Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
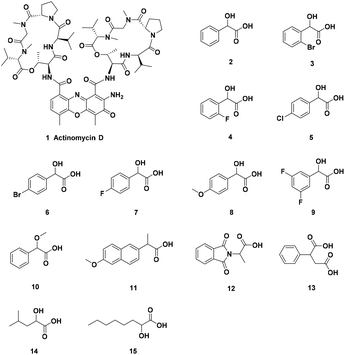
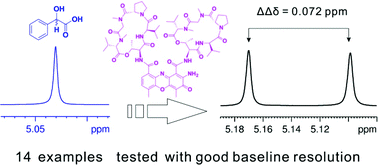
Correction: Enantiomeric NMR discrimination of carboxylic acids using actinomycin D as a chiral solvating agent
Liwen
Bai
,
Pian
Chen
,
Jiangxia
Xiang
,
Jiarui
Sun
and
Xinxiang
Lei
 *
*
School of Pharmaceutical Sciences, South Central University for Nationalities, Wuhan, 430074, P.R. China. E-mail: xxlei@mail.scuec.edu.cn
First published on 28th March 2019
Abstract
Correction for ‘Enantiomeric NMR discrimination of carboxylic acids using actinomycin D as a chiral solvating agent’ by Liwen Bai, et al., Org. Biomol. Chem., 2019, 17, 1466–1470.
The authors regret that an incorrect structure for actinomycin D was included in Scheme 1, the graphical abstract and the ESI. The correct graphics are shown below.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2019 |