Four-component acyloxy-trifluoromethylation of arylalkenes mediated by a photoredox catalyst†
Xiaocong
Zhou
ab,
Guijie
Li
 *a,
Zongzhou
Shao
a,
Kun
Fang
a,
Hongjun
Gao
b,
Yuanqiang
Li
b and
Yuanbin
She
*a
*a,
Zongzhou
Shao
a,
Kun
Fang
a,
Hongjun
Gao
b,
Yuanqiang
Li
b and
Yuanbin
She
*a
aCollege of Chemical Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, People's Republic of China. E-mail: guijieli@zjut.edu.cn; sheyb@zjut.edu.cn
bZhejiang Jiuzhou Pharmaceutical Technology Co., Ltd., Hangzhou, Zhejiang 310051, People's Republic of China
First published on 9th October 2018
Abstract
A four-component intermolecular trifluoromethylation–acyloxylation of arylalkenes induced by visible light has been developed in the presence of the photoredox catalyst Ru(bpy)3(PF6)2 under mild reaction conditions. A new Umemoto's reagent was used as a trifluoromethyl radical source, and this redox neutral reaction demonstrated good functional group tolerance for aryl alkenes with high yields up to 91%. The detailed reaction process was investigated based on control, deuterium and O18-labeling experiments to support that N,N-dimethylformamide (DMF)/H2O acted as an acyloxyl source.
Introduction
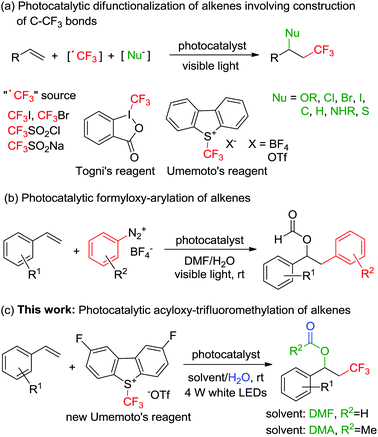
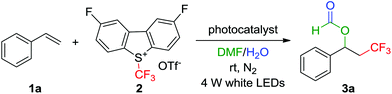
The trifluoromethyl (CF3) group is a useful structural motif in many biologically active molecules because it influences their chemical and metabolic stabilities, lipophilicity, and binding selectivities.1 Thus, the development of new methodologies for highly efficient introduction of the CF3 group into organic molecules has attracted plenty of attention in organic synthesis and drug discovery.2 Among the approaches, photocatalytic difunctionalization of unsaturated double bonds induced by visible light is considered as a useful protocol for the synthesis of diverse trifluoromethylated compounds.3,4 Over the past decade, various trifluoromethylation reactions of alkenes,5 like oxytrifluoromethylation,6 halotrifluoromethylation,7 carbotrifluoromethylation,8 hydrotrifluoromethylation,9 aminotrifluoromethylation,10 and thiotrifluoromethylation,11 have been reported using CF3I,7a,9c CF3Br,5k CF3SO2Cl,7b,c CF3SO2Na,9b,10c Togni's reagent,8a–d or Umemoto's reagent7d,8e,9a,10a,b as a CF3 radical source (Scheme 1a). Despite these achievements, other methods to make structurally complex trifluoromethylated compounds still need to be developed. There have been some reports employing N,N-dimethylformamide (DMF) as the solvent and acetylation reagent in a visible light-induced photocatalytic reaction.12 Recently, König's group reported an intermolecular tandem arylation/formyloxyarylation of alkenes by the photoredox Meerwein reaction (Scheme 1b).12d However, the acyloxy-trifluoromethylation of alkenes with DMF has not been reported. Here, we report the first visible light-promoted tandem acyloxy-trifluoromethylation of alkenes (Scheme 1c).Results and discussion
Recently, a new Umemoto's reagent was developed which was thermally stable, air-stable, and one-pot-preparable,13 and has been used as a CF3 reagent in several types of reactions by Umemoto and co-workers.13,14 We initially examined the photocatalytic reaction of styrene and the air-stable Umemoto's reagent using eosin Y as the photocatalyst irradiated with 4 W white LEDs for 2 h, and only a trace amount of product was obtained (Table 1, entry 1). Rose Bengal did not work for the reaction (entry 2). However, 52% yield could be obtained in the presence of 1 mol% of Ru(bpy)3(PF6)2 in the same reaction system (entry 3). Prolonging the reaction time to 6 h, Umemoto's reagent 2 could be consumed completely and 3a was obtained in 75% yield (entry 5). Moreover, both trifluoromethanesulfonate and tetrafluoroborate of 2 gave similar results (entries 4 and 5). The reaction yield could be further enhanced by increasing the amount of styrene (entries 7–9). However, the amount of water had little effect on the yields (entries 5, 10 and 11). Furthermore, the photocatalyst loading was also investigated, and 0.5 mol% of Ru(bpy)3(PF6)2 could also give a satisfactory result with a 74% isolated yield (entries 7, 12–14). Control experiments showed that both the photocatalyst and visible light were essential for the success of the tandem acyloxy-trifluoromethylation reaction (entries 15 and 16). Other CF3 reagents, like CF3SO2Cl, CF3SO2Na and Togni's reagent, were also investigated, and no product was detected (entries 17–19). These results demonstrated the advantage of the new Umemoto's reagent 2 as an electrophilic trifluoromethylating agent for this reaction using Ru(bpy)3(PF6)2 as the photocatalyst. The old Umemoto's reagent can also be used as an efficient CF3 radical source (entries 20 vs. 6). Additionally, different Ru(II) and Ir(III)-based photocatalysts as well as various additives were also investigated (see Table S1†).| Entry | Catalyst | 1a/eq. | H2O/eq. | Time/h | Yielda/% |
|---|---|---|---|---|---|
| a The yields were determined by 19F NMR spectroscopy of crude reaction mixtures with 1-chloro-4-(trifluoromethyl)benzene as an internal standard, and isolated yields are given in parentheses for some cases. b Tetrafluoroborate of 2 was used. c No light. d CF3SO2Cl was used as a CF3 reagent. e CF3SO2Na was used as a CF3 reagent. f Togni's reagent was used as a CF3 reagent. g Old Umemoto's reagent was used. | |||||
| 1 | Eosin Y (1.0 mol%) | 1.0 | 1.0 | 2 | Trace |
| 2 | Rose Bengal (1.0 mol%) | 1.0 | 1.0 | 2 | NR |
| 3 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 1.0 | 2 | 52 |
| 4 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 1.0 | 4 | 71 |
| 5 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 1.0 | 6 | 75 |
| 6b | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 1.0 | 6 | 74 |
| 7 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.2 | 1.0 | 6 | 78 (76) |
| 8 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.5 | 1.0 | 6 | 79 (79) |
| 9 | Ru(bpy)3(PF6)2 (1.0 mol%) | 2.0 | 1.0 | 6 | 80 |
| 10 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 2.0 | 6 | 74 |
| 11 | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 5.0 | 6 | 73 |
| 12 | Ru(bpy)3(PF6)2 (1.5 mol%) | 1.2 | 1.0 | 6 | 76 |
| 13 | Ru(bpy) 3 (PF 6 ) 2 (0.5 mol%) | 1.2 | 1.0 | 6 | 78 (74) |
| 14 | Ru(bpy)3(PF6)2 (0.1 mol%) | 1.2 | 1.0 | 6 | 72 |
| 15 | No photocatalyst | 1.2 | 1.0 | 6 | NR |
| 16c | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.2 | 1.0 | 6 | NR |
| 17d | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.2 | 1.0 | 6 | 0 |
| 18e | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.2 | 1.0 | 6 | 0 |
| 19f | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.2 | 1.0 | 6 | 0 |
| 20b,g | Ru(bpy)3(PF6)2 (1.0 mol%) | 1.0 | 1.0 | 6 | 70 |
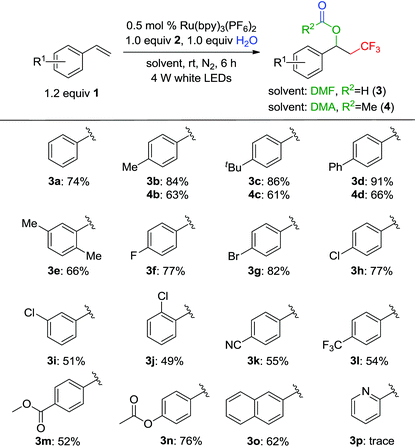
By employing the optimized conditions, a series of aryl alkenes were used to investigate the generality of the above visible light-promoted formyloxy-trifluoromethylation reaction in DMF (Table 2). The aryl alkenes were compatible with various electron-donating groups (3b–3e) and halogen substituents (3f–3j) to afford the desired products in 49–91% yields. However, the chlorine substituent on the meta- and ortho-positions resulted in a lower yield than that on the para-position. The aryl alkenes bearing a strong electron-withdrawing group, like the cyano or trifluoromethyl group, afforded products 3g–3m in 52–55% yields. The aryl alkene substrates with the ester or steric hindrance group could also proceed smoothly to give products 3n and 3o in 76% and 62% yields, respectively. However, 2-vinylpyridine was incompatible with this reaction and only a trace amount of product 3l was observed, which may be attributed to the poor stability of the 2-pyridinylmethyl cation compared with the benzyl cation.15 Notably, N,N-dimethylacetamide (DME) could also be used as a solvent and acetoxylation reagent to generate the corresponding products 4b–4d.
| a Isolated yields. |
|---|
|
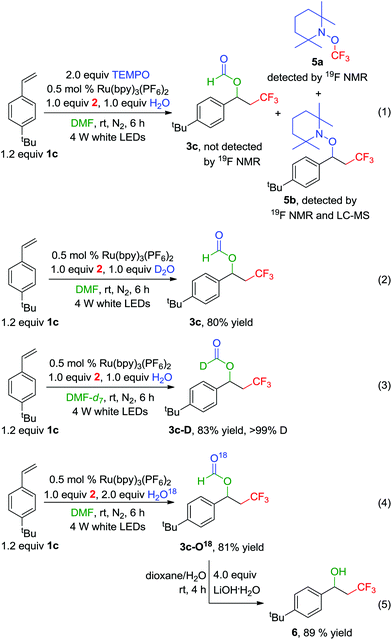
For gaining insight into the mechanism of the reaction, control experiments were conducted as shown in Scheme 2. In the presence of 2 equiv. of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), the acyloxy-trifluoromethylation of the 1-(tert-butyl)-4-vinylbenzene 1c was quenched, and no 3c could be detected by in situ19F NMR spectroscopy. However, as expected, the signals of both products 5a and 5b were observed in the in suit 19F NMR spectrum, whose chemical shifts were consistent with those reported in the literature16,17 (see the ESI†). 5b could also be detected through LC-MS (eqn (1)). These results indicated that a radical-mediated pathway is involved. Moreover, replacing H2O with D2O (99.9% D) just afforded no deuterated product 3c (eqn (2)); however, the deuterated product 3c-D was obtained in 83% yield with >99% D incorporation on using DMF-d7 (99.5% D) as the solvent (eqn (3)). These results demonstrated that the hydrogen atom of the formyloxy group was from the DMF, rather than H2O. What's more, O18-labeling experiments were also conducted using H2O18 (97% O18) instead of H2O. The O18-labeled product 3c-O18 was successfully isolated in 81% yield (eqn (4)), which was further hydrolyzed to give the no O18-labeled product benzyl alcohol 6 (eqn (5)). These results supported that the oxygen atom of the formyl group in 3c-O18 was from H2O, and the other oxygen atom originated from DMF. All the products 3c, 3c-D, 3c-O18, 5 and 6 were further confirmed by HRMS analyses (see the ESI†).
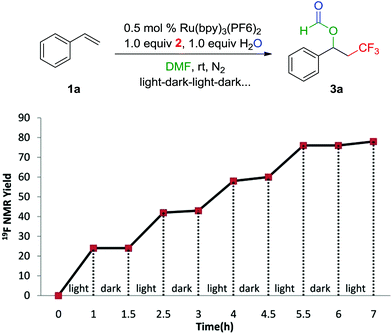
The light–dark interval experiment was also performed to investigate the possibility of the radical propagation. From Scheme 3, it can be clearly seen that continuous irradiation with visible light is essential for the transformation. Moreover, the quantum yield of this photochemical reaction was found to be 39%. Although a radical propagation cannot be completely excluded, these results demonstrated that the radical propagation (Scheme 4) is not a dominant reaction pathway, which were also described in the previous reports.6a,18–20
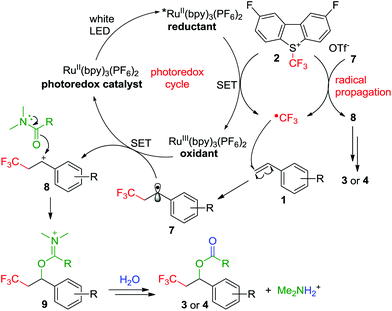
Based on the above results and previous literature reports,12 a plausible mechanism is depicted in Scheme 4. Photoexcitation of RuII(bpy)3(PF6)2 is initiated with a white LED, followed by single-electron reduction of 2 to generate RuIII(bpy)3(PF6)2 and the electrophilic trifluoromethyl radical through the single-electron transfer (SET) pathway. Then the trifluoromethyl radical rapidly combines with arylalkene 1 to furnish the stable benzyl radical 7, which was supported by the control experiment (Scheme 2, eqn (1)). Radical 7 can be oxidized by RuIII(bpy)3(PF6)2 through the other SET process to afford benzyl carbocation 8, which was subject to nucleophilic attack by DMF or DME to give imine intermediate 9. Hydrolysis of 9 yielded the desired product 3 or 4, which was also supported by the deuterated and O18-labeling experiments discussed above.
Conclusions
In conclusion, we have successfully developed a visible-light-induced four-component acyloxy-trifluoromethylation of arylalkenes using Ru(bpy)3(PF6)2 as the photoredox catalyst. This difunctionalization approach is a redox neutral reaction with complete regioselectivity. Moreover, the detailed reaction process of this transformation was studied based on the radical quenching experiment, in suit 19F NMR spectrum, LC-MS study, deuterium and O18-labeling experiments. This approach will be useful in organic synthesis and drug discovery fields owing to the mild reaction conditions, high efficiencies, reasonably broad substrate scope and good functional group tolerance, and also the compatibility with various substrates of the aryl alkenes and amides.Experimental
General procedure for the reaction of arylalkenes and Umemoto reagent
A 10 mL Schlenk tube equipped with a magnetic stir bar was charged with arylalkene 1 (1.2 equiv.), Umemoto reagent 2 (1.0 equiv.), DMF or DMA (5.0 mL mmol−12), H2O (1.0 equiv.) and Ru(bpy)3(PF6)2 (0.5 mol%). The mixture was evacuated and back-filled with nitrogen three times. Then the tube was irradiated for 6 h with a 4 W white LED lamp placed at a distance of 1–2 cm. After that, 20 mL of water was added to the reaction mixture, which was then extracted with ethyl acetate (50 mL × 3). The combined organic phases were washed with water, dried over MgSO4 and concentrated in a vacuum. The residue was purified by silica gel column chromatography to give the corresponding product 3 or 4.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (21602198, 21878276, 21776259 and 21476270) and the “Qianjiang Talents Plan” (Grant No. QJD1602017) for financial support. We thank Dr Teruo Umemoto for his technical instructions on the reaction of Umemoto's reagents and for reviewing the manuscript.Notes and references
- (a) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2004 CrossRef; (b) J.-P. Bégué and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley-VCH, Weinheim, 2008 CrossRef; (c) K. Müller, F. Fach and D. Diederich, Science, 2007, 317, 1881 CrossRef; (d) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC; (e) J. Wang, M. Sanchez-Rosello, J. Luis Acena, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS.
- For selected reviews and articles, see: (a) T. Umemoto, Chem. Rev., 1996, 96, 1757 CrossRef CAS; (b) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475 CrossRef CAS; (c) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470 CrossRef CAS; (d) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (e) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed; (f) C. Zhang, Org. Biomol. Chem., 2014, 12, 6580 RSC; (g) C. Zhang, Adv. Synth. Catal., 2014, 356, 2895 CrossRef CAS; (h) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513 CrossRef CAS; (i) S. Barata-Vallejo, B. Lantaño and A. Postigo, Chem. – Eur. J., 2014, 51, 16806 CrossRef; (j) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS; (k) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683 CrossRef CAS; (l) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS; (m) C. Alonso, E. Marigorta de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS; (n) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS; (o) S.-M. Wang, J.-B. Han, C.-P. Zhang, H.-L. Qin and J.-C. Xiao, Tetrahedron, 2015, 71, 7949 CrossRef CAS; (p) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765 CrossRef CAS; (q) G. Yin, X. Mu and G. Liu, Acc. Chem. Res., 2016, 49, 2413 CrossRef CAS; (r) T. Chatterjee, N. Iqbal, Y. You and E. J. Cho, Acc. Chem. Res., 2016, 49, 2284 CrossRef CAS; (s) M. Zhou, C. Ni, Z. He and J. Hu, Org. Lett., 2016, 18, 3754 CrossRef CAS; (t) T. Ide, S. Masuda, Y. Kawato, H. Egami and Y. Hamashima, Org. Lett., 2017, 19, 4452 CrossRef CAS; (u) J. B. Geri and N. K. Szymczak, J. Am. Chem. Soc., 2017, 139, 9811 CrossRef CAS; (v) H. Shen, Z. Liu, P. Zhang, X. Tan, Z. Zhang and C. Li, J. Am. Chem. Soc., 2017, 139, 9843 CrossRef CAS; (w) X. Tan, Z. Liu, H. Shen, P. Zhang, Z. Zhang and C. Li, J. Am. Chem. Soc., 2017, 139, 12430 CrossRef CAS; (x) M. Imiołek, G. Karunanithy, W.-L. Ng, A. J. Baldwin, V. Gouverneur and B. G. Davis, J. Am. Chem. Soc., 2018, 140, 1568 CrossRef; (y) J. A. Kautzky, T. Wang, R. W. Evans and D. W. C. MacMillan, J. Am. Chem. Soc., 2018, 140, 6522 CrossRef CAS; (z) X. Yang and G. C. Tsui, Org. Lett., 2018, 20, 1179 CrossRef CAS.
- For selected recent reviews on visible light photocatalysis, see: (a) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828 CrossRef CAS; (b) C. K. Prier, D. A. Rankic and D. W. Macmillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS; (c) D. M. Schultz and T. P. Yoon, Science, 2014, 343, 985 CrossRef CAS; (d) R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872 CrossRef CAS; (e) J. Xuan, Z.-G. Zhang and W.-J. Xiao, Angew. Chem., Int. Ed., 2015, 54, 15632 CrossRef CAS; (f) F. Tan and W.-J. Xiao, Acta Chim. Sin., 2015, 73, 85 CrossRef CAS; (g) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2016, 45, 2044 RSC.
- For selected reviews on difunctionalization of alkenes, see: (a) T.-T. Zeng, J. Xuan, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Imaging Sci. Photochem., 2014, 32, 415 CAS; (b) T. Koike and M. Akita, Top. Catal., 2014, 57, 967 CrossRef CAS; (c) T. Koike and M. Akita, Org. Chem. Front., 2016, 3, 1345 RSC; (d) T. Koike and M. Akita, Acc. Chem. Res., 2016, 49, 1937 CrossRef CAS; (e) X. Pan, H. Xia and J. Wu, Org. Chem. Front., 2016, 3, 1163 RSC; (f) T. Koike and M. Akita, Chem., 2018, 4, 409 CrossRef CAS.
- For selected recent examples of trifluoromethylation of alkenes, see: (a) F. Wang, D. Wang, X. Wan, L. Wu, P. Chen and G. Liu, J. Am. Chem. Soc., 2016, 138, 15547 CrossRef CAS; (b) K. Shen and Q. Wang, Org. Chem. Front., 2016, 3, 222 RSC; (c) Y. An, Y. Kuang and J. Wu, Org. Chem. Front., 2016, 3, 994 RSC; (d) Y. Cheng and S. Yu, Org. Lett., 2016, 18, 2962 CrossRef CAS; (e) L. Wu, F. Wang, X. Wan, D. Wang, P. Chen and G. Liu, J. Am. Chem. Soc., 2017, 139, 2904 CrossRef CAS; (f) Y. Tang, Q. Yu and S. Ma, Org. Chem. Front., 2017, 4, 1762 RSC; (g) L.-H. Wu, K. Zhao, Z.-L. Shen and T.-P. Loh, Org. Chem. Front., 2017, 4, 1872 RSC; (h) L. Li, L. Ye, S.-F. Ni, Z.-L. Li, S. Chen, Y.-M. Du, X.-H. Li, L. Deng and X.-Y. Liu, Org. Chem. Front., 2017, 4, 2139 RSC; (i) H.-Y. Zhang, C. Ge, J. Zhao and Y. Zhang, Org. Lett., 2017, 19, 5260 CrossRef CAS PubMed; (j) H. Jiang, Y. He, Y. Cheng and S. Yu, Org. Lett., 2017, 19, 1240 CrossRef CAS; (k) Y.-Y. Ren, X. Zheng and X. Zhang, Synlett, 2018, 29, 1028 CrossRef CAS; (l) B. Chang, Y. Su, D. Huang, K.-H. Wang, W. Zhang, Y. Shi, X. Zhang and Y. Hu, J. Org. Chem., 2018, 83, 4365 CrossRef CAS.
- (a) Y. Yasu, T. Koike and M. Akita, Angew. Chem., Int. Ed., 2012, 51, 9567 CrossRef CAS; (b) Y. Yasu, Y. Arai, R. Tomita, T. Koike and M. Akita, Org. Lett., 2014, 16, 780 CrossRef CAS; (c) Q.-H. Deng, J.-R. Chen, Q. Wei, Q.-Q. Zhao, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2015, 51, 3537 RSC; (d) M. Daniel, G. Dagousset, P. Diter, P.-A. Klein, B. Tuccio, A.-M. Goncalves, G. Masson and E. Magnier, Angew. Chem., Int. Ed., 2017, 56, 3997 CrossRef CAS.
- (a) J. D. Nguyen, J. W. Tucker, M. D. Konieczynska and C. R. J. Stephenson, J. Am. Chem. Soc., 2011, 133, 4160 CrossRef CAS; (b) S. H. Oh, Y. R. Malpani, N. Ha, Y.-S. Jung and S. B. Han, Org. Lett., 2014, 16, 1310 CrossRef CAS; (c) X. Tang and W. R. Dolbier, Angew. Chem., Int. Ed., 2015, 54, 4246 CrossRef CAS; (d) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Synthesis, 2015, 2439 CAS.
- (a) P. Xu, J. Xie, Q. Xue, C. Pan, Y. Cheng and C. Zhu, Chem. – Eur. J., 2013, 19, 14039 CrossRef CAS; (b) P. G. Janson, I. Ghoneim, N. O. IIchenko and K. J. Szabó, Org. Lett., 2012, 14, 2882–2885 CrossRef CAS; (c) H. Egami, R. Shimizu and M. Sodeoka, Tetrahedron Lett., 2012, 53, 5503–25506 CrossRef CAS; (d) Y. Yasu, T. Koika and M. Akita, Chem. Commun., 2013, 49, 2037–2039 RSC; (e) A. Carboni, G. Dagousset, E. Magnier and G. Masson, Chem. Commun., 2014, 50, 14197 RSC.
- (a) S. Mizuta, S. Verhoog, K. M. Engle, T. Khotavivattana, M. O'Duill, K. Wheelhouse, G. Rassias, M. Medebielle and V. Gouverneur, J. Am. Chem. Soc., 2013, 135, 2505 CrossRef CAS; (b) D. J. Wilger, N. J. Gesmundo and D. A. Nicewicz, Chem. Sci., 2013, 4, 3160 RSC; (c) N. J. W. Straathof, S. E. Cramer, V. Hessel and T. Noël, Angew. Chem., Int. Ed., 2016, 55, 15549 CrossRef CAS.
- (a) Y. Yasu, T. Koike and M. Akita, Org. Lett., 2013, 15, 2136 CrossRef CAS; (b) G. Dagousset, A. Carboni, E. Magnier and G. Masson, Org. Lett., 2014, 16, 4340 CrossRef CAS; (c) X.-L. Yu, J.-R. Chen, D.-Z. Chen and W.-J. Xiao, Chem. Commun., 2016, 52, 8275 RSC.
- D. B. Bagal, G. Kachkovskyi, M. Knorn, T. Rawner, B. M. Bhanage and O. Reiser, Angew. Chem., Int. Ed., 2015, 54, 6999 CrossRef CAS.
- (a) M. D. Konieczynska, C. Dai and C. R. Stephenson, Org. Biomol. Chem., 2012, 10, 4509 RSC; (b) C. Dai, J. M. Narayanam and C. R. Stephenson, Nat. Chem., 2011, 3, 140 CrossRef CAS; (c) Y.-Q. Zou, W. Guo, F.-L. Liu, L.-Q. Lu, J.-R. Chen and W.-J. Xiao, Green Chem., 2014, 16, 3787 RSC; (d) C.-J. Yao, Q. Sun, N. Rastogi and B. König, ACS Catal., 2015, 5, 2935 CrossRef CAS.
- T. Umemoto, B. Zhang, T. Zhu, X. Zhou, P. Zhang, S. Hu and Y. Li, J. Org. Chem., 2017, 82, 7708 CrossRef CAS.
- H. Wang, Q. Xu and S. Yu, Org. Chem. Front., 2018, 5, 2224 RSC.
- M. Gasonoo, R. R. Naredla, S. O. N. Lill and D. A. Klumpp, J. Org. Chem., 2016, 81, 11758 CrossRef CAS.
- (a) F. Wang, D. Wang, X. Mu, P. Chen and G. Liu, J. Am. Chem. Soc., 2014, 136, 10202 CrossRef CAS; (b) X. Dong, R. Sang, Q. Wang, X.-Y. Tang and M. Shi, Chem. – Eur. J., 2013, 19, 16910 CrossRef CAS.
- F. Wang, D. Wang, X. Wan, L. Wu and G. Liu, J. Am. Chem. Soc., 2016, 138, 15547 CrossRef CAS.
- E. Arceo, I. D. Jurberg, A. Álvarez-Fernández and P. Melchiorre, Nat. Chem., 2013, 5, 750 CrossRef CAS.
- M. Silvi, C. Verrier, Y. P. Rey, L. Buzzetti and P. Melchiorre, Nat. Chem., 2017, 9, 868 CrossRef CAS.
- X. Shen, Y. Li, Z. Wen, S. Cao, X. Hou and L. Gong, Chem. Sci., 2018, 9, 4562 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation and characterisation data as well as 1H, 13C, 19F NMR and HRMS spectra of all compounds. See DOI: 10.1039/c8ob02239a |
| This journal is © The Royal Society of Chemistry 2019 |