One-pot synthesis of 3D Cu2S–MoS2 nanocomposites by an ionic liquid-assisted strategy with high photocatalytic activity†
Ya
Chen
 a,
Penghe
Su
a,
Xiaotong
Liu
a,
Hongchi
Liu
a,
Baolin
Zhu
a,
Shoumin
Zhang
a,
Penghe
Su
a,
Xiaotong
Liu
a,
Hongchi
Liu
a,
Baolin
Zhu
a,
Shoumin
Zhang
 a and
Weiping
Huang
a and
Weiping
Huang
 *abc
*abc
aCollege of Chemistry, Nankai University, Tianjin 300071, China
bCollaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300071, China
cThe Key Laboratory of Advanced Energy Materials Chemistry (Ministry of Education), Nankai University, Tianjin 300071, China
First published on 15th November 2018
Abstract
Novel 3D Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) nanocomposites with different proportions of Cu2S (x) and MoS2 (y) are synthesized successfully by a one-step hydrothermal method with the assistance of the ionic liquid [BMIM]SCN. The characterization results show that the nanocomposites are self-assembled from nanosheets of Cu2S and MoS2, they display nanoflower morphology and a typical mesoporous structure. The fabrication mechanism of the nanocomposites is investigated using time-dependent experiments, which indicate the key role of the ionic liquid (IL) in the synthesis process. Furthermore, TAA is used as a sulfur source instead of the IL to form a Cu2S–MoS2 nanocomposite, with the aim of further investigating the effects of the IL on the morphology of the composite. Photodegradation of MB under visible light irradiation experiments were used as probe reactions to evaluate the photocatalytic performance of the as-prepared samples. All the nanocomposites show better catalytic activity than Cu2S and MoS2 monomers. Among the different Cu2S–MoS2(x
y) nanocomposites with different proportions of Cu2S (x) and MoS2 (y) are synthesized successfully by a one-step hydrothermal method with the assistance of the ionic liquid [BMIM]SCN. The characterization results show that the nanocomposites are self-assembled from nanosheets of Cu2S and MoS2, they display nanoflower morphology and a typical mesoporous structure. The fabrication mechanism of the nanocomposites is investigated using time-dependent experiments, which indicate the key role of the ionic liquid (IL) in the synthesis process. Furthermore, TAA is used as a sulfur source instead of the IL to form a Cu2S–MoS2 nanocomposite, with the aim of further investigating the effects of the IL on the morphology of the composite. Photodegradation of MB under visible light irradiation experiments were used as probe reactions to evaluate the photocatalytic performance of the as-prepared samples. All the nanocomposites show better catalytic activity than Cu2S and MoS2 monomers. Among the different Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) nanocomposites, the Cu2S–MoS2(1
y) nanocomposites, the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite exhibits the most excellent photocatalytic performance and cycling stability.
1) composite exhibits the most excellent photocatalytic performance and cycling stability.
1. Introduction
There are growing concerns over organic pollutants in daily life as these have a number of effects on human health. Methods to eradicate organic pollutants in water and air are being greatly investigated. Researchers have found that disintegration of organic pollutants by photocatalytic degradation using photocatalysts is an effective method.1–4 Semiconductor nanomaterials have been regarded as the most promising photocatalysts due to their efficient degradation activity for pollutants in water and air.5–7 Among the different semiconductors, TiO2 is the most famous one because of its superior properties of low cost, environment friendliness and recycling performance.8 However, the practical application of TiO2 is limited by its wide bandgap, unsatisfying response to visible light and short lifetime of light-induced charge-carriers.9,10 Hence, the development of simple, efficient and sustainable visible-light-induced photocatalysts has attracted much attention. Transition metal sulfides, such as CdS,11 CuS,12 Cu2S13 and ZnS,14 with controllable electronic structures due to their rich d electrons, have been used as effective photocatalytic semiconductors for pollutant degradation. Among these, cuprous sulfide (Cu2S), a p-type semiconductor with a bulk band gap of 1.2 eV, has excellent absorption ability in the visible wavelength region, which lies in the optimum range for solar energy utilization. Up to now, Cu2S has been reported as an ideal visible-light-absorbing and photoelectric material for solar cells,15 biosensors,16 solar energy conversion17 and efficient photocatalysis.18 However, the high rate of photo-generated electron–hole recombination limits the practical application of Cu2S as photocatalysts. It is well known that both the adsorption ability of light and recombination rate of photo-generated electron–hole pairs have significant effects on photocatalytic reactions.19 Therefore, intensive efforts have been made to develop heterojunctions or composites, which are aiming to extend the lifetimes of the photo-induced charge carriers for higher efficiency of degradation. Heterostructures or composites usually have larger specific surface areas than those of the two components and there are interfaces between the two components. These structural advantages provide an extended light response range, separation rate of photo-generated electron–hole pairs and lower the activation energy barrier for chemical reaction, which could effectively enhance the photocatalytic performance to the single-component semiconductor.20–22 In recent years, some semiconductor heterojunctions or composites such as Cu2S–Bi2WO6, Bi2WO6-g-C3N4, Cu2S–MoO3, V2O5/MoO3, Fe2O3–TiO2–graphene aerogel, CuS–Cu2S23–34etc. have been reported as photocatalysts with enhanced activities.Molybdenum disulfide (MoS2) has a layered structure consisting of Mo–S–Mo sandwiched in a graphite-like manner. MoS2 nanostructures have been found to have a superior response to visible-light illumination due to their layered structures. Hence, MoS2 nanostructures are usually used as co-catalysts to improve photocatalysts in terms of catalytic activity and stability against photo corrosion.35–39 Nano-MoS2 has a high conduction band energy level, which makes it an electronic trapper and it can easily transfer electrons to Cu2S. The extended electronic channels allow longer separation-times of photo-generated electron–holes. However, the reported Cu2S–MoS2 composites were usually synthesized by complex preparation procedures and have small specific surface areas.40,41
In this paper, Cu2S–MoS2 composites are facilely synthesized by a one-pot strategy with the assistance of an IL and hydrothermal conditions. Comparing with the other synthesis methods, a one-pot strategy is relatively simple and convenient. The fabrication mechanism of the nanocomposites with the assistance of the IL is investigated using time-dependent experiments. Furthermore, the photocatalytic performance of the Cu2S–MoS2 nanocomposites, Cu2S and MoS2 are investigated using the photodegradation of MB solution under visible-light irradiation. The influence of the proportions of Cu2S and MoS2 on the morphology, structure and photocatalytic performance are discussed. Moreover, a Cu2S–MoS2 composite is synthesized using TAA as a sulfur source instead of the IL, and the key role of the ionic liquid in the synthesis of Cu2S–MoS2 composites is also discussed.
2. Experimental
2.1 Synthesis of Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) y) materials
y) materials
Different amounts of Cu(CH3COO)2·H2O and (NH4)6Mo7O24·4H2O (0.033 g and 0.099 g; 0.044 g and 0.088 g; 0.066 g and 0.066 g; 0.088 g and 0.044 g; 0.099 g and 0.033 g) with 0.366 g [BMIM]SCN were dissolved in 16 mL deionized water. After a white precipitate was formed, the solution was stirred for 30 min. Then, the solution was transferred into a 20 mL autoclave for hydrothermal synthesis and heated at 200 °C for 24 h. Finally, the precipitate was washed with deionized water and ethanol, and then dried at 60 °C. The samples were labelled Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y), in which x
y), in which x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y is the ratio of Cu2S to MoS2 in the samples.
y is the ratio of Cu2S to MoS2 in the samples.
Cu2S was synthesized under the same conditions with 0.066 g Cu(CH3COO)2·H2O, 0.366 g [BMIM]SCN and 16 mL deionized water without the molybdenum precursor.
MoS2 was also synthesized under the same conditions with 0.066 g (NH4)6Mo7O24·4H2O, 0.366 g [BMIM]SCN and 16 mL deionized water.
Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T was synthesized using thioacetamide (TAA) as the sulfur source instead of [BMIM]SCN: 0.066 g Cu(CH3COO)2·H2O, 0.066 g (NH4)6Mo7O24·4H2O and 0.134 g TAA were dispersed in 16 mL deionized water. After stirring for 30 min, the solution was transferred into a 20 mL autoclave for hydrothermal synthesis at 200 °C for 24 h. Cu2S–MoS2(1
1)-T was synthesized using thioacetamide (TAA) as the sulfur source instead of [BMIM]SCN: 0.066 g Cu(CH3COO)2·H2O, 0.066 g (NH4)6Mo7O24·4H2O and 0.134 g TAA were dispersed in 16 mL deionized water. After stirring for 30 min, the solution was transferred into a 20 mL autoclave for hydrothermal synthesis at 200 °C for 24 h. Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T was obtained after washing and drying procedures.
1)-T was obtained after washing and drying procedures.
2.2 Characterization
The crystalline structures of the as-prepared samples were characterized using X-ray powder diffraction (XRD) (Bruker D8 FOCUS, Cu Kα radiation, λ = 0.15056 nm). SEM images were obtained using a ZEISS MERLIN Compact (Field Emission) scanning electron microscope. TEM images of the samples were collected using a Tecnai G2 F20 transmission electron microscope. Elemental analysis was performed using an Elementar vario EL cube. A Varian 725-ES inductively coupled plasma spectrometer was used to measure the metal content in the as-prepared samples. Ultraviolet-visible (UV-Vis) diffuse reflectance spectroscopy (DRS) of the samples was carried out using a Cary 100 UV-Vis spectrophotometer and the scanning range was 200–800 nm. X-ray photoelectron spectra (XPS) and Auger electron spectroscopy (AES) experiments were conducted using a Kratos Axis Ultra DLD multi-technique X-ray photoelectron spectrometer. The specific surface area (SSA) and pore size of the resultant samples were investigated using an Autosorb-1-MP 1530VP automatic surface area and porosity analyzer. Samples were degassed at 200 °C for 3 h and then analyzed at −196 °C.2.3 Photocatalytic measurements
The photocatalytic performance of the as-prepared Cu2S–MoS2 samples was evaluated using the photocatalytic degradation of MB aqueous solution under visible-light illumination using a 500 W Xe lamp. The temperature inside the reactor was maintained at 20 °C via a continuous circulation of water surrounding the reactor. In the experiment, 50 mg of the photocatalyst was dispersed in 60 mL of MB aqueous solution (10−4 mol L−1). The suspension was stirred with a magnetic stirrer for 60 min in darkness before irradiation, which ensured the adsorption–desorption equilibrium of the MB aqueous solution on the photocatalyst. 60 mL of MB aqueous solution (10−4 mol L−1) without photocatalyst was used as a control reaction with continuous magnetic stirring. At the 15 min interval, an aliquot of the mixture was taken out and centrifuged at 8000 rpm for 3 min. Then, the supernatant solution was analysed using a UV-Vis spectrophotometer (Shimadzu UV-2450). The characteristic absorption peak of MB was 664 nm in the UV-Vis absorption spectra.3. Results and discussion
3.1 Characterization of the photocatalysts
Fig. 1a–e shows the SEM images of Cu2S–MoS2 samples with different proportions of Cu2S and MoS2 (calculated from ICP measurements). It can be seen that all the samples are assembled from nanosheets, showing the nanoflower morphology. The average diameters of nanoflowers in Fig. 1a–e vary from 0.3 μm to 1.5 μm along with the variation of the Cu–Mo ratio. In addition, the dispersity of the samples is different. Among the samples, Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displays the most regular structure with a diameter of 0.8–1 μm. The X-ray diffraction (XRD) diagram gives the specific composite of Cu, Mo and S elements in Fig. 1f. As shown by the red line of PDF card 53-0522 (Fig. 1f), the peaks at 15.9°, 27.7°, 36.1°, 46.2° and 54.7° could be attributed to the {100}, {111}, {210}, {220} and {311} planes of Cu2S. The characteristic peaks at 39.5°, 60.1° and 70.1° correspond to the {103}, {008} and {108} planes of MoS2 (blue line of PDF card 37-1492). The broad peak at 32.3° is an overlapping peak which is caused by the overlap of the {200} plane of Cu2S and the {100} plane of MoS2. Moreover, the peaks at 9.0° and 17.6° are attributed to low crystallinity of the MoS2{002} plane.42 After annealing treatment, the two peaks shifted to 14.3°, which is attributed to the {002} plane reflection. In Fig. 1f, diffraction peak intensity increases when the ratio of Cu2S to MoS2 increases from 1
1) displays the most regular structure with a diameter of 0.8–1 μm. The X-ray diffraction (XRD) diagram gives the specific composite of Cu, Mo and S elements in Fig. 1f. As shown by the red line of PDF card 53-0522 (Fig. 1f), the peaks at 15.9°, 27.7°, 36.1°, 46.2° and 54.7° could be attributed to the {100}, {111}, {210}, {220} and {311} planes of Cu2S. The characteristic peaks at 39.5°, 60.1° and 70.1° correspond to the {103}, {008} and {108} planes of MoS2 (blue line of PDF card 37-1492). The broad peak at 32.3° is an overlapping peak which is caused by the overlap of the {200} plane of Cu2S and the {100} plane of MoS2. Moreover, the peaks at 9.0° and 17.6° are attributed to low crystallinity of the MoS2{002} plane.42 After annealing treatment, the two peaks shifted to 14.3°, which is attributed to the {002} plane reflection. In Fig. 1f, diffraction peak intensity increases when the ratio of Cu2S to MoS2 increases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then decreases dramatically when the ratio increases from 1
1, and then decreases dramatically when the ratio increases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This means that the Cu2S–MoS2(1
1. This means that the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite exhibits the best long-term ordered structure, which is in agreement with the SEM results. The XRD pattern of the prepared pure Cu2S is shown in Fig. S1 (ESI†), which corresponds with PDF card 53-0522. The Cu2S exhibits nanoflower morphology assembled with some nanosheets as shown in Fig. S2 (ESI†). The XRD pattern and SEM image of pure MoS2 are shown in Fig. S3 and S4 (ESI†), respectively. It can be seen that its XRD pattern can be assigned to PDF card 37-1492 with a rod-shaped morphology. Compared with pure Cu2S and MoS2, the composites show a more regular morphology, which may have an impact on the photocatalytic performance of Cu2S–MoS2(x
1) composite exhibits the best long-term ordered structure, which is in agreement with the SEM results. The XRD pattern of the prepared pure Cu2S is shown in Fig. S1 (ESI†), which corresponds with PDF card 53-0522. The Cu2S exhibits nanoflower morphology assembled with some nanosheets as shown in Fig. S2 (ESI†). The XRD pattern and SEM image of pure MoS2 are shown in Fig. S3 and S4 (ESI†), respectively. It can be seen that its XRD pattern can be assigned to PDF card 37-1492 with a rod-shaped morphology. Compared with pure Cu2S and MoS2, the composites show a more regular morphology, which may have an impact on the photocatalytic performance of Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) composites.
y) composites.
 | ||
Fig. 1 (a–e) SEM images of Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y): (a) 1 y): (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (b) 1 3, (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (c) 1 2, (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (d) 2 1, (d) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and (e) 3 1 and (e) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (f) XRD patterns of samples. 1; (f) XRD patterns of samples. | ||
N2 adsorption–desorption experiments were carried out to investigate the textural and structural characteristics of the resultant samples, and the results are shown in Fig. 2 and Table 1. All the samples exhibit the typical type IV adsorption–desorption isotherm with a H3 hysteresis. This means that the samples are characterized by a mesoporous structure and slit-like pores. In addition, all of the nanocomposites show a similar pore size distribution with the maximum values located at 3.8 nm. As the proportion of Cu2S increases, the SBET of the samples increases continuously (Table 1) and all of the composites with different proportions exhibit a much higher specific surface area than that of pure Cu2S and MoS2. The differences in specific surface area may affect the catalytic performance of the composites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) samples
y) samples
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| S BET (m2 g−1) | 21.94 | 35.87 | 49.31 | 67.52 |
x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y y |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Cu2S | MoS2 | (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T 1)-T |
| S BET (m2 g−1) | 81.93 | 5.55 | 16.67 | 28.94 |
TEM images of the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite are shown in Fig. 3a and b, and two different lattice stripes can be found in the HRTEM image of the sample. In Fig. 3b, two orthogonal lattice fringes in the top inset show crystal spacing of 0.39 and 0.56 nm, which can be assigned to the {110} and {100} planes of Cu2S (53-0522), respectively. According to the literature,43 the layered spacing of 0.89 nm in the bottom inset should be the classical {002} plane of MoS2 (37-1492), which maybe resulted from hydrothermal synthesis of MoS2 without annealing treatment. The existence of Cu, Mo and S is confirmed by the EDS spectrum and elemental mapping shown in Fig. 3c. As the X-ray can penetrate 1 μm thickness, we could deduce that Mo, Cu and S are all distributed in the sample with high abundance. Meanwhile, the interlayer distance of the peak at 9.0° in the XRD pattern is about 0.9 nm according to the Bragg equation, which agrees with the result of the TEM. From the electron microscopy images, EDS-mapping and XRD analysis, it is demonstrated that nanoflower like composite materials have been successfully prepared in one-pot, and these are made up of Cu2S and MoS2.
1) nanocomposite are shown in Fig. 3a and b, and two different lattice stripes can be found in the HRTEM image of the sample. In Fig. 3b, two orthogonal lattice fringes in the top inset show crystal spacing of 0.39 and 0.56 nm, which can be assigned to the {110} and {100} planes of Cu2S (53-0522), respectively. According to the literature,43 the layered spacing of 0.89 nm in the bottom inset should be the classical {002} plane of MoS2 (37-1492), which maybe resulted from hydrothermal synthesis of MoS2 without annealing treatment. The existence of Cu, Mo and S is confirmed by the EDS spectrum and elemental mapping shown in Fig. 3c. As the X-ray can penetrate 1 μm thickness, we could deduce that Mo, Cu and S are all distributed in the sample with high abundance. Meanwhile, the interlayer distance of the peak at 9.0° in the XRD pattern is about 0.9 nm according to the Bragg equation, which agrees with the result of the TEM. From the electron microscopy images, EDS-mapping and XRD analysis, it is demonstrated that nanoflower like composite materials have been successfully prepared in one-pot, and these are made up of Cu2S and MoS2.
 | ||
Fig. 3 (a and b) HRTEM images and (c) EDS spectrum and elemental mapping of S, Cu and Mo of the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite. 1) nanocomposite. | ||
In order to further confirm the composition of the composite materials, XPS and AES were performed to investigate the valence state of the elements in Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the results are displayed in Fig. 4. In Fig. 4a, the high-resolution spectrum of Cu 2p shows two peaks with binding energy (BE) of 932.2 eV and 952.2 eV, which correspond to Cu 2p1/2 and Cu 2p3/2, respectively, indicating the existence of Cu1+. Because no satellite peaks are detected, the existence of Cu2+ is excluded.44 The AES (Fig. 4b) further confirms that the valence state of Cu is +1, since the characteristic peak associated with LMM at 569.6 eV is in accordance with the literature.45 The two peaks of Mo with BE of 229.3 eV and 232.5 eV in Fig. 4c are assigned to Mo 3d3/2 and Mo 3d5/2, respectively, which indicate that the Mo in the composite is tetravalent.46 According to a previous report,47 the peaks of the S 2p spectra at 162.3 eV (2p1/2) and 161.1 eV (2p3/2) in Fig. 4d are characteristic of S2−. The results demonstrate that the nanoflower like composite materials consist of Cu2S and MoS2. The BE values of the Cu and Mo spectra shift slightly compared with the literature as mentioned. This can be attributed to the interaction of electrons between the Cu2S and MoS2 components.
1), the results are displayed in Fig. 4. In Fig. 4a, the high-resolution spectrum of Cu 2p shows two peaks with binding energy (BE) of 932.2 eV and 952.2 eV, which correspond to Cu 2p1/2 and Cu 2p3/2, respectively, indicating the existence of Cu1+. Because no satellite peaks are detected, the existence of Cu2+ is excluded.44 The AES (Fig. 4b) further confirms that the valence state of Cu is +1, since the characteristic peak associated with LMM at 569.6 eV is in accordance with the literature.45 The two peaks of Mo with BE of 229.3 eV and 232.5 eV in Fig. 4c are assigned to Mo 3d3/2 and Mo 3d5/2, respectively, which indicate that the Mo in the composite is tetravalent.46 According to a previous report,47 the peaks of the S 2p spectra at 162.3 eV (2p1/2) and 161.1 eV (2p3/2) in Fig. 4d are characteristic of S2−. The results demonstrate that the nanoflower like composite materials consist of Cu2S and MoS2. The BE values of the Cu and Mo spectra shift slightly compared with the literature as mentioned. This can be attributed to the interaction of electrons between the Cu2S and MoS2 components.
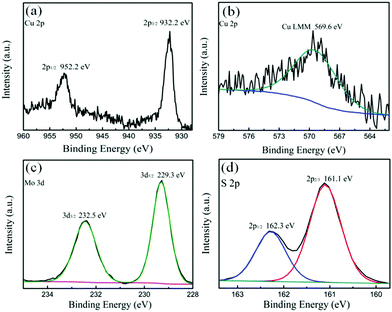 | ||
Fig. 4 (a, c and d) High resolution X-ray photoelectron spectra of Cu 2p, Mo 3d and S 2p in the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite, respectively; (b) LMM spectrum of Cu in the Cu2S–MoS2(1 1) nanocomposite, respectively; (b) LMM spectrum of Cu in the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite. 1) nanocomposite. | ||
In order to explore the formation mechanism of Cu2S–MoS2 nanocomposites, a series of time dependent experiments during the preparation of Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were performed by intercepting the intermediates during continuous reaction time. Fig. 5a is the SEM image of the white precipitate collected after 2 h hydrothermal reaction. The image shows the morphology and the micro-bulks are about 10 μm and micro-rods about 40 μm in size. When the reaction time is prolonged to 4 h, the micro-rods become shorter around 20 μm and form hollow structures, while the micro-bulks decompose to nanoparticles (Fig. 5b). When the reaction time reached 8–12 h, the nanoparticles gather to form nanoflowers with size about 200 nm, which are assembled with nanosheets (Fig. 5c and d), while the micro-rods dissolve to less than 10 μm. After 16 h, bulges begin to form on the surfaces of nanoflowers (Fig. 5e) and the micro-rods dissolve to less than 5 μm. After 20 h, the micro-rods are fully dissolved to form nanosheets and grow together with the nanosheets in the nanoflowers. When the time is extended to 24 h, the two kinds of nanosheet keep growing together, showing the flower like appearance of the final products (Fig. 1c). As confirmed by the XRD results (Fig. 5g), the XRD curves at 2 h should be mixed peaks of micro-bulks and micro-rods, which mainly show the signal of [BMIM]2Mo4O13 according to the literature.43 With the reaction time prolonged from 4 h to 8 h, the [BMIM]2Mo4O13 peaks (8.6°–10.5° and 21°–49°) become weaker. After 12 h, the peaks of Cu2S and MoS2 appear. After 16 h, only Cu2S and MoS2 peaks remain, which is in agreement with the final XRD curves. UV-Vis DRS was carried out to investigate the optical properties of the as-prepared samples and the results are shown in Fig. 5h. When the synthetic reaction time was extended from 2 h to 24 h, the light absorption in the visible region becomes more and more broad and the absorbance capability of the composite becomes higher and higher. Meanwhile, the light absorption of the composite in the UV region is maintained. This result could be due to the more regular morphology and increased crystallinity obtained from the prolonged reaction time. So the well-developed Cu2S–MoS2(1
1) were performed by intercepting the intermediates during continuous reaction time. Fig. 5a is the SEM image of the white precipitate collected after 2 h hydrothermal reaction. The image shows the morphology and the micro-bulks are about 10 μm and micro-rods about 40 μm in size. When the reaction time is prolonged to 4 h, the micro-rods become shorter around 20 μm and form hollow structures, while the micro-bulks decompose to nanoparticles (Fig. 5b). When the reaction time reached 8–12 h, the nanoparticles gather to form nanoflowers with size about 200 nm, which are assembled with nanosheets (Fig. 5c and d), while the micro-rods dissolve to less than 10 μm. After 16 h, bulges begin to form on the surfaces of nanoflowers (Fig. 5e) and the micro-rods dissolve to less than 5 μm. After 20 h, the micro-rods are fully dissolved to form nanosheets and grow together with the nanosheets in the nanoflowers. When the time is extended to 24 h, the two kinds of nanosheet keep growing together, showing the flower like appearance of the final products (Fig. 1c). As confirmed by the XRD results (Fig. 5g), the XRD curves at 2 h should be mixed peaks of micro-bulks and micro-rods, which mainly show the signal of [BMIM]2Mo4O13 according to the literature.43 With the reaction time prolonged from 4 h to 8 h, the [BMIM]2Mo4O13 peaks (8.6°–10.5° and 21°–49°) become weaker. After 12 h, the peaks of Cu2S and MoS2 appear. After 16 h, only Cu2S and MoS2 peaks remain, which is in agreement with the final XRD curves. UV-Vis DRS was carried out to investigate the optical properties of the as-prepared samples and the results are shown in Fig. 5h. When the synthetic reaction time was extended from 2 h to 24 h, the light absorption in the visible region becomes more and more broad and the absorbance capability of the composite becomes higher and higher. Meanwhile, the light absorption of the composite in the UV region is maintained. This result could be due to the more regular morphology and increased crystallinity obtained from the prolonged reaction time. So the well-developed Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite at 24 h should have the best response capability and light trapping properties in the whole visible region. In addition, the UV-Vis DRS results are in accordance with the SEM analysis (Fig. 5a–f).
1) composite at 24 h should have the best response capability and light trapping properties in the whole visible region. In addition, the UV-Vis DRS results are in accordance with the SEM analysis (Fig. 5a–f).
Based on the above results, the growth mechanism of the Cu2S–MoS2 composite materials can be described as a four-step process (Fig. 6). In the first step (within 2 h), two kinds of metal soluble salt react with the ionic liquid. The Mo4O132− transformed from (NH4)6Mo7O24·4H2O could react with [BMIM]+, forming [BMIM]2Mo4O13 micro-rods, while Cu2+ reacts with SCN−, forming Cu2S micro-bulks. The morphologies of the sample in this step are micro-rods and micro-bulks (Fig. 5a) and the XRD curves show the strong characteristic peaks of [BMIM]2Mo4O13 (Fig. 5g). The second step is the reaction within 8 h. In this step, the [BMIM]2Mo4O13 micro-rods begin to react with H2S on their surfaces and form MoS2 crystal nuclei, while the inner part of the micro-rods would diffuse to the surface forming hollow structures by the Kirkendall effect (Fig. 5b). At the same time, the Cu2S micro-bulks dissolve into nanoparticles and gather to form nanoflowers by Ostwald ripening (Fig. 5c). The XRD curves of this step show that the characteristic peaks of the Mo precursor become weaker (Fig. 5g). When the reaction time was prolonged to 12–16 h, the sample is in the third reaction step. In this step, [BMIM]2Mo4O13 decomposes rapidly. The micro-hollow-rods are hard to see, while the Mo precursor just remains as nanoplates (Fig. 5d and e). The main morphology of the sample is nanoflowers with ridges on their surfaces. These should be the MoS2 crystal nuclei adsorbed on the Cu2S nanosheets. The XRD peaks of [BMIM]2Mo4O13 disappeared and Cu2S–MoS2 peaks appeared, which can support the above view (Fig. 5g). The last step is the reaction from 16 h to 24 h. The MoS2 nanosheets grow together with the Cu2S nanosheets (Fig. 1c), forming the final composite material. As a result, the composite material is made up of Cu2S and MoS2, and should exhibit good photocatalytic activity.
To further explore the effects of the IL on the morphology of Cu2S–MoS2 composites, TAA was used as a substitute for the IL to synthesize Cu2S–MoS2, this was labeled Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T. Its XRD pattern and SEM image are shown in Fig. 7a and b, respectively. As seen in Fig. 7a, Cu2S–MoS2(1
1)-T. Its XRD pattern and SEM image are shown in Fig. 7a and b, respectively. As seen in Fig. 7a, Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T exhibits similar diffraction peaks to Cu2S–MoS2(1
1)-T exhibits similar diffraction peaks to Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), but the intensity of the diffraction peaks is obviously weaker than those of Cu2S–MoS2(1
1), but the intensity of the diffraction peaks is obviously weaker than those of Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This phenomenon demonstrates that the addition of the ionic liquid could improve the long-term order of the compound structure. In addition, compared with the irregular morphology and smaller specific surface area of Cu2S–MoS2(1
1). This phenomenon demonstrates that the addition of the ionic liquid could improve the long-term order of the compound structure. In addition, compared with the irregular morphology and smaller specific surface area of Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T (Fig. 7b and Table 1), Cu2S–MoS2(1
1)-T (Fig. 7b and Table 1), Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) shows a regular 3D nanoflower morphology and much larger specific surface area (Fig. 1c and Table 1). This indicates that the ionic liquid plays an important role in controlling the morphology of the composites during the preparation process, which is consistent with the literature.48 The different morphologies and specific surface areas may have impacts on the photocatalytic performance.
1) shows a regular 3D nanoflower morphology and much larger specific surface area (Fig. 1c and Table 1). This indicates that the ionic liquid plays an important role in controlling the morphology of the composites during the preparation process, which is consistent with the literature.48 The different morphologies and specific surface areas may have impacts on the photocatalytic performance.
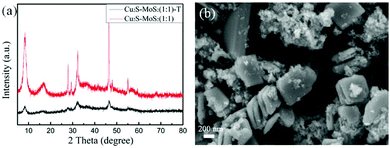 | ||
Fig. 7 XRD patterns of Cu2S–MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and Cu2S–MoS2 (1 1) and Cu2S–MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T (a); SEM image of Cu2S–MoS2 (1 1)-T (a); SEM image of Cu2S–MoS2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T (b). 1)-T (b). | ||
3.2 Photocatalytic activity evaluation
Before light irradiation, adsorption experiments were carried out in darkness to obtain the adsorption equilibrium of MB on the Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) materials. To eliminate the effect of the substrate on the photocatalytic reaction, an experiment with MB solution (60 mL, 10−4 mol L−1) without any photocatalyst was performed as a blank experiment, which indicated that the effect of MB on the photocatalytic reaction can be neglected. P-25, Cu2S and MoS2 were used along with the resultant Cu2S–MoS2 composites.
y) materials. To eliminate the effect of the substrate on the photocatalytic reaction, an experiment with MB solution (60 mL, 10−4 mol L−1) without any photocatalyst was performed as a blank experiment, which indicated that the effect of MB on the photocatalytic reaction can be neglected. P-25, Cu2S and MoS2 were used along with the resultant Cu2S–MoS2 composites.
The photocatalytic results for all the catalysts are shown in Fig. 8 as are their corresponding curves of ln(C0/C) vs. irradiation time. The rate constant values (k) of the different samples are listed in Tables 2 and 3. As seen in Fig. 8a, all of the Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) samples show excellent adsorption capacity to methylene blue in darkness while there is great difference in photocatalytic performance. All the Cu2S–MoS2(x
y) samples show excellent adsorption capacity to methylene blue in darkness while there is great difference in photocatalytic performance. All the Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) composites present better photocatalytic performance than Cu2S or MoS2. This result indicates that the existence of MoS2 could obviously enhance the photoactive performance of the composites under visible irradiation. The more regular 3D nanoflower structure and larger specific surface area could be the reason for the enhanced photocatalytic performance compared to that of pure Cu2S and MoS2. In addition, the efficient electron transport between the Cu2S and MoS2 interface of the composites may have a positive influence on photocatalysis. Among the Cu2S–MoS2(x
y) composites present better photocatalytic performance than Cu2S or MoS2. This result indicates that the existence of MoS2 could obviously enhance the photoactive performance of the composites under visible irradiation. The more regular 3D nanoflower structure and larger specific surface area could be the reason for the enhanced photocatalytic performance compared to that of pure Cu2S and MoS2. In addition, the efficient electron transport between the Cu2S and MoS2 interface of the composites may have a positive influence on photocatalysis. Among the Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) samples, the Cu2S–MoS2(1
y) samples, the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite exhibits the most outstanding performance with k = 0.0471 min−1. As the ratio of Cu2S to MoS2 increases from 1
1) nanocomposite exhibits the most outstanding performance with k = 0.0471 min−1. As the ratio of Cu2S to MoS2 increases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the photocatalytic efficiency increased obviously. This result may be due to the increased proportion of Cu2S to MoS2 which results in larger specific surface area and more regular morphology. The regular 3D structure and larger specific surface area could supply more active sites on the surface for transfer of charge-carriers and a larger contact area for substrates, thus promoting the photocatalytic performance. In addition, the number of transferred electrons on the interface of Cu2S–MoS2 composites could increase with the increased proportion of Cu2S. In this case, the interface between Cu2S and MoS2 could improve the electron transfer efficiency and effectively inhibit charge recombination. However, as the ratio of Cu2S to MoS2 increases further from 1
1, the photocatalytic efficiency increased obviously. This result may be due to the increased proportion of Cu2S to MoS2 which results in larger specific surface area and more regular morphology. The regular 3D structure and larger specific surface area could supply more active sites on the surface for transfer of charge-carriers and a larger contact area for substrates, thus promoting the photocatalytic performance. In addition, the number of transferred electrons on the interface of Cu2S–MoS2 composites could increase with the increased proportion of Cu2S. In this case, the interface between Cu2S and MoS2 could improve the electron transfer efficiency and effectively inhibit charge recombination. However, as the ratio of Cu2S to MoS2 increases further from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the degradation rate of MB decreased dramatically despite of the ever-increasing specific surface area. This phenomenon should be caused by excessive MoS2 covering the Cu2S component, and the decreased exposed amount of Cu2S could hinder electron transfer on the interface between Cu2S and MoS2, thus in turn leading to the decrease in photoactivity. Furthermore, as the ratio of Cu2S to MoS2 increases from 1
1, the degradation rate of MB decreased dramatically despite of the ever-increasing specific surface area. This phenomenon should be caused by excessive MoS2 covering the Cu2S component, and the decreased exposed amount of Cu2S could hinder electron transfer on the interface between Cu2S and MoS2, thus in turn leading to the decrease in photoactivity. Furthermore, as the ratio of Cu2S to MoS2 increases from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the morphology of the composites becomes irregular, which can also negatively affect the photoactivity. MoS2 has excellent absorption ability for visible light and transfer ability for electrons, which could result in shortened lifetimes of photo-induced charge-carriers. Therefore, a low MoS2 proportion could depress the composite's photocatalytic performance. On the other hand, the gradual irregular morphology (shown in Fig. 1) may have a negative impact on the photocatalytic activity of the nanocomposites.
1, the morphology of the composites becomes irregular, which can also negatively affect the photoactivity. MoS2 has excellent absorption ability for visible light and transfer ability for electrons, which could result in shortened lifetimes of photo-induced charge-carriers. Therefore, a low MoS2 proportion could depress the composite's photocatalytic performance. On the other hand, the gradual irregular morphology (shown in Fig. 1) may have a negative impact on the photocatalytic activity of the nanocomposites.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) samples and Cu2S–MoS2(1
y) samples and Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) samples with different reaction times at 200 °C for the degradation of MB solution
1) samples with different reaction times at 200 °C for the degradation of MB solution
| Sample | k (min−1) | Sample | k (min−1) |
|---|---|---|---|
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.0135 | 2 h | 0.0027 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.0247 | 4 h | 0.0049 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.0471 | 8 h | 0.0085 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.0077 | 12 h | 0.0162 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.0058 | 16 h | 0.0223 |
| P-25 | 0.0006 | 20 h | 0.0360 |
| Blank | 0.0002 | 24 h | 0.0471 |
| Cu2S | 0.0037 | MoS2 | 0.0032 |
Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T 1)-T |
0.0049 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in a cycling experiment for the degradation of MB solution
1) in a cycling experiment for the degradation of MB solution
| Cycle | 1st | 2nd | 3rd | 4th |
|---|---|---|---|---|
| k (min−1) | 0.0471 | 0.0381 | 0.0288 | 0.0245 |
In Fig. 8a, the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite shows much higher catalytic activity than Cu2S–MoS2(1
1) nanocomposite shows much higher catalytic activity than Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)-T, which corresponds with the XRD, SEM and BET results. This indicates that ionic liquid-assisted Cu2S–MoS2 nanocomposites have excellent photocatalytic performance for the degradation of MB under visible light.
1)-T, which corresponds with the XRD, SEM and BET results. This indicates that ionic liquid-assisted Cu2S–MoS2 nanocomposites have excellent photocatalytic performance for the degradation of MB under visible light.
Fig. 8b shows the photocatalytic results of the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite with different reaction times at 200 °C. With prolonged reaction time, the photocatalytic performance of the samples is significantly enhanced and the sample within 24 h exhibits the best catalytic performance. With the extension of the reaction, the samples exhibit more regular morphology and their response ability to visible light enhance obviously. The catalytic results are also in accordance with the SEM and UV-Vis DRS analysis.
1) nanocomposite with different reaction times at 200 °C. With prolonged reaction time, the photocatalytic performance of the samples is significantly enhanced and the sample within 24 h exhibits the best catalytic performance. With the extension of the reaction, the samples exhibit more regular morphology and their response ability to visible light enhance obviously. The catalytic results are also in accordance with the SEM and UV-Vis DRS analysis.
Cycling experiments were carried out to investigate the lifetime and cycle stability of the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite and the results are shown in Fig. 8c. It can be seen that the sample still exhibits good degradation of MB after four cycles. The decreased photocatalytic performance may result from the adsorption of the substrate and products on the catalyst in reaction, which may decrease the number of active sites. In addition, this result is also attributed to the loss of catalyst in the recovery process.
1) nanocomposite and the results are shown in Fig. 8c. It can be seen that the sample still exhibits good degradation of MB after four cycles. The decreased photocatalytic performance may result from the adsorption of the substrate and products on the catalyst in reaction, which may decrease the number of active sites. In addition, this result is also attributed to the loss of catalyst in the recovery process.
After the cycling experiment, the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) nanocomposite was characterized by XRD and XPS, and the results are shown in Fig. S5 and S6 (ESI†), respectively. As can be seen from the XRD pattern of the composite in Fig. S5 (ESI†), the composite still retains its structural characteristics after the experiment although peak intensity was slightly different from that before the reaction. From Fig. S6 (ESI†), it can be seen that the valence states of the elements in the Cu2S–MoS2(1
1) nanocomposite was characterized by XRD and XPS, and the results are shown in Fig. S5 and S6 (ESI†), respectively. As can be seen from the XRD pattern of the composite in Fig. S5 (ESI†), the composite still retains its structural characteristics after the experiment although peak intensity was slightly different from that before the reaction. From Fig. S6 (ESI†), it can be seen that the valence states of the elements in the Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) composite did not change during the experiment. This means that the nanocomposite still retains its internal structure after cycling and that it is an excellent and stable catalyst for the degradation of MB under visible irradiation.
1) composite did not change during the experiment. This means that the nanocomposite still retains its internal structure after cycling and that it is an excellent and stable catalyst for the degradation of MB under visible irradiation.
4. Conclusion
Novel Cu2S–MoS2(x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y) nanocomposites with different ratios of Cu2S (x) and MoS2 (y) are synthesized successfully by a one-step hydrothermal method with the assistance of the ionic liquid [BMIM]SCN. The results show that the ratio of x
y) nanocomposites with different ratios of Cu2S (x) and MoS2 (y) are synthesized successfully by a one-step hydrothermal method with the assistance of the ionic liquid [BMIM]SCN. The results show that the ratio of x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) y has an obvious influence on the specific surface area as well as the photocatalytic performance of the Cu2S–MoS2 nanocomposites. The formation mechanism of Cu2S–MoS2 nanocomposites is investigated by time-dependent experiments during the synthesis of Cu2S–MoS2(1
y has an obvious influence on the specific surface area as well as the photocatalytic performance of the Cu2S–MoS2 nanocomposites. The formation mechanism of Cu2S–MoS2 nanocomposites is investigated by time-dependent experiments during the synthesis of Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). It could be deduced that the ionic liquid (IL) plays an important role in the formation of [BMIM]2Mo4O13 intermediates, which could interact with H2S on their surfaces to form MoS2 crystal nuclei. Among the composites, Cu2S–MoS2(1
1). It could be deduced that the ionic liquid (IL) plays an important role in the formation of [BMIM]2Mo4O13 intermediates, which could interact with H2S on their surfaces to form MoS2 crystal nuclei. Among the composites, Cu2S–MoS2(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibits the most outstanding photocatalytic activity and reusability performance.
1) exhibits the most outstanding photocatalytic activity and reusability performance.
Due to the structural advantages and excellent absorption ability for light in the whole region, these Cu2S–MoS2 nanocomposites may be expected to be promising catalysts in other fields of photocatalysis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (21373120 and 21071086) and 111 Project (B12015).Notes and references
- J. Di, J. X. Xia, Y. P. Ge, H. P. Li, H. Y. Ji, H. Xu, Q. Zhang, H. M. Li and M. N. Li, Appl. Catal., B, 2015, 168, 51–61 CrossRef.
- T. Boningari, S. N. R. Inturi, M. Suidan and P. G. Sminiotis, Chem. Eng. J., 2018, 339, 249–258 CrossRef CAS.
- M. Yan, Y. Q. Hua, F. F. Zhu, W. Gu, J. H. Jiang, H. Q. Shen and W. D. Shi, Appl. Catal., B, 2017, 202, 518–527 CrossRef CAS.
- R. Atchudan, T. N. J. I. Edison, S. Perumal, N. Karthik, D. Karthikeyan, M. Shanmugam and Y. R. Lee, J. Photochem. Photobiol., A, 2018, 350, 75–85 CrossRef CAS.
- S. G. Kumar and K. S. R. K. Rao, Appl. Surf. Sci., 2017, 391, 124–148 CrossRef CAS.
- C. L. Yu, G. Li, S. Kumar, K. Yang and R. C. Jin, Adv. Mater., 2014, 26, 892–898 CrossRef CAS PubMed.
- R. Saravanan, E. Sacari, F. Gracia, M. M. Khan, E. Mosquera and V. K. Gupta, J. Mol. Liq., 2016, 221, 1029–1033 CrossRef CAS.
- X. Liu, Y. D. Shi, Y. M. Dong, H. X. Li, Y. M. Xia and H. J. Wang, New J. Chem., 2017, 41, 13382–13390 RSC.
- R. Daghrir, P. Drogui and D. Robert, Ind. Eng. Chem. Res., 2013, 52, 3581–3599 CrossRef CAS.
- S. K. Kansal, P. Kundu, S. Sood, R. Lamba, A. Umar and S. K. Mehta, New J. Chem., 2014, 38, 3220–3226 RSC.
- N. Qutub, B. M. Pirzada, K. Umar and S. Sabir, Chem. Eng. J., 2016, 4, 808–817 CAS.
- Y. X. Cui, C. Y. Wei, J. Q. Yang, J. Zhang and W. J. Zheng, CrystEngComm, 2016, 18, 6245–6253 RSC.
- Q. Cao, R. C. Che and N. Chen, Appl. Catal., B, 2015, 162, 187–195 CrossRef CAS.
- W. T. Wang, X. L. Liu, D. Li, L. Z. Fan and Y. C. Li, Phys. Chem. Chem. Phys., 2015, 17, 14532–14541 RSC.
- H. T. Zhang, G. Wu and X. H. Chen, Langmuir, 2005, 21, 4281–4282 CrossRef CAS PubMed.
- S. K. Maji, A. K. Dutta, G. R. Bhadu, P. Paul, A. Mondal and B. Adhikary, J. Mater. Chem. B, 2013, 1, 4127–4134 RSC.
- C. H. Lai, M. Y. Lu and L. J. Chen, J. Mater. Chem., 2012, 22, 19–30 RSC.
- Y. Liu, Y. H. Deng, Z. K. Sun, J. Wei, G. F. Zheng, A. M. Asiri, S. B. Khan, M. M. Rahman and D. Y. Zhao, Small, 2013, 9, 2702–2708 CrossRef CAS PubMed.
- Y. H. Si, Y. Xia, S. K. Shang, X. B. Xiong, X. R. Zeng, J. Zhou and Y. Y. Li, Nanomaterials, 2018, 8, 526 CrossRef PubMed.
- J. Zhang, Z. Dai, F. Qin, H. P. Zhao, S. Zhao and R. Chen, Appl. Catal., B, 2017, 205, 281–291 CrossRef.
- Q. Zhang, Z. Dai, G. Chen, Y. L. Liu and R. Chen, Ceram. Int., 2017, 43, 11296–11304 CrossRef CAS.
- Z. Dai, F. Qin, H. P. Zhao, F. Tian, Y. L. Liu and R. Chen, Nanoscale, 2015, 7, 11991–11999 RSC.
- U. Shamraiz, A. Badshah, R. A. Hussain, M. A. Nadeem and S. Saba, J. Saudi Chem. Soc., 2017, 21, 390–398 CrossRef CAS.
- W. Guo, K. Fan, J. J. Zhang and C. J. Xu, Appl. Surf. Sci., 2018, 447, 125–134 CrossRef CAS.
- H. Y. Chuai, D. F. Zhou, X. F. Zhu, Z. H. Li and W. P. Huang, Chin. J. Catal., 2015, 36, 2194–2202 CrossRef CAS.
- J. J. Zhang, P. Qi, J. Li, X. C. Zheng, P. Liu, X. X. Guan and G. P. Zheng, J. Ind. Eng. Chem., 2015, 61, 407–415 CrossRef.
- I. J. L. Plante, A. Teitelboim, I. Pinkas, D. Own and T. Mokari, J. Phys. Chem. Lett., 2014, 5, 590–596 CrossRef PubMed.
- H. Wang, L. Liu, Y. F. Wang, S. L. Lin, W. J. An, W. Q. Cui and Y. H. Liang, Mater. Lett., 2015, 160, 351–354 CrossRef CAS.
- S. B. Patil, B. Kishore, K. Manjunath, V. Reddy and G. Nagaraju, Int. J. Hydrogen Energy, 2018, 43, 4003–4014 CrossRef CAS.
- X. W. Wang, Y. A. Li, M. R. Wang, W. J. Li, M. F. Chen and Y. Zhao, New J. Chem., 2014, 38, 4182–4189 RSC.
- Y. F. Zhang and S. J. Park, J. Catal., 2018, 361, 238–247 CrossRef CAS.
- K. Zhang, J. K. Kim, B. Park, S. F. Qia, B. Ji, X. W. Sheng, H. Zeng, H. Shin, S. H. Oh, C. L. Lee and J. H. Park, Nano Lett., 2017, 17, 6676–6683 CrossRef CAS PubMed.
- Y. F. Zhang and S. J. Park, Appl. Catal., B, 2019, 240, 92–101 CrossRef CAS.
- Y. F. Zhang and S. J. Park, J. Catal., 2016, 355, 1–10 CrossRef.
- M. Joy, A. P. Mohamed, K. G. K. Warrier and U. S. Hareesh, New J. Chem., 2017, 41, 3432–3442 RSC.
- J. M. Wu, W. E. ChNG, Y. T. Chang and C. K. Chang, Adv. Mater., 2016, 28, 3718–3725 CrossRef CAS PubMed.
- L. X. Zheng, S. C. Han, H. Liu, P. P. Yu and X. S. Fang, Small, 2016, 12, 1527–1536 CrossRef CAS PubMed.
- K. Chang, Z. W. Mei, T. Wang, Q. Kang, S. X. Ouyang and J. H. Ye, ACS Nano, 2014, 8, 7078–7087 CrossRef CAS PubMed.
- J. He, L. Chen, F. Wang, Y. Liu, P. Chen, C. K. Au and S. F. Yin, ChemSusChem, 2016, 9, 624–630 CrossRef CAS PubMed.
- X. J. Zhang, Y. C. Guo, J. Tian, B. T. Sun, Z. Q. Liang, X. S. Xu and H. Z. Cui, Appl. Catal., B, 2018, 232, 355–364 CrossRef CAS.
- X. Sun, H. T. Deng, W. G. Zhu, Z. Yu, C. Z. Wu and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 1704–1709 CrossRef CAS PubMed.
- X. Y. Yu, H. Hu, Y. W. Wang, H. Y. Chen and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 7395–7398 CrossRef CAS PubMed.
- W. H. Luo, G. F. Zhang, Y. X. Cui, Y. Sun, Q. Qin, J. Zhang and W. J. Zheng, J. Mater. Chem. A, 2017, 5, 11278–11285 RSC.
- G. S. Selopal, I. Gurpreet, R. Milan, M. M. Natile, G. Sberveglieri and A. Vomiero, Nano Energy, 2014, 6, 200–210 CrossRef CAS.
- M. D. Ye, C. Chen, N. Zhang, X. R. Wen, W. X. Guo and C. J. Lin, Adv. Energy Mater., 2014, 4, 1301564 CrossRef.
- C. B. Liu, L. L. Wang, Y. H. Tang, S. L. Luo, Y. T. Liu, S. Q. Zhang, Y. X. Zeng and Y. Z. Xu, Appl. Catal., B, 2015, 164, 1–9 CrossRef CAS.
- A. C. Poulose, S. Veeranarayanan, M. S. Mohamed, Y. Nagaoka, R. R. Aburto, T. Mitcham, P. M. Ajayan, R. R. Bouchard, Y. Sakamoto, Y. Yoshida, T. Maekawa and D. S. Kumar, Nanoscale, 2015, 7, 8378–8388 RSC.
- R. M. Wang, G. Cheng, Z. Dai, J. Ding, Y. L. Rong and R. Chen, Chem. Eng. J., 2017, 327, 371–386 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nj05229h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |




