of carbon atoms from the ZnO surface. The OA/PEG2k-b-PVPA1k and ZnO/OA/PEG2k-b-PVPA1k samples show 31P chemical shift anisotropy that is averaged to zero in the PEG2k-b-PVPA1k polymer alone by local motions. The 31P NMR resonances showed a shift of 6.6 ppm between the OA/PEG2k-b-PVPA1k and ZnO/OA/PEG2k-b-PVPA1k sample due to the interaction of PVPA with the ZnO surface.
All the data collected by NMR, DLS and TEM experiments demonstrate that, in THF, the block copolymers were adsorbed on the surface of ZnO NPs. Additionally, PEG2k-b-PAA1k and PEG2k-b-PVPA1k interact with OA through acid–base reaction.
C. Transfer of ZnO NPs in water
In order to transfer ZnO NPs from THF to water, THF was first evaporated. The resulting powder was dispersed in water and sonicated. The pH of the obtained colloidal solutions was found between 6.8 and 7.2. The presence of the copolymers enabled the transfer of ZnO NPs from THF to water (Fig. 4B and C) whereas ZnO NPs covered by OA alone could not be redispersed in water (Fig. 4A). In the presence of polymers, the transfer was visualized by yellow luminescence of ZnO and the number of NPs solubilized in the aqueous phase was evaluated from measurements of the absorbance. The absorbance spectra display clear evidence of the presence of ZnO/copolymer nanohybrids dispersed in the water solution. TEM observations of the ZnO NPs before and after transfer showed that the morphology of the ZnO NPs (diameter 6 ± 3 nm, Fig. S14 in ESI†) is not significantly modified. DLS (Fig. S15 in ESI†) was used to determine a number average hydrodynamic diameter of around 20 nm. This value is comparable to that obtained in THF before transfer. Hence a successful transfer of modified ZnO NPs as single NPs from THF to water was obtained.To further investigate the effect of polymer structure on the stabilization of ZnO NPs we added a concentrated NaCl solution to reach a final concentration of 5 mmol L−1 (Fig. 5). Under these conditions initial OA-covered ZnO NPs aggregate quickly and then totally precipitate in one day. The same phenomenon is observed in the presence of the homopolymers PAA1k and PEG2k. In all these cases, electrostatic repulsions should be responsible (at least in part) for the colloidal stability. Only the use of copolymer PEG2k-b-PAA1k produced solution that were stable for 10 days, even in the presence of salt. The same behavior was observed for PEG2k-b-PVPA1k. This clearly demonstrates that the block structure of the polymer with a PEG block is necessary to obtain nanohybrids with enhanced colloidal stability in water.
The luminescence of the NP/copolymer systems in water was then studied over several days. For both copolymers, a decrease of the absorbance (Fig. 6A) and of the luminescence (Fig. 6B and Fig. S16 in ESI†) were observed through time. This could be related to two phenomena: (i) a partial precipitation or (ii) a structural modification or digestion of ZnO particles over time.
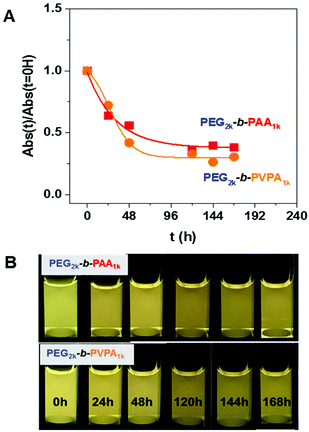 | ||
| Fig. 6 (A) Evolution through time of normalized absorbance at 350 nm of ZnO NPs coated by n-octylamine or by OA/PEG2k-b-PAA1k or OA/PEG2k-b-PVPA1k after transfer in water after 0, 24, 48, 120, 144 and 168 hours (normalized absorbance was obtained from Fig. S16, see ESI† for details). (B) Corresponding luminescence of these solutions (samples are irradiated at 365 nm). | ||
When the NMR study is performed in D2O for ZnO/OA, the signals of strongly bound ligand at the ZnO surface are observed (with a diffusion filter experiment as depicted in Fig. S17 in ESI†) but disappears quickly with time. Moreover a release of OA and THF molecules in the solution is observed that induces the precipitation of the nanoparticles over time. Such results should be related to hydroxylation of the nanoparticle surface.27 Interestingly, in the presence of PEG2k-b-PAA1k, the release of OA and THF is significantly slower (Fig. S18 in ESI†). The hydroxylation of the surface is delayed. Interaction of OA with PAA may account for this result. The system is slightly less stable in the presence of PEG2k-b-PVPA1k (Fig. S19 in ESI†) as shown by 1D diffusion filter experiments that showed a faster loss of the population of slow diffusing species (OA and polymer in interaction with NPs) over time in presence of PEG2k-b-PVPA1k than in presence of PEG2k-b-PAA1k. During this time, only release from the NP surface of THF molecules and of a small quantity of OA molecules is observed by 1D 1H NMR, ruling out OA and PVPA precipitation. These results suggest a digestion of ZnO NPs over time with PEG2k-b-PVPA1k.
To summarize, in water, the interaction between PEG2k-b-PAA1k or PEG2k-b-PVPA1k and OA is favorable since the presence of the polymer prevents fast hydroxylation of the surface. The release of OA from the surface is significantly slower than for ZnO/OA and the precipitation of the nanoparticles is delayed. However, in the case of PVPA digestion of the nanoparticles over time is suggested.
D. Stability of ZnO nanorods
Further experiments were performed with ZnO nanorods, whose shape will enable us to have further insight on this digestion process. ZnO nanorods were obtained by hydrolysis of the zinc precursor in presence of 1 molar equivalent of OA without additional solvent at room temperature over 12 hours. Fig. 7 depicts a TEM image of the obtained nanorods. Their dimensions are 36 ± 14 nm by 6 ± 1 nm.The nanorods were further modified by PEG2k-b-PAA1k or PEG2k-b-PVPA1k in a similar manner to that described for isotropic ZnO NPs in THF and then transferred to water. TEM images were then recorded at different aging times to follow the evolution of shape. Fig. 8 and Table 2 give an overview of the obtained results. In THF, a decrease of length of the ZnO nanorods covered by PEG2k-b-PAA1k is observed (Fig. 8A and B). This decrease is more significant when PEG2k-b-PVPA1k is used as a stabilizing agent. Indeed, 2D-plots show a strong decrease of nanoparticle length over time (from 45 ± 26 nm to 20 ± 17 nm after 7 days).
| No polymers (nm) | +PEG2k-b-PAA1k (nm) | +PEG2k-b-PVPA1k (nm) | |
|---|---|---|---|
| Length (0 h) | 36 ± 14 | 45 ± 26 | 34 ± 20 |
| Width (0 h) | 6 ± 1 | 6 ± 2 | 6 ± 1 |
| Length (7 days) | — | 20 ± 17 | 13 ± 8 |
| Width (7 days) | — | 5 ± 1 | 5 ± 2 |
In water, this phenomenon is less pronounced (Fig. S20 and Table S1†), confirming NMR results that show a less reactive surface than in the case of THF. This digestion is slow and could therefore be of great interest for biological applications, as it will guarantee the disappearance of residual NPs after use and thus minimize long-term toxicity issues.
3. Experimental section
A. Materials and methods
Size exclusion chromatography (SEC) was performed on an Agilent 1100 HPLC system, an 18-angle Multi-Angle Light Scattering (MALS) DAWN-Heleos-II (Wyatt Technology, Santa Barbara, CA, US), an OptilaRex Refractometer (Wyatt Technology, Santa Barbara, CA, US) and a set of 2 columns (Shodex SB-806 M and SB-802.5) thermostated at 30 °C. Water (NaCl 100 mmol L−1, NaH2PO4 25 mmol L−1, Na2HPO4 25 mmol L−1, buffer solution at pH = 7) was used as eluent with a flow rate of 1.0 mL min−1. The dn/dc of PVPA (0.144 mL g−1) was measured at 620 nm using a DNDC-2010 differential refractometer (PSS).
Solid state NMR experiments were recorded on a Bruker AvanceIII 400 spectrometer. Samples were packed into 4 mm zirconia rotors inside a glove box. The rotors were spun at 8 or 10 kHz at 293 K. 13C MAS with direct polarization were acquired with a small flip angle of 30° and a recycle delay of 10 s. 13C and 31P with Cross Polarization (CP) were recorded with a recycle delay of 1.5 s and a contact time of 2 ms. 1H and 13C chemical shifts are relative to TMS and 31P chemical shifts were referenced to an external 85% H3PO4 sample.
B. Synthesis
Synthesis of PEG-Br. PEG-X1 was obtained using commercially available CH3(OCH2CH2)45OH (PEG-OH, M = 2012 g mol−1). PEG-OH (20 g, 0.01 mol), Et3N (1.77 g; 0.03 mol), CH2Cl2 (50 mL) were placed in the flask and the mixture was degassed by bubbling argon for 10 min. Then, 2-bromopropionyl bromide was added dropwise at 0 °C. The reaction mixture temperature was raised to room temperature and stirred for 20 h. The white precipitate (Et3NHBr) was filtered and the solvent was evaporated under reduced pressure. 200 mL of CH2Cl2 was added and the mixture was washed with a saturated ammonium chloride solution (2 × 30 mL), next with a sodium carbonate saturated solution (4 × 40 mL) and finally with pure water (5 × 50 mL). The organic phase was dried over Na2SO4. After filtration and evaporation of the solvent, 17.2 g (80%) of a white powder was obtained.
Synthesis of PEG-X1. PEG-Br (16.7 g, 7.64 mmol) was placed in a flask under of argon atmosphere. The solvent (anhydrous CH2Cl2, 100 mL) and ethyl potassium xanthate (3.75 g, 23.4 mmol) were added. The reaction mixture was stirred at room temperature for 17 h. Then, half of the solvent was evaporated under reduced pressure, the solution was filtered to remove precipitated salt (KBr). The resulting solution was viscous and yellow. The product was precipitated from pentane (3 × 200 mL) and dried under reduced pressure. 12.93 g of beige powder was obtained (77%). PEG2K-X1 1H NMR δ (ppm) (D2O, 300.13 MHz): 1.33 (t, 3H, –CH2–CH3), 1.49 (d, 3H, CH–CH3), 3.32 (s, 3H, –O–CH3), 3.50–3.70 (m, –O–CH2–CH2–O–), 4.32–4.42 (m, 1H, CH–CH3), 4.55–4.65 (m, 2H, –CH2–CH3).
C. Modification of ZnO NPs by polymer and transfer in water
250 μL of ZnO NPs THF solution were mixed with THF solutions of polymers (with a molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 between OA and acidic function AA or VPA). The volume was adjusted to 1 mL and sonicated for 5 min. Then 50 μL of this mixture was diluted 40-times by THF and sonicated for 2 min. For transferring NPs into water, 50 μL of the pristine solution in THF was taken and THF was removed by vacuum pump. 2 mL of water was added to the residue and sonicated for 2 min.
1 between OA and acidic function AA or VPA). The volume was adjusted to 1 mL and sonicated for 5 min. Then 50 μL of this mixture was diluted 40-times by THF and sonicated for 2 min. For transferring NPs into water, 50 μL of the pristine solution in THF was taken and THF was removed by vacuum pump. 2 mL of water was added to the residue and sonicated for 2 min.
4. Conclusions
Low molar mass poly(ethylene glycol)-poly(vinylphosphonic acid) and poly (ethylene glycol)-poly(acrylic acid) block copolymers (PEG-b-PVPA and PEG-b-PAA) were synthesized by RAFT/MADIX polymerization and characterized. These two types of polymers were then used to modify the surface of preformed ZnO NPs covered by amine ligands.After modification, stable colloidal solutions were obtained in THF. Interestingly, whereas luminescence remains constant for pristine ZnO NPs, an increase of luminescence is observed for ZnO NPs modified by the two types of polymers. These modifications may be ascribed to changes in the ZnO surface induced by polymer adsorption and displacement of OA. NMR experiments showed the adsorption of PEG2k-b-PAA1k and PEG2k-b-PVPA1k on the surface of ZnO NPs and additional interaction with OA through acid–base reaction. A higher affinity with OA is observed in the case of PVPA-based polymers but this does not significantly impact the colloidal and luminescent properties in THF compared to PAA-based polymers.
In water, whereas pristine ZnO NPs covered by OA quickly precipitate, stable colloidal solutions were obtained for polymer-modified ZnO NPs as a result of their diblock structure. In both cases, the release of OA from the surface is significantly slower than for ZnO/OA and the precipitation of the nanoparticles is consequently delayed. Moreover the interactions between PEG2k-b-PAA1k or PEG2k-b-PVPA1k and ZnO surface and OA prevent fast hydroxylation of the surface. Nevertheless for both copolymers, a decrease of the absorbance and of the luminescence are observed over a long period of time (typically several days). As demonstrated on ZnO nanorods, a slow digestion mechanism is suggested. This phenomenon occurred more rapidly in the case of ZnO NPs covered with PVPA-based copolymer than for the PAA-based copolymer. This mechanism could be of great interest in biological applications to avoid the accumulation of NPs in vivo. This will be the focus of further studies.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to thank the China Scholarship Council (CSC) and the CNRS for funding.References
- S. Saliba, C. Valverde Serrano, J. Keilitz, M. L. Kahn, C. Mingotaud, R. Haag and J.-D. Marty, Chem. Mater., 2010, 22, 6301 CrossRef CAS.
- W. C. Chan and S. Nie, Science, 1998, 281, 2016 CrossRef CAS; M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisato, Science, 1998, 281, 2013 CrossRef.
- Y. Wu, C. Lim, S. Fu, A. Tok, H. Lau, F. Boey and X. Zeng, Nanotechnology, 2007, 18, 215604 CrossRef.
- M. Beija, N. Lauth-de Viguerie, R. Salvayre and J.-D. Marty, Trends Biotechnol., 2012, 30(9), 485 CrossRef CAS.
- L. Spanhel and M. A. Anderson, J. Am. Chem. Soc., 1991, 113, 2826 CrossRef CAS.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55 RSC.
- P. Zrazhevskiy, M. Sena and X. Gao, Chem. Soc. Rev., 2010, 39, 4326 RSC.
- S. A. Kulinich, J. S. Qiu, W. J. Qin, R. Li, J. Sun and J. Liu, J. Am. Chem. Soc., 2007, 129, 16029 CrossRef.
- H. M. Xiong, Y. Xu, Q. G. Ren and Y. Y. Xia, J. Am. Chem. Soc., 2008, 130, 7522 CrossRef CAS.
- (a) N. R. Jana, H. H. Yu, E. M. Ali, Y. Zheng and J. Y. Ying, Chem. Commun., 2007, 14, 1406 RSC; (b) R. O. Moussodia, L. Balan and R. Schneider, New J. Chem., 2008, 32, 1388 RSC.
- X. Tang, E. S. G. Choo, L. Li, J. Ding and J. Xue, Chem. Mater., 2010, 22, 3383 CrossRef CAS.
- J. Rubio-Garcia, Y. Coppel, P. Lecante, C. Mingotaud, B. Chaudret, F. Gauffre and M. L. Kahn, Chem. Commun., 2011, 47, 988 RSC.
- M. Beija, J.-D. Marty and M. Destarac, Prog. Polym. Sci., 2011, 36, 845 CrossRef CAS.
- W. Brullot, N. K. Reddy, J. Wouters, V. K. Valev, B. Goderis, J. Vermont and T. J. Verbiest, J. Magn. Magn. Mater., 2012, 324, 1919 CrossRef CAS.
- R. Quiñones, K. Rodriguez and R. Iuliucci, Thin Solid Films, 2014, 565, 155 CrossRef.
- (a) K. Markiewicz, L. Seiler, I. Misztalewska, K. Winkler, S. Harrisson, A. Wilczewska, M. Destarac and J.-D. Marty, Polym. Chem., 2016, 7, 6391 RSC; (b) T. Sahoo, H. Pizem, T. Fried, D. Golodnitsky, L. Burstein, C. M. Subenik and G. Markovich, Langmuir, 2001, 17, 7907 CrossRef; (c) C. Boyer, V. Bulmus, P. Priyanto, W. Yang Teoh, R. Amal and T. P. Davis, J. Mater. Chem., 2009, 19, 111 RSC; (d) L. Qi, A. Sehgal, J.-C. Castaing, J.-P. Chapel, J. Fresnais, J.-F. Berret and F. Cousin, ACS Nano, 2008, 2, 879 CrossRef CAS; (e) C. Ghorbil, G. Popa, A. Parat, C. Billotey, J. Taleb, P. Bonazza, S. Begin-Colin and D. Felder-Flesch, Chem. Commun., 2013, 49, 9158 RSC.
- G. Pound, F. Aguesse, J. B. McLeary, R. F. Lange and B. Klumperman, Macromolecules, 2007, 40, 8861 CrossRef CAS.
- (a) G. Layrac, C. Gerardin, D. Tichit, S. Harrisson and M. Destarac, Polymer, 2015, 72, 292 CrossRef CAS; (b) L. Seiler, J. Loiseau, F. Leising, P. Boustingorry, S. Harrisson and M. Destarac, Polym. Chem., 2017, 8, 3825 RSC.
- M. Monge, M. L. Kahn, A. Maisonnat and B. Chaudret, Angew. Chem., Int. Ed., 2003, 42, 5321 CrossRef CAS.
- R. O. Moussodia, L. Balan, C. Merlin, C. Mustin and R. Schneider, J. Mater. Chem., 2010, 20, 1147 RSC.
- (a) M. Vaseem, A. Umar and Y. B. Hahn, in Metal Oxide Nanostructures and Their Applications, Vol 5, ed. A. Umar and Y.-B. Hahn, American Scientific Publishers, 2010, ch. 4, pp. 1–36 Search PubMed; (b) M. A. Vergés, A. Mifsud and C. Serna, J. Chem. Soc., Faraday Trans., 1990, 86, 959 RSC.
- Z. Zhao, Z. Zheng, C. Roux, C. Delmas, J.-D. Marty, M. L. Kahn and C. Mingotaud, Chem. – Eur. J., 2016, 22, 12424 CrossRef CAS.
- A. Van Dijken, E. Meulenkamp, D. Vanmaekelbergh and A. Meijerink, J. Lumin., 2000, 90, 123 CrossRef CAS.
- Y. Coppel, G. Spataro, B. Chaudret, A. Maisonnat and M. L. Kahn, Chem. – Eur. J., 2012, 18, 5384 CrossRef CAS.
- B. Fritzinger, I. Moreels, P. Lommens, R. Koole, Z. Hens and J. C. Martins, J. Am. Chem. Soc., 2009, 131, 3024 CrossRef CAS.
- Y. Coppel, G. Spataro, V. Collière, B. Chaudret, C. Mingotaud, A. Maisonnat and M. L. Kahn, Eur. J. Inorg. Chem., 2012, 2691 CrossRef CAS.
- C. N. Valdez, A. M. Schimpf, D. R. Gamelin and J. M. Mayer, ACS Nano, 2014, 8, 9463 CrossRef CAS.
- I. Blidi, R. Geagea, O. Coutelier, M. Mazières, F. Violleau and M. Destarac, Polym. Chem., 2012, 3, 609 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8py01387j |
| ‡ Actual address: Science on Nuclear Wastes and Environmental Safety Laboratory, Sichuan Civil-military Integration Institute, School of life Science and Engineering, Southwest University of Science and Technology, Mianyang, China. |
| This journal is © The Royal Society of Chemistry 2019 |