Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Substituent-controlled racemization of dissymmetric coordination capsules
Kentaro
Harada
,
Ryo
Sekiya
,
Takeshi
Maehara
and
Takeharu
Haino
*
Department of Chemistry, Graduate School of Science, Hiroshima University, 1-3-1, Kagamiyama, Higashi-Hiroshima, Hiroshima, 739-8526, Japan. E-mail: haino@hiroshima-u.ac.jp
First published on 24th April 2019
Abstract
Correction for ‘Substituent-controlled racemization of dissymmetric coordination capsules’ by Kentaro Harada et al., Org. Biomol. Chem., 2019, DOI: 10.1039/c9ob00388f.
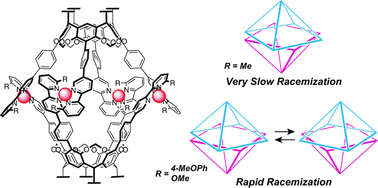
The authors regret that there were errors in Fig. 7 and the graphic abstract. The correct graphics are shown below.
| This journal is © The Royal Society of Chemistry 2019 |