Magnetic ionic liquids based on transition metal complexes with N-alkylimidazole ligands†
Deepak
Chand
a,
Muhammad Qamar
Farooq
a,
Arjun K.
Pathak
b,
Jingzhe
Li
a,
Emily A.
Smith
 a and
Jared L.
Anderson
a and
Jared L.
Anderson
 *a
*a
aDepartment of Chemistry, Iowa State University, 1605 Gilman Hall, Ames, IA 50011, USA. E-mail: andersoj@iastate.edu; Tel: +1-515-294-8356
bDivision of Materials Science and Engineering, Ames Laboratory, Iowa State University, 233 Spedding, Ames, IA 50011, USA
First published on 13th November 2018
Abstract
In this study, magnetic ionic liquids (MILs) consisting of Ni(II), Co(II), and Mn(II) and paired with the bis[(trifluoromethyl)sulfonyl]imide [NTf2−] anion were synthesized from their water soluble chloride intermediates. The MILs feature low viscosity, high hydrophobicity, and hydrolytic stability making them attractive candidates for a number of highly interdisciplinary applications.
Ionic liquids (ILs) are salts possessing a wide liquid range from cryogenic temperatures up to 100 °C. ILs are often composed of large asymmetric cations and anions, and can be designed for specific applications through the choice of the respective cation and anion combination.1 In addition to their wide liquid range and the tunability of physicochemical properties, they have garnered significant attention due to their negligible vapor pressure, high thermal and chemical stability, wide electrochemical window, and unique solvation capabilities.2
MILs are a subclass of ILs that originate by incorporating high-spin transition metals and rare earth metals into their structure. MILs combine the properties of ionic liquids with magnetic, optical or catalytic properties that originate from the metal incorporated in the complex anion.3–6 MILs have been exploited in a wide range of applications including catalysis,7,8 fabrication of nanostructured and reduced dimensionality materials such as thin films, tubes, wires or particles;9 electrochemical and medical devices;10 and in numerous analytical applications including liquid–liquid extractions (LLE) and stationary phases for gas chromatography (GC).11–14
Heavily alkylated phosphonium cations and weakly-coordinating fluorinated anions such as bis[(trifluoromethyl)sulfonyl]imide [NTf2−] are often employed to impart hydrophobicity to MILs.15 The majority of MILs contain anionic metal centers where the hydrophobicity is typically imparted through the inclusion of nonpolar moieties in the cation.16 Metallocenium ionic liquids containing a cationic metal center paired with weakly-coordinating [NTf2−] anions are generally less viscous.17 However, they are prone to hydrolysis because of the hydrolytic instability of the employed ligands used, which often limits their applicability.18,19 Ligands that possess better water stability can be helpful in improving the hydrolytic stability in the case of MILs containing cationic metal centers. Imidazole-based ligands, in combination with other auxiliary ligands that coordinate to the metal center, have been shown to produce MILs with good hydrolytic stability.20
The magnetic susceptibility of MILs can be largely tuned for a specific application by incorporating different paramagnetic metals into the chemical structure.21–23 Mn(II) based MILs possess higher magnetic susceptibilities than Co(II) and Ni(II) MILs. Moreover, Mn(II) is known to exhibit interesting photophysical properties such as photoluminescence (fluorescence and phosphorescence),24 triboluminescence25 as well as electroluminescence.26
In this study, we report the efficient synthesis of low viscosity and hydrophobic MILs based on Ni(II), Mn(II) and Co(II) that feature only imidazole ligands and the [NTf2−] anion. SQUID measurements provide insight into their magnetic behavior and the thermal properties of each MIL were evaluated using thermogravimetric (TG) analysis and differential scanning calorimetry (DSC). Viscosity measurements revealed that MILs possessing 1-octylimidazole ligands are significantly less viscous (η = 152 cP). This study is likely to open up many new areas of opportunity in the fields of chemical separations, opto-electronics, electrochemistry, among others.
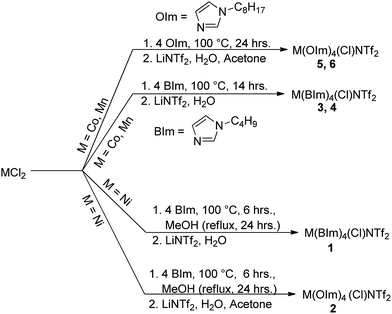
Transition metal (Ni(II), Co(II), and Mn(II)) MILs were synthesized according to Scheme 1. The precursor chloride-based MILs were obtained in a solvent free reaction by treating the anhydrous metal chlorides with 1-butylimidazole (BIm) or 1-octylimidazole (OIm). The obtained MILs, in chloride form, were then treated with an aqueous solution of lithium bis[(trifluoromethyl)sulfonyl]imide to obtain the corresponding [NTf2−]-based MILs (1–6) via metathesis reaction. Detailed synthetic methods as well as elemental analysis, Raman, and IR spectroscopy data are provided in ESI.†
The thermal properties of all [NTf2−]-based compounds were investigated by differential scanning calorimetry (DSC) and thermogravimetric (TG) analysis. Thermal data are summarized in Table 1. All synthesized [NTf2−]-based MILs have moderate thermal stability ranging from 149–178 °C and decompose in a two-step thermal pathway (see ESI†). The MILs composed of octylimidazole ligands appear to have slightly better thermal stability than their 1-butylimidazole counterparts. The identity of gases evolved during the heating process was determined by mass spectrometry detection and shows that fluorinated hydrocarbons are the major decomposition products (see ESI†). Therefore, the thermal stability of these ionic liquids is limited by the strength of metal–heteroatom bond, otherwise [NTf2−]-based ILs generally have very good thermal stability.27,28
| MIL | Abbreviation | Physical state at room temperature (RT), 25 °C | Melting point [°C] | Decomposition temperature [°C] |
|---|---|---|---|---|
| 1 | Ni(BIm)4(Cl)NTf2 | Solid | 65.8 | 164 |
| 2 | Ni(OIm)4(Cl)NTf2 | Liquid | <25 | 178 |
| 3 | Co(BIm)4(Cl)NTf2 | Solid | <40 | 159 |
| 4 | Mn(BIm)4(Cl)NTf2 | Solid | <40 | 149 |
| 5 | Co(OIm)4(Cl)NTf2 | Liquid | <25 | 168 |
| 6 | Mn(OIm)4(Cl)NTf2 | Liquid | <25 | 168 |
MILs of low viscosity are desirable in applications such as liquid–liquid extraction (LLE),29 electrodeposition of metals,30 and in the design of equipment such as heat exchangers.31,32 For MILs that contain an anionic metal center, hydrogen bonding and van der Waals interactions are often the limiting factors that determine viscosity.33 The viscosity has been found to generally increase with an increase in alkyl chain length for MILs containing the 1-alkyl-3-methylimidazolium cation.34 Viscosities of MILs 1–6 are presented in Table 2. It can be seen that the viscosity decreases with an increase in carbon chain length of the imidazole ligand. MILs with 1-octylimidazole ligands are significantly less viscous than MILs comprised of 1-butylimidazole ligands. The Co(OIm)4(Cl)NTf2 MIL exhibited a viscosity of 152 cP at 21.7 °C and is the least viscous among the synthesized MILs in this study. MILs 1, 3 and 4 are highly viscous liquids and were observed to solidify when stored for extended periods of time at room temperature. Therefore, these MILs, and in particular those with octyl-imidazole ligands, are less viscous than previous generations of MILs. For example, the viscosity of phosphonium metal halide based MILs is on the order of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cP,35 while the viscosities of phosphonium metal-diketonate MILs range between 400–1000 cP.16
000 cP,35 while the viscosities of phosphonium metal-diketonate MILs range between 400–1000 cP.16
| MIL | Abbreviation | Viscositya [cP] | Solubility |
|---|---|---|---|
| a Viscosity measurements were performed at 21.7 °C; additional details are provided in ESI. b I = insoluble in hexane at 0.01% (w/v) and insoluble in water at 0.001% (w/v) MIL to solvent ratio. c S = soluble in acetone, acetonitrile, chloroform, dichloromethane, ethanol, ethyl acetate, methanol at 0.01% (w/v) MIL to solvent ratio. d S = 50% soluble in toluene and diethyl ether at 0.01% (w/v) MIL to solvent ratio (v/v) MIL to solvent ratio. e The viscosity of the MILs was measured after synthesis/purification. Over time, the BIm-based MILs formed solids (see Table 1). | |||
| 1 | Ni(BIm)4(Cl)NTf2 | 2501e | Ib, Scd |
| 2 | Ni(OIm)4(Cl)NTf2 | 680 | Ib, Sc |
| 3 | Co(BIm)4(Cl)NTf2 | 1256e | Ib, Scd |
| 4 | Mn(BIm)4(Cl)NTf2 | 1245e | Ib, Scd |
| 5 | Co(OIm)4(Cl)NTf2 | 152 | Ib, Sc |
| 6 | Mn(OIm)4(Cl)NTf2 | 303 | Ib, Sc |
The solubility data presented in Table 2 shows that, independent of the nature of the ligand, MILs 1–6 were immiscible with water at compositions as low as 0.001% (w/v) MIL-to-solvent ratio. Despite their hydrophobic nature, these MILs are insoluble in nonpolar solvents such as hexane at 0.01% (w/v) MIL-to-solvent ratio. Therefore, the hydrophobic properties of these MILs are ideal for applications such as aqueous liquid−liquid microextractions, where extremely low phase ratios of extraction solvent are employed; photoluminescent materials,36 where water could quench the luminescence due to vibrionic coupling and magnetic ionic liquid membranes for gas separations.35 MILs bearing the 1-butylimidazole ligand, (e.g., Ni(BIm)4(Cl)NTf2, Co(BIm)4(Cl)NTf2, and Mn(BIm)4(Cl)NTf2), were found to be partially soluble in toluene and diethyl ether. All of the reported MILs dissolved completely in higher polarity solvents such as acetone, acetonitrile, chloroform, dichloromethane, ethanol, ethyl acetate, and methanol.
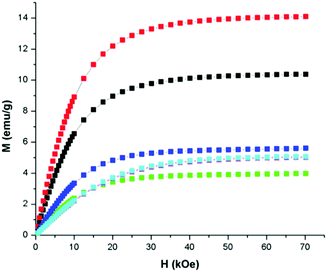
Magnetic properties were measured using a Quantum Design MPMS SQUID magnetometer in the temperature range of 2–300 K with an external magnetic field as high as 70 kOe. The magnetization of MILs 1–6, measured at 2 K as a function of applied magnetic field, show a tendency of saturation which is typical for ferromagnetic behavior (Fig. 1); however, the magnetization values are rather small. The observation that these MILs can be manipulated by a hand-held neodymium magnet also confirms their magnetic nature.
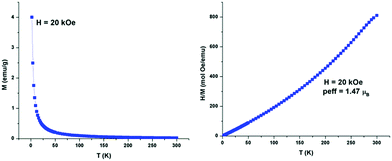
Fig. S17 and videos provided as ESI† show manipulation of the MILs using a 0.66 T handheld magnet. Fig. 2 shows the temperature dependence of magnetization and a plot of the reciprocal susceptibility versus temperature for the Ni(OIm)4(Cl)NTf2 (2) MIL, confirming an inverse relationship between magnetic susceptibility and temperature as defined by the Curie–Weiss law. The calculated μeff for the Ni(OIm)4(Cl)NTf2 MIL was 1.47 μB which indicates that the compound exists in an equilibrium state between square planer (no unpaired electron)–tetrahedral (two unpaired electrons) because of the noncoordinating nature of the [NTf2−] anions.37,38 Similar magnetic behaviour has been observed for the Ni(BIm)4(Cl)NTf2 MIL (see ESI†).
Similarly, Co(OIm)4(Cl)NTf2 (5) and Mn(OIm)4(Cl)NTf2 (6) exhibit normal paramagnetic behavior, as shown in Fig. 3 and Fig. S12 (ESI†), respectively. The calculated μeff for 5 and 6 were 3.24 and 4.04 μB, respectively. Plots of magnetization versus temperature and reciprocal molar susceptibility versus temperature for MILs 1, 3 and 4 are reported in the ESI.† It can be observed that Co(BIm)4(Cl)NTf2 (3) displays a phase transition above 270 K. The magnetic susceptibility values of MILs 1–6 are 0.31, 0.37, 0.78, 2.55, 1.32, and 2.17 emu K mol−1, respectively, while the reported values for (P66614)2CoCl4 and (P66614)2MnCl4 are 2.10 and 4.23 emu K mol−1, respectively.15 These lower magnetic susceptibilities could be the result of an equilibrium between the square planar and tetrahedral geometries for the [NTf2−]-based MILs.
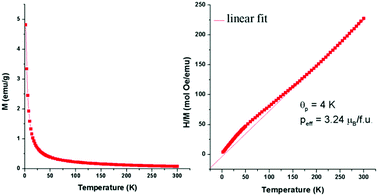 | ||
| Fig. 3 Magnetization vs. temperature plot for the Co(OIm)4(Cl)NTf2 MIL (left) at H = 20 kOe and Curie–Weiss fit of the linear portion of the reciprocal susceptibility (right). | ||
MILs comprised of the [NTf2−] anion and cations based on transition-metal complexes with 1-alkylimidazole ligands were prepared using an efficient synthetic methodology involving the solvent free synthesis of chloride intermediates. The metal containing chloride intermediates were found to be stable in water and were converted into their [NTf2−] form through a simple metathesis reaction pathway. The resulting MILs were found to be hydrophobic and significantly less viscous, especially for those bearing 1-octylimidazole ligands. Co(OIm)4(Cl)NTf2 was found to be the least viscous MIL with a viscosity of 152 cP at 21.7 °C. SQUID magnetic measurements performed on the reported ionic liquids confirm their magnetic nature. These MILs exhibit moderate thermal stability and are expected to be useful new materials in diverse areas of catalysis, chemical separations, and opto-electronics.
Conflicts of interest
The authors declare no conflicts.Acknowledgements
The authors acknowledge assistance from Stephen A. Pierson in the initial synthesis of these compounds. The authors thank Yaroslav Mudryk for interpretation of the magnetic properties. J. L. A. acknowledges funding from the Chemical Measurement and Imaging Program at the National Science Foundation (Grant No. CHE-1709372). The magnetic susceptibility measurements (A. P.) were performed at Ames Laboratory. The Ames Laboratory is operated for the U.S. Department of Energy (DOE) by Iowa State University of Science and Technology under contract No. DE-AC02-07CH11358.References
- Z. Lei, B. Chen, K. Yoon-Mo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS.
- S. Hayashi and H.-O. Hamaguchi, Chem. Lett., 2004, 33, 1590–1591 CrossRef CAS.
- M. S. Sitze, E. R. Schreiter, E. V. Patterson and R. G. Freeman, Inorg. Chem., 2001, 40, 2298–2304 CrossRef CAS.
- E. Santos, J. Albo and A. Irabien, RSC Adv., 2014, 4, 40008–40018 RSC.
- Y. Yoshida and G. Saito, Phys. Chem. Chem. Phys., 2010, 12, 1675–1684 RSC.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS.
- M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391–1398 CAS.
- Z. Ma, J. Yu and S. Dai, Adv. Mater., 2010, 22, 261–285 CrossRef CAS.
- K. D. Clark, O. Nacham, J. A. Purslow, S. A. Pierson and J. L. Anderson, Anal. Chim. Acta, 2016, 934, 9–21 CrossRef CAS.
- A. Joseph, Z. Źyła, V. I. Thomas, P. R. Nair, A. S. Padmanabhan and S. J. Mathew, J. Mol. Liq., 2016, 218, 319–331 CrossRef CAS.
- H. Wang, R. Yan, Z. Li, X. Zhang and S. Zhang, Catal. Commun., 2010, 11, 763–767 CrossRef CAS.
- K. D. Clark, O. Nacham, H. Yu, T. Li, M. M. Yamsek, D. R. Ronning and J. L. Anderson, Anal. Chem., 2015, 87, 1552–1559 CrossRef CAS PubMed.
- K. D. Clark, M. M. Yamsek, O. Nacham and J. L. Anderson, Chem. Commun., 2015, 51, 16771–16773 RSC.
- R. E. Del Sesto, T. M. McCleskey, A. K. Burrell, G. A. Baker, J. D. Thompson, B. L. Scott, J. S. Wilkes and P. Williams, Chem. Commun., 2008, 447–449 RSC.
- S. A. Pierson, O. Nacham, K. D. Clark, H. Nan, Y. Mudryk and J. L. Anderson, New J. Chem., 2017, 41, 5498–5505 RSC.
- T. Inagaki and T. Mochida, Chem. Lett., 2010, 39, 572–573 CrossRef CAS.
- T. Inagaki and T. Mochida, Chem. – Eur. J., 2012, 18, 8070–8075 CrossRef CAS.
- Y. Miura, F. Shimizu and T. Mochida, Inorg. Chem., 2010, 49, 10032–10040 CrossRef CAS.
- M. Okuhata, Y. Funasako, K. Takahashi and T. Mochida, Chem. Commun., 2013, 49, 7662–7664 RSC.
- S. Hayashi, S. Saha and H. Hamaguchi, Chem. Lett., 2004, 33, 159–1591 Search PubMed.
- S. Hayashi, S. Saha and H. Hamaguchi, IEEE Trans. Magn., 2006, 42, 12–14 CAS.
- B. Mallick, B. Balke, C. Felser and A.-V. Mudring, Angew. Chem., Int. Ed., 2008, 47, 7635–7638 CrossRef CAS.
- S. Pitula and A.-V. Mudring, Chem. – Eur. J., 2010, 16, 3355–3365 CrossRef CAS.
- F. A. Cotton, D. M. L. Goodgame and M. Goodgame, J. Am. Chem. Soc., 1962, 84, 167–172 CrossRef CAS.
- K. Sato, M. Morita, S. Okamoto, S. Morita, T. Kambara, K. I. Gondaira and H. Takenoshita, Prog. Cryst. Growth Charact., 1984, 10, 311 CrossRef.
- M. L. Mutch and J. S. Wilkes, Proc. – Electrochem. Soc., 1998, 98, 254–260 Search PubMed.
- P. Bonhôte, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef.
- A. Joseph, G. Żyła, V. I. Thomas, P. R. Nair, A. S. Padmanabhan and S. Mathew, J. Mol. Liq., 2016, 218, 319–331 CrossRef CAS.
- K. Binnemans, Chem. Rev., 2007, 107, 2592–2614 CrossRef CAS.
- V. M. B. Nunes, M. J. V. Lourenco, F. J. V. Santos and C. J. Nieto de Castro, J. Chem. Eng. Data, 2003, 48, 446–450 CrossRef CAS.
- E. Hendriks, G. M. Kontogeorgis, R. Dohrn, J. C. de Hemptine, I. G. Economou, L. F. Zilnik and V. Vesovic, Ind. Eng. Chem. Res., 2010, 49, 11131–11141 CrossRef CAS.
- R. E. Del Sesto, C. Corley, A. Robertson and J. S. Wilkes, J. Organomet. Chem., 2005, 690, 2536–2542 CrossRef CAS.
- Y. Yoshida and G. Saito, J. Mater. Chem., 2006, 16, 1254–1262 RSC.
- E. Santos, J. Albo, A. Rosatella, C. A. M. Afonso and C. Á. Irabien, J. Chem. Technol. Biotechnol., 2014, 89, 866–871 CrossRef CAS.
- A. Babai and A.-V. Mudring, Chem. Mater., 2005, 17, 6230–6238 CrossRef CAS.
- F. H. Field and W. C. Vosburgh, J. Am. Chem. Soc., 1949, 71, 2398–2401 CrossRef CAS.
- V. V. Sushev, A. N. Kornev, Y. Shvedenkov, Y. Kurskii, G. K. Fukin, E. V. Baranov and G. A. Abakumov, Dokl. Chem., 2005, 403, 136–139 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nj05176c |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |