Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Trehalose-cored amphiphiles for membrane protein stabilization: importance of the detergent micelle size in GPCR stability
Manabendra
Das
a,
Yang
Du
b,
Jonas S.
Mortensen
c,
Manuel
Ramos
d,
Lubna
Ghani
a,
Ho Jin
Lee
a,
Hyoung Eun
Bae
a,
Bernadette
Byrne
e,
Lan
Guan
d,
Claus J.
Loland
c,
Brian K.
Kobilka
b and
Pil
Seok Chae
*a
aDepartment of Bionanotechnology, Hanyang University, Ansan, 155-88, Korea. E-mail: pchae@hanyang.ac.kr
bMolecular and Cellular Physiology, Stanford University, Stanford, CA 94305, USA. E-mail: kobilka@stanford.edu
cDepartment of Neuroscience, University of Copenhagen, DK-2200, Copenhagen, Denmark. E-mail: cllo@sund.ku.dk
dDepartment of Cell Physiology and Molecular Biophysics, Center for Membrane Protein Research, School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA. E-mail: lan.guan@ttuhsc.edu
eDepartment of Life Sciences, Imperial College London, London, SW7 2AZ UK. E-mail: b.byrne@imperial.ac.uk
First published on 7th May 2019
Abstract
Correction for ‘Trehalose-cored amphiphiles for membrane protein stabilization: importance of the detergent micelle size in GPCR stability’ by Manabendra Das et al., Org. Biomol. Chem., 2019, 17, 3249–3257.
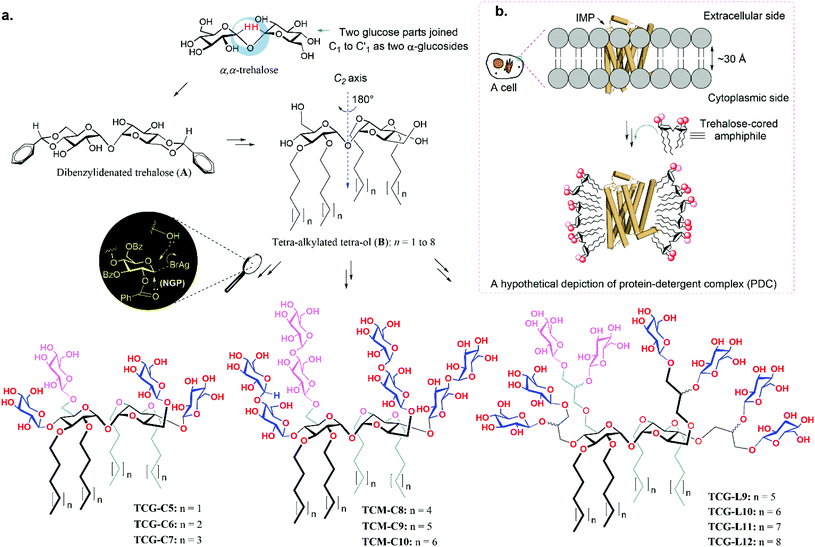
The authors regret that there were errors in the chemical structures of the amphiphiles in Fig. 2a and 3a. All the sugar units of TCG-C5 to TCM-C10 are identical (i.e. β-D-glucose for TCG-C5 to TCG-C7 and β-D-maltose for TCM-C8 to TCM-C10). The correct figures are shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |