Cations mediating proton conductivity in an oxalate based microporous coordination polymer†
Xing
Meng
 *a,
Hai-Ning
Wang
a,
Xiao-Kun
Wang
b,
Long-Zhang
Dong
*a,
Hai-Ning
Wang
a,
Xiao-Kun
Wang
b,
Long-Zhang
Dong
 b and
Yan-Hong
Zou
a
b and
Yan-Hong
Zou
a
aSchool of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, Shandong, 255049, P. R. China. E-mail: mengxing@sdut.edu.cn
bJiangsu Collaborative Innovation Centre of Biomedical Functional Materials, Jiangsu, Key Laboratory of New Power Batteries, College of Chemistry and Materials Science, Nanjing Normal University, Nanjing 210023, P. R. China
First published on 16th November 2018
Abstract
Through cation substitution in a zirconium based coordination polymer, an isostructural framework 1@NH4+ has been prepared without any apparent structural change. The proton conductivity of 1@NH4+ is successfully improved. It exhibits high proton conductivity (1.39 × 10−2 S cm−1) at 98% relative humidity and 60 °C.
Proton conductive coordination polymers (CPs) have drawn more and more attention within the last dozen years due to their crystallinity and ordered structure, which motivates us to understand proton conduction mechanisms clearly.1–10 Oxalates, as simple bis-bidentate type ligands, have been widely explored in the construction of CPs. Oxalate based CPs are widely known to possess various structures, such as one-dimensional (1D) chain structures and two-dimensional (2D) layers as well as three-dimensional (3D) architectures. Due to the small size of oxalate, these CPs do not usually contain available cavities. However, they are important components of proton conductive materials owing to their responsibility for mediating the transport of protons. Up to now, major achievements based on oxalate ligands have been made.11–22 Among them, bimetallic oxalate based CPs attract more interest,12,14,17 because bimetallic CPs have a high prospect of becoming functional materials in this field. In addition, an anionic network which is able to host a wide variety of cations, which can carry protons, provides a new route for the rational synthesis of proton conductive materials. To date, the mobility of proton carriers can be imparted or improved by pore filling, postmodification, and other methods.23 Proton conduction relies on the introduction of acidic or hydrophilic residues into the framework in order to construct proton-conductive pathways. Recently, Pardo et al. described an oxalate-based CP with the formula (NH4)4[MnCr2(ox)6]·4H2O, whose proton conduction was related to the presence of ammonium and/or isolated water molecules within 1D channels.15 To demonstrate cations tuning the proton conductive behavior, it is necessary that cations should be exchanged within the CP. Furthermore, it is ideal that the structural skeleton remains unchanged in the process of ion substitution, because the structural change may influence the proton conductivity. Based on the reported achievements, our strategy for producing bimetallic proton-conductive materials based on oxalates focuses on introducing new cationic residues into the framework so as to construct new proton-conductive pathways.
Ca2Zr(C2O4)4·5.5H2O (1) was synthesized according to the reported procedure.24 Then about 0.500 g of sample 1 was added to 5 mol L−1 of NH4Cl in a solution containing 20 mL of water. The contents were placed in a 20 mL screw-capped vial, which was heated to 45 °C in a water bath for 12 h. A white precipitate was obtained by filtration and washed with water several times to remove excess NH4Cl. In the present study, this is a new approach to mediate the proton conductivity through generating effective proton conduction pathways by cation substitution without changing the main framework. We employ an oxalate based CP Ca2Zr(C2O4)4·5.5H2O (1) owing to the two following considerations: (i) it is an anionic framework, as well as having Ca2+ ions inside its framework; and (ii) the channels are full of water molecules, which may facilitate the generation of hydrogen-bonding networks or act as proton carriers (Fig. 1). Here we mainly describe the influence of cation substitution on the proton conductivity of 1.
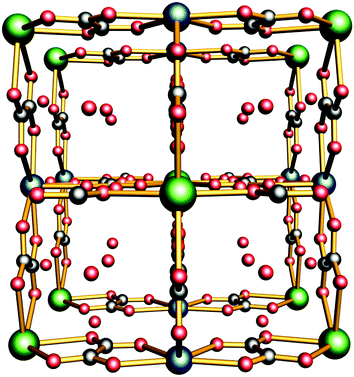 | ||
| Fig. 1 The three-dimensional framework of 1 (the 1D channel existing along the c axis, and the Ca2+ ions, Zr4+ ions and free water molecules marked in dark teal, dark green and red, respectively). | ||
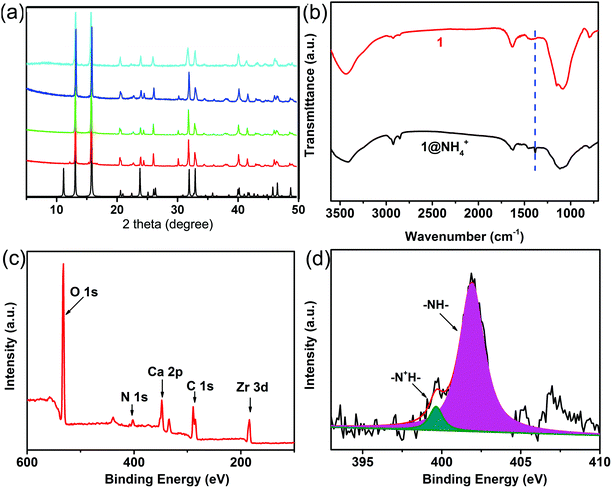
Inspired by the previous work, the sample exchanged with NH4+ ions has been synthesized and labeled as 1@NH4+. The structure of 1@NH4+ has been determined to be unchanged with the help of powder X-ray diffraction (PXRD) analyses (Fig. 2a). The different reflection intensities between the simulated and experimental patterns might be attributed to small sizes of as-synthesized powder samples as well as a certain degree of preferred orientation of the crystalline samples during data collection. Additionally, the compositions of the prepared 1 and 1@NH4+ have also been characterized by Fourier transform infrared (FT-IR) and X-ray photoelectron spectra (XPS). The FTIR spectra of 1 and 1@NH4+ are shown in Fig. 2b. The characteristic bands at approximately 1400 cm−1 in 1@NH4+ are ascribed to the stretching vibrations of NH4+.25 Meanwhile, XPS spectra of 1@NH4+ have been used to evaluate its composition, and demonstrate the coexistence of C, N, O, Ca and Zr elements (Fig. 2c). The N 1s XPS spectrum successfully confirms the existence of NH4+, and could be divided into two peaks, which can be ascribed to –NH– segments (399.7 eV) and the protonation states (–NH+–) at 402 eV (Fig. 2d), confirming that NH4+ ions are immobilized into the channels.26 The amounts of Ca and Zr in 1@NH4+ have been determined by inductively coupled plasma atomic emission spectroscopy (ICP-AES) (Table S4, ESI†). Combined with thermogravimetric analysis, we calculate that 1 mol compound 1 loads 0.3 mol NH4+ ions.
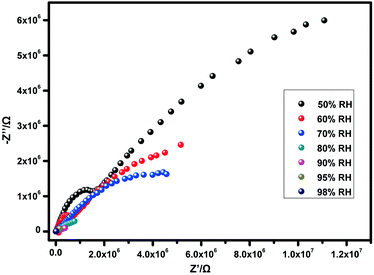
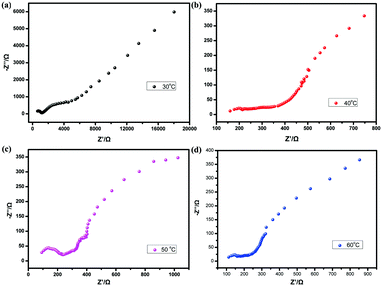
To determine the proton conductivity of both samples, the proton conductivities of the two solid samples have been measured by AC impedance spectroscopy using pelletized samples. For 1, due to disordered data points, it is impossible to find the corresponding impedance value meaning the proton conductivity is very low. While, for 1@NH4+, the proton conductivities are 1.07 × 10−6 S cm−1, 3.05 × 10−5 S cm−1, 2.93 × 10−4 S cm−1, 3.93 × 10−4 S cm−1, 4.57 × 10−4 S cm−1, 5.73 × 10−4 S cm−1, and 1.42 × 10−3 S cm−1 at 30 °C, under different relative humidity conditions (50% RH, 60% RH, 70% RH, 80% RH, 90% RH, 95% RH, and 98% RH) (Fig. 3 and Fig. S3, ESI†). Additionally, in order to evaluate the temperature influence, the temperature-dependent proton conductivities have also been measured. These measurements have been carried out in the temperature range of 30–60 °C and 98% RH (Fig. 4). As the temperature increases, the proton conductivity for 1@NH4+ reaches 1.39 × 10−2 S cm−1 under 60 °C and 98% RH, which is comparable to the reported MOFs (Table S3, ESI†). In addition, the PXRD pattern of the tested sample matches well with the original sample, indicating that the sample exhibits good stability before and after the impedance tests (Fig. 2a). The FTIR spectrum of 1@NH4+ after AC impedance measurements has been investigated. As shown in Fig. S5 (ESI†), a characteristic band at approximately 1400 cm−1 in 1@NH4+ exists, which is ascribed to the stretching vibrations of NH4+. Considering that 1 and 1@NH4+ are isostructural, they exhibit different proton conductivities. The difference in proton conductivity may result from the introduced NH4+ ions. The NH4+ ions play an important role in improving proton conductivity. We speculate that NH4+ ions have two roles in the process of proton conduction: increasing proton carriers in the channels, and promoting the formation of a hydrogen-bonding network.19 Additionally, the motion of ammonium ions may also contribute to the high proton conductivity.27 It is worth mentioning that this is the first example of successful control of proton conductivity using cation substitution in zirconium based CPs.
The conductivity of 1@NH4+ increases with increasing RH. This phenomenon suggests that proton conductive behaviors are related to the concentration of water. Inferred from this, the framework could adsorb water molecules into the channel slowly, facilitating the establishment of hydrogen-bond networks and proton-transport pathways. For 1@NH4+, water vapor adsorption and desorption isotherms are collected at room temperature. As shown in Fig. S2 (ESI†), with the increase of vapor pressure, the adsorbed water molecules increase, and the maximum water vapor uptake of 141.1 cm3 g−1 is calculated from the adsorption isotherms, demonstrating that water molecules are able to enter into its channels.
The Grotthuss and vehicle proton conduction mechanisms have been generally used for explaining the proton transfer process in solids.28 The reported activation energy (Ea) is in the range 0.1–0.4 eV for the Grotthuss mechanism and in the range 0.5–0.9 eV for the vehicle mechanism. The results of Ea and the structural analysis of the compound provide an opportunity to further get an insight into the possible proton-conduction pathway and mechanism. A linear correlation (ln(σ) versus 1000/T) is utilized to calculate the Ea of proton transport. In compound 1, the proton conductivity is very low. There exist water molecules in the channel, and the distances between free water molecules along the c-axis direction are about 4.22 Å. This means that a successive hydrogen-bonding network could not be formed solely in compound 1, which results in low conductivity. While in 1@NH4+, the proton conductivity reaches up to 1.39 × 10−2 S cm−1 at 60 °C under 98% RH, and the activation energy is 0.61 eV (Fig. S4, ESI†). This indicates that the proton transport of 1@NH4+ is mainly governed by the vehicle mechanism. The introduced NH4+ and adsorbed H2O molecules as vehicles accept and transport H+ protons under AC impedance measurements, resulting in the vehicle mechanism.
Conclusions
In conclusion, we have prepared an isostructural framework exchanged with NH4+ ions that exhibits a profound proton conduction property, while the origin one cannot obtain proton conductivity. The observed conductivity of 1.39 × 10−2 S cm−1 at 60 °C and 98% RH is comparable to most excellent proton conduction materials. The exchange of cations succeeds in altering the conductivity significantly based on the integrity of the skeleton. Proton conductivity control using cation substitution in zirconium based CPs is reported by us for the first time. This experimental result may provide a general approach to enhance the proton-conducting properties of CPs through ion substitution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the financial aid from the National Natural Science Foundation of China (No. 21601109).Notes and references
- X. Meng, H. N. Wang, S. Y. Song and H. J. Zhang, Chem. Soc. Rev., 2017, 46, 464–480 RSC.
- F. M. Zhang, L. Z. Dong, J. S. Qin, W. Guan, J. Liu, S. L. Li, M. Lu, Y. Q. Lan, Z. M. Su and H. C. Zhou, J. Am. Chem. Soc., 2017, 139, 6183–6189 CrossRef CAS PubMed.
- X. Meng, M. J. Wei, H. N. Wang, H. Y. Zang and Z. Y. Zhou, Dalton Trans., 2018, 47, 1383–1387 RSC.
- D. X. Gui, X. Dai, Z. Tao, T. Zheng, X. X. Wang, M. A. Silver, J. Shu, L. H. Chen, Y. L. Wang, T. T. Zhang, J. Xie, L. Zou, Y. H. Xia, J. J. Zhang, J. Zhang, L. Zhao, J. Diwu, R. H. Zhou, Z. F. Chai and S. Wang, J. Am. Chem. Soc., 2018, 140, 6146–6155 CrossRef CAS PubMed.
- Y. X. Wang, Z. T. Tao, X. M. Yin, J. Shu, L. H. Chen, D. P. Sheng, Z. F. Chai, T. E. Albrecht-Schmitt and S. Wang, Inorg. Chem., 2015, 54, 10023–10029 CrossRef CAS PubMed.
- D. X. Gui, T. Zheng, J. Xie, Y. W. Cai, Y. X. Wang, L. H. Chen, J. Diwu, Z. F. Chai and S. Wang, Inorg. Chem., 2016, 55, 12508–12511 CrossRef CAS PubMed.
- X. Wang, Y. L. Wang, M. A. Silver, D. X. Gui, Z. L. Bai, Y. X. Wang, W. Liu, L. H. Chen, J. Diwu, Z. F. Chai and S. Wang, Chem. Commun., 2018, 54, 4429–4432 RSC.
- C. L. Xiao, Y. X. Wang, L. H. Chen, X. M. Yin, J. Shu, D. P. Sheng, Z. F. Chai, T. E. Albrecht-Schmitt and S. Wang, Chem. – Eur. J., 2015, 21, 17591–17595 CrossRef CAS PubMed.
- B. Q. Song, D. Q. Chen, Z. G. Ji, J. H. Tang, X. L. Wang, H. Y. Zang and Z. M. Su, Chem. Commun., 2017, 53, 1892–1895 RSC.
- B. Q. Song, X. L. Wang, G. S. Yang, H. N. Wang, J. Liang, K. Z. Shao and Z. M. Su, CrystEngComm, 2014, 16, 6882–6888 RSC.
- T. Yamada and T. Nankawa, Inorg. Chem., 2016, 55, 8267–8270 CrossRef CAS PubMed.
- S. Tominaka, F. X. Coudert, T. D. Dao, T. Nagao and A. K. Cheetham, J. Am. Chem. Soc., 2015, 137, 6428–6431 CrossRef CAS PubMed.
- Q. Tang, Y. Liu, S. Liu, D. He, J. Miao, X. Wang, G. Yang, Z. Shi and Z. Zheng, J. Am. Chem. Soc., 2014, 136, 12444–12449 CrossRef CAS PubMed.
- H. Ōkawa, M. Sadakiyo, T. Yamada, M. Maesato, M. Ohba and H. Kitagawa, J. Am. Chem. Soc., 2013, 135, 2256–2262 CrossRef PubMed.
- C. Maxim, S. Ferlay, H. Tokoro, S. Ohkoshi and C. Train, Chem. Commun., 2014, 50, 5629–5632 RSC.
- F. Guo, C. Chen, K. Wang, Q. Zhang and Z. Lin, Inorg. Chem., 2016, 55, 7817–7819 CrossRef CAS PubMed.
- H. kawa, M. Sadakiyo, K. Otsubo, K. Yoneda, T. Yamada, M. Ohba and H. Kitagawa, Inorg. Chem., 2015, 54, 8529–8535 CrossRef PubMed.
- M. Sadakiyo, T. Yamada, K. Honda, H. Matsui and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 7701–7707 CrossRef CAS PubMed.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 13166–13169 CrossRef CAS PubMed.
- S. S. Nagarkar, S. M. Unni, A. Sharma, S. Kurungot and S. K. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2638–2642 CrossRef CAS PubMed.
- C. H. Guo, C. Chen, K. C. Wang, H. M. Zeng and Z. E. Lin, Inorg. Chem. Commun., 2017, 85, 96–99 CrossRef CAS.
- W. Wang, L. D. Luan, M. Yang, K. C. Wang and Z. E. Lin, Inorg. Chem. Commun., 2017, 75, 46–48 CrossRef CAS.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906–9907 CrossRef CAS PubMed.
- N. Audebrand, E. Jeanneau, T. Bataille, S. Raite and D. Louër, Solid State Sci., 2004, 6, 579–591 CrossRef CAS.
- G. G. Zeelani and S. L. Pal, Int. Res. J. Eng. Technol., 2016, 8, 1251–1256 Search PubMed.
- M. Boota, M. P. Paranthaman, A. K. Naskar, Y. Li, K. Akato and Y. Gogotsi, ChemSusChem, 2015, 8, 3576–3581 CrossRef CAS PubMed.
- S. Miyatsu, M. Kofu, A. Nagoe, T. Yamada, M. Sadakiyo, T. Yamada, H. Kitagawa, M. Tyagi, V. G. Sakai and O. Yamamuro, Phys. Chem. Chem. Phys., 2014, 16, 17295–17304 RSC.
- A. Shigematsu, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2011, 133, 2034–2036 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, water vapor adsorption and desorption isotherms, the TGA plot, and experimental details of the proton conductivities. See DOI: 10.1039/c8nj04763d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |