TiO2 nanotrees for the photocatalytic and photoelectrocatalytic phenol degradation†
Juan
Xie
a,
Wei
Wen
 b,
Qi
Jin
a,
Xiao-Bo
Xiang
a and
Jin-Ming
Wu
b,
Qi
Jin
a,
Xiao-Bo
Xiang
a and
Jin-Ming
Wu
 *a
*a
aState Key Laboratory of Silicon Materials and School of Materials Science and Engineering, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: msewjm@zju.edu.cn
bCollege of Mechanical and Electrical Engineering, Hainan University, Haikou 570228, P. R. China
First published on 13th June 2019
Abstract
Growing branches on one-dimensional TiO2 nanostructures to construct nanotrees is an effective tactic to promote their photoelectrical performance for various applications in photoelectrocatalysis and solar cells. The appropriate choice of the trunk and branch to achieve excellent properties is of general interest. Herein we report a comparative study on TiO2 nanotrees for photocatalytic (PC) and photoelectrocatalytic (PEC) degradation of phenol in water under UV light illumination. An alkali-hydrothermal technique was adopted to grow anatase TiO2 arrays of polycrystalline nanobelts and single-crystalline nanowires, separately, on metallic Ti substrates. Using a precursor solution derived from solution combustion synthesis, few-layer TiO2 nanosheets were then deposited to construct sheet-on-belt (SOB) and sheet-on-wire (SOW) TiO2 nanotrees. We found that the PEC efficiency is promoted upon constructing the nanotrees; whilst the improvement in the PC activity is relatively insignificant. The length and atomic arrangement of the trunk readily affect the PEC performance. With a fixed branch precipitation duration, the SOW TiO2 nanotrees with a film thickness of 3 μm exhibited the best PEC capability towards phenol degradation. The PEC reaction rate constant is 0.40 h−1, which is 1.7 times that of the pristine alkali-hydrothermal nanowire film (0.23 h−1). This PEC reaction rate constant is even 47 times that of the pristine nanowire film to assist the PC degradation of phenol in water (0.0085 h−1). The present study suggests that the single-crystalline trunk plays a key role in the photoelectrical performance of TiO2 nanotrees.
Introduction
With the ever-increasing environmental and energy crisis, the development in highly efficient functional materials for environmental remediation, green energy production and energy storage is accelerating nowadays. Undoubtedly, new materials should impose the least environmental impact, and titanium dioxide (TiO2) is a suitable choice because of its earth-abundance, low cost and eco-friendliness. It is also very stable for most applications in fields such as photocatalysis,1 sensors,2 dye-sensitized solar cells3 and energy storage devices.4 In the last several decades, TiO2 nanostructures have witnessed the development from zero-dimensional (0D) nanoparticles, 1D nanorods, nanotubes, nanowires and nanobelts, to 3D nanoflowers and hierarchical spheres. When serving as a photocatalyst, rapid charge migration along the axial direction makes 1D nanowires and nanobelts extremely suitable for highly efficient photocatalytic reactions; yet their light harvesting capability, specific surface area and catalytic active sites are still to be enhanced.5Efforts have thus been made to design and construct hetero-structures on 1D TiO2 surfaces. Nanostructured semiconductors like Co3O4,6 AgI,7 g-C3N4,8 Bi2WO69 and even TiO2 itself,10–25 were precipitated on 1D TiO2, which enlarged the specific surface area, extended the responsive light wavelength and introduced phase junctions favoring the charge separations. Compared to powders, branched nanowires or nanobelts which quasi-aligned vertically on conductive substrates are more desirable for dye-sensitized solar cells, energy storage and photoelectrochemical catalysis, because of the easier collection of electrons to the external circuit. Thin films with such a nanostructure are generally termed as nanotrees.11,12 Arrays of TiO2 nanowires and nanobelts on metallic Ti foils or transparent conductive glasses are common choices for trunks to construct nanotrees. The trunk is synthesized hydrothermally using the reactants of either TiO2 in NaOH,11–15 inorganic/organic Ti-complexes in acidic environments,16–18 or direct oxidation of metallic Ti in H2O2 aqueous solutions under mild conditions.19–21 TiO2 nanotrees can be constructed by immersing the hydrogen titanate nanowires (the trunk) in an acidic environment and under a hydrothermal condition, which achieved the in situ branch growth via a dissolution–precipitation route.11–21 An alternative is to achieve firstly the TiO2 nanorod arrays (the trunk), which are then subjected to a subsequent alkali-hydrothermal treatment to finally obtain TiO2 branches.17,18
The ex situ branch growth on pre-formed trunks from certain precursor solutions provides feasibility to control the TiO2 nanotrees in either phase compositions or nanostructures. There are not too many choices of precursors to precipitate nanostructured branches. Recently, we obtained a water-proof black Ti-complex via solution combustion synthesis (SCS).22 The precursor obtained by adding the Ti-complex in an H2O2 aqueous solution at ambient temperature precipitated few-layer TiO2 nanosheets on hydrothermally synthesized TiO2 nanowires,22,23 nanorods24 and nanobelts.22,25
The performance of TiO2 nanotrees is undoubtedly dependent on the architecture of their trunks and branches. Wu et al. reviewed recently the structural aspects (length tuning and branch modification) affecting the performance of TiO2 nanotrees for solar cell applications.11,12 To our limited knowledge, studies on the effects of nanostructural aspects on the photocatalytic (PC) and photoelectrocatalytic (PEC) characteristics of TiO2 nanotrees are scarce. Herein, we reported our comparative study on PC and PEC characteristics of anatase TiO2 nanotrees on metallic Ti substrates with sheet-on-belt and sheet-on-wire architectures, respectively. With carefully controlled compositional and structural parameters, the effect of nanotree architectures on PC and PEC efficiencies towards degradation of phenol in water is clarified. It concludes that the PEC efficiency is more likely to be promoted upon constructing nanotrees using single-crystalline trunks.
Experimental
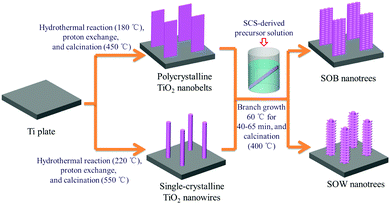
Scheme 1 illustrates the synthetic route of the TiO2 nanotrees. All chemicals are of analytical grade and used as-received, without further purifications.Preparation of the precursor solution via SCS
According to Wen et al.,22 1.75 g of C2H5NO2, 0.6 mL of HNO3 (65 wt%) and 1.25 g of TiOSO4 were dissolved in 10 mL deionized water in a beaker under magnetic stirring. The solution was placed in a muffle furnace for 16 min at 400 °C to fulfill the combustion, leading to black Ti-complex powders. 0.5 g of the black powder was mixed with 50 mL of H2O2 solution (30 wt%) and stored at room temperature for 72 h. The suspension was then removed via centrifugation and the remaining solution was utilized as the precursor solution for branch precipitation.Preparation of the TiO2 nanobelt and nanowire arrays via alkali-hydrothermal approaches
To synthesize the nanobelt array, surface cleaned metallic Ti plates with a size of 2.5 × 2.5 × 0.1 cm3 were immersed in a Teflon-lined stainless-steel autoclave (45 mL in volume) filled with 37 mL NaOH (5 M) aqueous solution. The autoclave was heated to 180 °C and maintained for 20 h. The Ti plate covered with sodium titanate nanobelt arrays was then immersed in 0.1 M HCl solution for 40 min repetitively for 3 times to finish the proton-exchange. Final calcination in air at 450 °C for 1 h decomposed the hydrogen titanate nanobelts into anatase TiO2 nanobelts. The anatase TiO2 nanowire arrays on Ti substrates were synthesized following a similar procedure, except that the concentration of the NaOH aqueous solution was 1.25 M and the autoclave was maintained at 220 °C for 20 h. Air calcination to obtain the TiO2 nanowires was carried out in air at 550 °C for 3 h, according to Wen et al.23Synthesis of the sheet-on-belt (SOB) and sheet-on-wire (SOW) hierarchical TiO2 nanotrees
The arrays of nanobelts and nanowires on Ti substrates, separately, were immersed in the precursor solution and maintained at 60 °C for 40, 50 and 65 min. Final calcination at 400 °C for 1 h was carried out to obtain the anatase TiO2 sheet-like branches. For simplicity, the samples are designated as NB/x min or NW/x min (x = 40, 50, 65), where x refers to the branch precipitation duration.Characterizations
The X-ray diffraction (XRD) patterns were collected by using a XRD-6000 diffractometer with Cu Kα radiation, operated at 40 kV and 40 mA (λ = 0.15406 nm). The morphology was observed by using a field emission scanning electron microscope (FESEM, Hitachi S-4800) and a transmission electron microscope (TEM, JEM-2100). The Raman spectra in the range 800–100 cm−1 were collected using an Almega dispersive Raman system (Nicolet) and a Nd:YAG intracavity doubled laser operating at 532 nm. The incident power was set at 10 mW. The UV-vis diffuse reflectance spectra were obtained using a UV-vis near-infrared spectrometer (UV-3150, Shimadzu).Photocurrent measurements
The photocurrent measurement under the illumination of simulated solar light was carried out in a standard three-electrode configuration with a Pt plate as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode, using an electrochemical workstation (CHI 660, China). The active area of the working electrode is 1.5 × 1.5 cm2 and the electrolyte is 0.1 M Na2SO4 aqueous solution. The simulated sun light was provided by a 500 W Xe lamp equipped with an AM 1.5 filter.Photocatalytic (PC) and photoelectrocatalytic (PEC) activity evaluations
The PC and PEC performance was evaluated by using phenol in water as the target molecule. The initial concentration is 10 ppm. For the PC evaluation, the TiO2 film 1.5 × 1.5 cm2 in area was immersed in 20 mL of the phenol solution. The UV light was provided by a LED UV lamp (18 W) and the average light density was ca. 12 mW cm−2. For the PEC evaluation, a bias potential of 0.6 V vs. SCE was provided using an electrochemical workstation (CHI 660, China) and 0.1 M Na2SO4 aqueous solution was used as the supporting electrolyte. Every two hours, 0.4 mL of the solution was sampled to record the change in phenol concentration, which was measured using a liquid chromatography apparatus (Wufeng LC100, WondaCract ODS-2 column, China). A mixed solution of methanol and acetic acid (methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid = 700
acetic acid = 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume) was used as the mobile phase.
1 in volume) was used as the mobile phase.
Results and discussion
Material synthesis and characterizations
Fig. 1 shows the FESEM images of the alkali-hydrothermally achieved TiO2 nanobelt array and the corresponding SOB nanotrees achieved by subsequently immersing in the precursor solution at 60 °C for various durations. The nanobelts are quasi-aligned on the Ti substrates with a layer thickness of ca. 8 μm, exhibiting a rectangular cross-section with a size of ca. 400 nm × 20 nm (Fig. 1a and b). Few-layer nanosheets can be observed growing vertically on the nanobelts, forming the SOB architecture. The branches nucleated and grew at a slower rate within the initial 40 min, achieving an average length of ca. 50 nm (Fig. 1c and d). In the following 10 min, the sparse branches became dense and the length increased averagely to ca. 80–100 nm (Fig. 1e and f), which remained almost unchanged upon increasing the precipitation duration to 65 min (Fig. 1g and h), due to the limited supply of Ti(IV) ions in the precursor solution.Similar morphology evolution was observed for the SOW nanotrees (Fig. 2). The alkali-hydrothermally achieved TiO2 nanowires exhibited an average diameter of 100 nm and the layer thickness was controlled by the hydrothermal reaction time to be near to that of the nanobelt array. The nanosheet branches grew radially along the nanowires with the prolonged immersing duration in the precursor solution. The branch length achieved for different durations of 40, 50 and 65 min was 60, 100 and 200 nm, respectively.
The nanostructures of both NB/65 and NW/65 were further characterized by TEM observations. Fig. 3a shows the nanosheets with a length of ca. 80 nm growing vertically on both sides of the nanobelt trunk. The sheets are only a few nanometers in thickness, which is much thinner than that of the nanobelt trunk (ca. 20 nm in thickness). The corresponding selected area electron diffraction (SAED) pattern shows multi-rings arising from multi-crystallites, which can be attributed to anatase TiO2 (Fig. 3b). The high resolution TEM (HRTEM) illustrates domains with clear lattices, all of which can be ascribed to the (101) facet of anatase TiO2 (Fig. 3c).
Nanosheet branches with similar morphologies can be seen growing radially on the nanowire trunk (Fig. 3d). Unlike SOB nanotrees of NB/65, the SAED of the SOW nanotrees of NW/65 is a characteristic of the combination of single-crystallites and polycrystallites (Fig. 3e). The inset in Fig. 3d shows the single-crystalline nature of the nanowire trunk and its growing direction can be determined to be along the [100] direction (Fig. 3e and f).23 The HRTEM observation of the nanosheet branch reveals also domains corresponding to the (101) facet of anatase TiO2 (Fig. 3f). It thus concludes that the trunks of nanobelts and nanowires are polycrystallites and single-crystallites, respectively; whilst the branched nanobelts are polycrystalline.
The phase compositions revealed by the TEM observations are further confirmed by the XRD patterns (Fig. 4a) and Raman spectra (Fig. 4b). All the diffraction peaks can be well-indexed to the anatase TiO2 (JCPDS 71-1167), either before or after the branch precipitation. For the nanowires and the corresponding SOW nanotrees, a weak XRD peak located at 27.5° which corresponds to rutile TiO2 can also be observed, which was obtained from the direct oxidation of the metallic Ti substrate in air at 550 °C for 3 h.23 The corresponding Raman spectra show only peaks at 144, 196, 396, 514 and 640 cm−1 for the nanobelts and nanowires, before and after the branch precipitation, all of which can be attributed to anatase TiO2.26,27
 | ||
| Fig. 4 XRD patterns (a) and Raman spectra (b) of the TiO2 arrays of nanobelts (NB) and nanowires (NW), and the corresponding nanotrees (NB/65 min and NW/65 min). Precipitation duration: 65 min. | ||
UV-vis diffuse reflectance spectra of the nanobelts and nanowires after growing the branches for various durations are shown in Fig. 5. The difference in the reflectance can be ascribed to the difference in the film thickness, refractive index and surface roughness of the TiO2 film on metallic Ti substrates.28 The insets in Fig. 5 show the Tauc plot to evaluate the band gaps.29,30 All the TiO2 films exhibited a similar band gap of ca. 3.2 eV, which is near to that of the bulk TiO2. This suggests that the light harvesting capability should have induced less impact on the photocatalytic behavior of the present TiO2 films.
The effects of the trunk nanostructure and branch length on PC and PEC performance
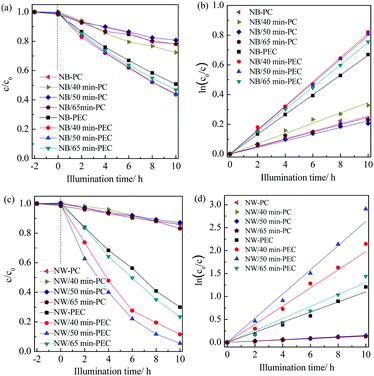
Fig. 6a and c show the PC and PEC degradation curves of phenol in water in the presence of SOB and SOW TiO2 nanotrees. The dark adsorption of phenol on all the TiO2 films can be neglected. Fig. 6b and d show the corresponding fitting results assuming a pseudo-first order reaction. It suggests that all the degradation kinetics followed roughly the pseudo-first-order reaction,31,32| −ln(ct/ct=0) = kt | (1) |
Both SOB and SOW TiO2 nanotrees exhibited much increased efficiency when a bias potential of 0.6 V vs. SCE was applied to enhance the charge separation. Fig. 7a shows that, when compared with the PC efficiency, there is a 159% increase in the PEC efficiency for the pristine TiO2 nanobelts; whereas an outstanding 614% increase was recorded for the pristine TiO2 nanowires (Fig. 7b). This is a strong hint that TiO2 with a more conductive nanostructure, which preferably is single-crystalline, is more effective to induce PEC reactions.
The most interesting finding from Fig. 7 is the distinct PEC behaviors arising from the branch precipitations on the various trunks of the polycrystalline nanobelts and single-crystalline nanowires. The enhancing factor, defined as the ratio of k-PEC to k-PC, is not affected readily by the branch precipitation duration for the SOB nanotrees; whilst for the SOW nanotrees, the ratio increased from the original 7.14 to 14.4 and 18.0 after the branch precipitation for 40 and 50 min, respectively. Upon further increasing the precipitation duration to 65 min, the enhancing factor decreased to 9.7. It thus concludes that the construction of nanotrees is effective to enhance the PEC efficiency using a single-crystalline trunk.
Fig. S1 in the ESI† shows the linear scanning voltage curves and transient current response curves under a bias potential of 0.6 V vs. SCE for the SOB and SOW TiO2 nanotrees, respectively, under UV light illumination. The dark current for all the samples is near to zero. Fig. S1b and d (ESI†) show that the transient photocurrent curve of SOW nanotrees reached a saturated value more quickly than that of the SOB nanotrees, suggesting a much enhanced electron collecting capability for the single-crystalline nanowires. The photocurrent under a bias potential of 0.6 V vs. SCE is shown in Fig. 7 as a function of the precipitation time, which in general is consistent with the change in the PEC efficiency. Fig. 7b shows that the SOW TiO2 nanotrees exhibited a higher photocurrent than that of the pristine nanowires and there is an optimum value for the growing duration of the TiO2 nanosheet branch. The photocurrent increased from 0.019 mA cm−2 for NW to 0.027 mA cm−2 for NW/50 min. However, Fig. 7a shows that the SOB TiO2 nanotrees exhibited a reduced photocurrent about half that of the pristine nanobelts (0.018 mA cm−2), suggesting that under the UV light illumination, it is relatively difficult for the charges excited from the nanosheet branch to transfer to the nanobelt trunk and in sequence to the Ti substrates. The reduced photocurrent upon the subsequent branch precipitation was also reported by Ning et al. on rutile TiO2 nanorod arrays, which was attributed to the interface between the backbone and the branch because of the defects therein disrupting the electron transport.33
It can thus be discerned that the nanostructured trunk with an improved electron conductivity facilitates the electron collection from the branch to the trunk and hence to the metallic Ti substrate. As illustrated in Scheme 2, the single-crystalline nanowires are free of grain boundaries, which disturb the directional migration of photogenerated electrons to the Ti substrate;34 therefore, a faster electron migration and in turn reduced charge recombination can be anticipated for the SOW nanotrees when compared with SOB constructed using multi-crystalline trunks. This allows more opportunities for highly oxidative holes to reach the very surface to be involved, either directly or indirectly through surface hydroxyl groups, in the PEC degradation of phenol in water.32,35,36 However, the PEC efficiency cannot be simply related to the photocurrent. For the SOB nanotrees, the photocurrent reduced to nearly a half upon branching; yet the PEC efficiency increased (Fig. 7a). It is noted that the charge separation is not the sole factor affecting the PEC efficiency. Other factors like light harvesting capability and charge migration path also affect readily the PEC behavior. The few-layer nanosheets reduce greatly the charge migration path and therefore contribute to the PEC efficiency of the SOB nanotrees. Recently, Butburee et al. reported that the order in the photoactivity of TiO2 films towards CO2 photoreduction shows an opposite trend to the PEC water splitting test.37
 | ||
| Scheme 2 A schematic image showing the enhanced PEC activity for the SOW nanotrees compared to the SOB nanotrees. | ||
The effects of the film thickness on PC and PEC performance
Fig. S2a and b (ESI†) show the cross-sectional SEM images of the TiO2 nanowire film obtained using an alkali-hydrothermal technique for durations of 4 h and 8 h, which exhibited a film thickness of 1 μm and 3 μm, respectively. The top-view SEM images of the corresponding SOW nanotrees synthesized using a precipitation method for a duration of 50 min are shown in Fig. S2c and d (ESI†). The nanotree morphology is similar to that of the specimen NW/50 min (Fig. 2e and f). Fig. 8 shows the PC and PEC efficiency of the SOW nanotrees with different film thickness. It can be seen that all the nanowire films exhibited similar PC efficiency and the subsequent precipitation of the TiO2 nanosheet branch increased slightly the PC activity, suggesting that the PC activity is insensitive to the film thickness for the alkali-hydrothermal nanowires 1–8 μm in thickness. This is a hint that only the surface of ca. 1 μm exposed to both the UV light and phenol aqueous solution was effective to assist the PC degradation. In contrast, the PEC efficiency differs significantly for both the nanowire film and the SOW nanotrees. The 3 μm-thick TiO2 nanowire film possessed the highest PEC activity (k = 0.23 h−1) among the three films and it is 27 times that of the corresponding PC activity (k = 0.0085 h−1). The reaction rate constants of the SOW nanotrees for PEC degradation of phenol in water are 0.13, 0.40 and 0.26 h−1, respectively, for film thicknesses of 1, 3 and 8 μm. The optimized PEC efficiency for the SOW nanotrees is thus 47 times that of the corresponding PC activity for the nanowire film with the same thickness of 3 μm. The fact that there is an optimized film thickness for the PEC efficiency of TiO2 films agrees well with our previous report on PC and PEC degradation of rhodamine B in water under UV light illumination, which can be explained by the fact that only TiO2 on the surface is involved in the PEC reaction and the electron migration to the metallic Ti substrates under the bias potential is inhibited with too long a charge migration path.38 In that study, the optimized film thickness is 0.46 μm for the compact nanoparticulate anatase TiO2 film; whilst in the current investigation, the optimized film thickness shows a much larger value of ca. 3 μm. This can be explained by the deeper UV light penetration length for the nanowire and nanotrees films. In addition, solely increasing the length of either the branch (Fig. 7) or the trunk (Fig. 8), which should have resulted in increased surface area, does not lead to a linearly increasing activity towards PC or PEC degradation of phenol in water. It is thus safe to neglect the possible effects arising from the various surface areas of the thin films derived in the current investigation.The current investigation suggests that constructing TiO2 nanotrees is a more effective method to promote the PEC efficiency rather than to enhance the PC activity. To obtain the TiO2 nanotrees with a high PEC efficiency, a conductive trunk with optimized length, together with optimized branches, is favourable. The photocurrent reflects the photogenerated charge density and the charge separation rate; but it does not serve as the sole indicator for the PEC efficiency. The current investigation can give hints for constructing TiO2 nanotrees for other photoelectrical applications like photocatalytic water splitting39 and dye-sensitized solar cells.40–43
Conclusions
Anatase TiO2 thin films of polycrystalline nanobelts and single-crystalline nanowires aligning vertically on metallic Ti substrates were synthesized by an alkali-hydrothermal technique. Few-layer anatase TiO2 nanosheets were then precipitated from a SCS-derived precursor solution to construct the corresponding TiO2 nanotrees. It is found that, when utilized to assist phenol degradation under UV light illumination, constructing TiO2 nanotrees is a more effective method to promote the PEC efficiency rather than to enhance the PC activity. The single-crystalline trunk is the most important factor to obtain TiO2 nanotrees with an excellent PEC efficiency. With an optimized branch precipitation duration of 50 min, the TiO2 nanotrees constructed using single-crystalline nanowires with an optimized film thickness of ca. 3 μm exhibited the best PEC capability towards phenol degradation, achieving a reaction rate constant of 0.40 h−1. This value is 47 times that of the pristine alkali-hydrothermal TiO2 nanowire film (3 μm) to assist the PC degradation of phenol in water.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial supports for this work from the National Natural Science Foundation of China (No. 51862005).References
- Z. Shayegan, C. S. Lee and F. Haghighat, Chem. Eng. J., 2018, 334, 2408–2439 CrossRef CAS.
- G. Dubourg and M. Radovic, ACS Appl. Mater. Interfaces, 2019, 11, 6257–6266 CrossRef CAS PubMed.
- Y. Wang, X. Q. Li, Z. Li, Y. Cao, Y. C. Li and Y. L. Zhao, ChemNanoMat, 2017, 3, 58–64 CrossRef CAS.
- J. F. Ni, S. D. Fu, Y. F. Yuan, L. Ma, Y. Jiang, L. Li and J. Lu, Adv. Mater., 2018, 30, 1704337 CrossRef PubMed.
- J. Tian, Z. H. Zhao, A. Kumar, R. I. Boughton and H. Liu, Chem. Soc. Rev., 2014, 43, 6920–6937 RSC.
- J. Liu, J. Ke, Y. Li, B. J. Liu, L. D. Wang, H. N. Xiao and S. B. Wang, Appl. Catal., B, 2018, 236, 396–403 CrossRef CAS.
- J. H. Yi, L. L. Huang, H. J. Wang, H. Yu and F. Peng, J. Hazard. Mater., 2015, 284, 207–214 CrossRef CAS PubMed.
- J. Ma, W. Zhou, X. Tan and T. Yu, Nanotechnology, 2018, 29, 215706 CrossRef PubMed.
- G. L. Fang, J. Liu, J. D. Wu, M. Y. Li, X. H. Yan and D. Y. Wang, Appl. Surf. Sci., 2019, 475, 785–792 CrossRef CAS.
- X. F. Yang, J. L. Zhuang, X. Y. Li, D. H. Chen, G. F. Ouyang, Z. Q. Mao, Y. X. Han, Z. H. He, C. L. Liang, M. M. Wu and J. C. Yu, ACS Nano, 2009, 3, 1212–1218 CrossRef CAS PubMed.
- W. Q. Wu, H. L. Feng, H. Y. Chen, D. B. Kuang and C. Y. Su, J. Mater. Chem. A, 2017, 5, 12699–12717 RSC.
- W. Q. Wu, Y. F. Xu, H. Y. Chen, D. B. Kuang and C. Y. Su, Acc. Chem. Res., 2019, 52, 633–644 CrossRef CAS PubMed.
- X. S. Peng and A. C. Chen, Adv. Funct. Mater., 2006, 16, 1355–1362 CrossRef CAS.
- F. Shao, J. Sun, L. Gao, S. Yang and J. Luo, J. Mater. Chem. A, 2014, 22, 6824–6830 RSC.
- L. L. Lai, W. Wen, B. Fu, X. Y. Qian, J. B. Liu and J. M. Wu, Mater. Des., 2016, 108, 581–589 CrossRef CAS.
- B. Liu and E. S. Aydil, J. Am. Chem. Soc., 2009, 131, 3985–3990 CrossRef CAS PubMed.
- F. Zhu, H. Dong, Y. Wang, D. P. Wu, J. M. Li, J. L. Pan, Q. Li, X. C. Ai, J. P. Zhang and D. S. Xu, Phys. Chem. Chem. Phys., 2013, 15, 17798–17803 RSC.
- X. Wang, Z. Wang, M. Zhang, X. Jiang, Y. Wang, J. Lv, G. He and Z. Sun, J. Alloys Compd., 2017, 725, 1166–1174 CrossRef CAS.
- J. Sun, W. Wen and J. M. Wu, J. Am. Ceram. Soc., 2013, 96, 2109–2116 CrossRef CAS.
- J. M. Wu and J. X. Yin, RSC. Adv., 2015, 5, 3465–3469 RSC.
- Y. Yu, W. Wen, X. Y. Qian, J. B. Liu and J. M. Wu, Sci. Rep., 2017, 7, 41253 CrossRef CAS PubMed.
- W. Wen, J. M. Wu, Y. Z. Jiang, S. L. Yu, J. Q. Bai, M. H. Cao and J. Cui, Sci. Rep., 2015, 5, 11804 CrossRef PubMed.
- W. Wen, J. M. Wu, Y. Z. Jiang, J. Q. Bai and L. L. Lai, J. Mater. Chem. A, 2016, 4, 10593–10600 RSC.
- W. Wen, J. C. Yao, Y. J. Gu, T. L. Sun, H. Tian, Q. L. Zhou and J. M. Wu, Nanotechnology, 2017, 28, 465602 CrossRef PubMed.
- H. Xing, W. Wen and J. M. Wu, Beilstein J. Nanotechnol., 2018, 9, 1550–1557 CrossRef CAS PubMed.
- T. Ohsaka and I. Fujio, J. Raman Spectrosc., 1987, 7, 321–324 CrossRef.
- J. C. Parker and R. W. Siegel, J. Mater. Res., 1990, 5, 1246–1252 CrossRef CAS.
- A. B. Murphy, Sol. Energy Mater. Sol. Cells, 2007, 91, 1326–1337 CrossRef CAS.
- S. Sakthivel and H. Kisch, ChemPhysChem, 2003, 4, 487–490 CrossRef CAS PubMed.
- Y. Xu, W. Wen and J. M. Wu, J. Hazard. Mater., 2018, 343, 285–297 CrossRef CAS PubMed.
- H. Xing, W. Wei and J. M. Wu, Mater. Res. Bull., 2019, 109, 98–102 CrossRef CAS.
- A. Turki, C. Guillard, F. Dappozze, Z. Ksibi, G. Berhault and H. Kochkar, Appl. Catal., B, 2015, 163, 404–414 CrossRef CAS.
- X. Ning, J. Huang, S. Li, L. Li, Y. Gu, X. Li and B. H. Kim, Mater. Sci. Semicond. Process., 2019, 94, 156–163 CrossRef CAS.
- D. P. Wu, Y. X. Wang, N. N. Ma, K. Cao, W. C. Zhang, J. L. Chen, D. Q. Wang, Z. Y. Gao, F. Xu and K. Jiang, Electrochim. Acta, 2019, 305, 474–483 CrossRef CAS.
- J. J. Murcia, M. C. Hidalgo, J. A. Navío, J. Araña and J. M. Doña-Rodríguez, Appl. Catal., B, 2015, 179, 305–312 CrossRef CAS.
- L. Wang, D. P. Wu, Z. Guo, J. J. Yan, Y. S. Hu, Z. Chang, Q. P. Yuan, H. Ming and J. S. Wang, J. Alloys Compd., 2018, 745, 26–32 CrossRef CAS.
- T. Butburee, P. Kotchasarn, P. Hirunsit, Z. Sun, Q. Tang, P. Khemthong, W. Sangkhun, W. Thongsuwan, P. Kumnorkaew, H. Wang and K. Faungnawakij, J. Mater. Chem. A, 2019, 7, 8156–8166 RSC.
- X. M. Song, J. M. Wu and M. Yan, Mater. Chem. Phys., 2008, 112, 510–515 CrossRef CAS.
- J. Liu, X. L. Yu, Q. Y. Liu, R. L. Liu, X. K. Shang, S. S. Zhang, W. H. Li, W. Q. Zheng, G. J. Zhang, H. B. Cao and Z. J. Gu, Appl. Catal., B, 2014, 158, 296–300 CrossRef.
- D. P. Wu, X. J. Shi, H. Dong, F. Zhu, K. Jiang, D. S. Xu, X. C. Ai and J. P. Zhang, J. Mater. Chem. A, 2014, 2, 16276–16284 RSC.
- D. P. Wu, J. J. He, S. Zhang, K. Cao, Z. Y. Gao, F. Xu and K. Jiang, J. Power Sources, 2015, 282, 202–210 CrossRef CAS.
- W. Q. Wu, H. L. Feng, H. Y. Chen, D. B. Kuang and C. Y. Su, J. Mater. Chem. A, 2017, 5, 12699–12717 RSC.
- P. F. Cheng, Y. Liu, P. Sun, S. S. Du, Y. X. Cai, F. M. Liu, J. Zheng and G. Y. Lu, J. Power Sources, 2014, 268, 19–24 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Other SEM images and photoelectrochemical results to support the discussion. See DOI: 10.1039/c9nj02219h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |