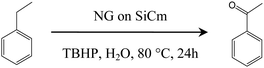Chemical modification of vertically aligned graphene standing on SiC microspheres for selective oxidation†
Jun
Ma
 *ab,
Yiwei
Fei
a,
Jianqiang
Hu
a,
Nan
Wu
a,
Shian
Sun
a,
Feng
Xie
a,
Gongyi
Li
b,
Xiaodong
Li
b and
Yuelun
Wang
*c
*ab,
Yiwei
Fei
a,
Jianqiang
Hu
a,
Nan
Wu
a,
Shian
Sun
a,
Feng
Xie
a,
Gongyi
Li
b,
Xiaodong
Li
b and
Yuelun
Wang
*c
aAir Force Logistics College, Xuzhou, 221000, People's Republic of China. E-mail: manudt@nudt.edu.cn
bCollege of Science, National University of Defense Technology, Changsha, 410073, People's Republic of China
cKey Laboratory of Coal Processing and Efficient Utilization, Ministry of Education, China University of Mining & Technology, Xuzhou, 221116, People's Republic of China. E-mail: wangyuelun@126.com
First published on 21st November 2018
Abstract
Vertically aligned multi-layer graphene standing on SiC microspheres could be chemically modified to prepare nitrogen doped graphene standing on SiC microspheres (NG on SiCm). The unique structure consisted of a nitrogen doped graphene layer (the lateral dimension is ∼200 nm) and a solid SiC core (the diameter is in the range from 0.2 μm to 0.8 μm). Based on XPS analysis, the nitrogen element is controllably doped in the nanostructure of the graphene layer of NG on SiCm. The NG on SiCm is found to be an effective recyclable carbocatalyst for selective oxidation reactions. Owing to the unique nanostructure of the nitrogen doped graphene grown on the inert SiC core, the nanomaterial is not only tough enough to hardly change in chemical modification reactions, but also expected to serve as a predispersed recyclable high performance carbocatalyst.
1. Introduction
Compared with noble metal catalysts, graphene-based catalysts have considerable advantages such as low cost and green and environmental protection. In the catalytic reactions such as oxygen evolution/reduction reactions (OER or ORR1–8), selective oxidation reactions,9,10 and transition metal oxide redox reactions,11 the nitrogen doped graphene exhibits highly efficient catalytic performance and selectivity. Usually, the pristine graphene needs further modification (including grafting, covering or doping) of the nanostructure to acquire designed properties in catalytic reactions. Chemical modification is widely accepted as a facile way of heteroatom doping12 and an efficient method to acquire modified graphene that can be dispersed in solution on a lab-scale.13,14 However, the graphene tends to aggregate in the modification process and further application in heterogeneous catalytic reactions due to the high specific surface area (∼2600 m2 g−1 in theory) and ultra-high intermolecular forces inside the sheets of graphene.13In order to find the effective methods to avoid or reduce stacking, aggregation, and precipitation in the application of graphene and modified graphene, both physical and chemical strategies are developed. In the field of physical aspects, the crumpled15–17 and vertically aligned18–21 graphene provide a better way to face the challenges, especially in heterogeneous reaction systems. Research about crumpled graphene found that the physical method could help graphene stably disperse in oil to improve the tribological properties,22 and formed self-standing crumpled graphene anodes to retain better diffusion paths for insertion of Li or Na when used as electrodes in rechargeable batteries.23 The vertically aligned graphene is another effective structure to fully stimulate the advantages of graphene-based catalysts in heterogeneous reactions, including the oxygen evolution reaction catalyzed by nitrogen doped vertically aligned graphene which are in situ grown on carbon cloth6 and air-breathing electrodes for rechargeable zinc–air batteries supported by free-standing vertically-aligned nitrogen-doped carbon nanotube arrays/graphene.24 On the other hand, chemical modification could also be applied to promote the dispersivity of graphene. The alkylated graphenes prepared by the coupling of alkylamine with carboxylic groups of graphene oxide have much better dispersivity in organic solvents.25 With the surfactant Triton X-100, the in situ chemical exfoliated graphene could highly stably disperse in water.26
Our team has successfully prepared the MG on SiC27 and NG on SiC28 simply by the direct pyrolysis of o-PS or acetonitrile and o-PS. The nitrogen content of the NG on SiC is mainly controlled by the acetonitrile to some extent. However, an acetonitrile amount of 5% is difficult to exceed, otherwise the evaporation of low molecules could considerably influence the production of NG on SiC. In order to simultaneously control the amount of preparation and nitrogen doping content, the chemical modification of MG on SiC is applied to prepare NG on SiCm. Based on the research, the as-prepared NG on SiCm is a predispersed nitrogen doped graphene that unlikely aggregates in the catalytic reactions since the graphene is already dispersed on the surface of SiC microspheres before reactions. NG on SiCm prepared by the chemical modification of MG on SiC provides a tunable alternative way to obtain predispersed nitrogen doped graphene, which is more feasible and controllable than the direct preparation of NG on SiC by pyrolysis of acetonitrile and o-PS.28
2. Experimental section
2.1 Materials
Nitric acid (HNO3, 65 wt%, A. R.), hydrazine hydrate (N2H4·H2O, 65 wt%, A. R.), tert-butyl hydroperoxide (TBHP, 70% aq. soln bought from Alfa Aesar), ethyl benzene (Alfa Aesar, 99%), benzyl alcohol (Alfa Aesar, 99%), n-dodecane (Alfa Aesar, 99%), dichloromethane (A. R.), oligomer polysilane (o-PS, a gift from the National University of Defense Technology, Changsha, China), and argon (Ar, 99.999%).2.2 Preparation of NG on SiC by chemical modification (NG on SiCm)
NG on SiCm is prepared by chemical modification of MG on SiC. As reported previously, MG on SiC could be prepared by a one-pot method.27 In this paper, MG on SiC is prepared by pyrolyzing o-PS via the continuous preparation system. Briefly, o-PS (5.4 mL) was injected into a horizontal alumina furnace while the temperature inside had been maintained at ∼1300 °C for 30 minutes. The injection speed of the syringe pump was set as 1 μL per second (3.6 mL per hour). After the completion of the injection process, the furnace was held at 1300 °C for another 30 minutes to thoroughly pyrolyze the raw material. Finally, the furnace was allowed to cool down to room temperature at a rate of 5 °C min−1. It should be noted that the whole preparation process was protected by high purified argon. The gram-scale MG on SiC could be readily collected from the surface of graphite boats, which were placed in the central area of the furnace in advance.Based on research studies on MG on SiC,27 the typical chemical modification process could be simply described as follows. The MG on SiC (20 mg) was firstly sonicated in concentrated HNO3 (2 mL) for 30 min to fully disperse in the solution. Then the solution was magnetically stirred for 6, 12 and 96 hours at room temperature, respectively. After oxidation treatment, the product (graphene oxide standing on SiC microspheres, MG on SiC oxide) was centrifuged and washed with distilled water until the pH of the filtered water was nearly neutral. After that, 1 mL hydrazine was applied to chemically reduce the MG on SiC oxide and the reduced product was annealed at 900 °C in highly purified N2 for 120 min. The final product is NG on SiCm.
2.3 The selective oxidation activity of NG on SiCm
NG on SiCm exhibits high catalytic activity in the activation of specific C–H bonds. The details are described as follows. TBHP was added into the reactants (ethyl benzene or benzyl alcohol) as the oxidant, and mixed with NG on SiC (20 mg) in water medium (3 mL). The molar ratio of TBHP and the reactants is 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in all reactions. Then the reactions were carried out in glass pressure bottles at 80 °C for 24 hours. After the reaction, 0.5 mmol n-dodecane was added into the products and extracted by dichloromethane (0.5 mL).
1 in all reactions. Then the reactions were carried out in glass pressure bottles at 80 °C for 24 hours. After the reaction, 0.5 mmol n-dodecane was added into the products and extracted by dichloromethane (0.5 mL).
The products obtained in this study were characterized by Scanning Electron Microscopy (SEM, Hitachi S-4800 FE), Transmission Electron Microscopy (TEM, TECNAI-F20), Selected Area Electron Diffraction (SAED), Raman spectrometry (Senterra&Veate X70), Gas Chromatography and Mass Spectrometry (GC-MS, Agilent 7890A/5975C and PerkinElmer Clarus 680/SQ 8T) and X-ray Photoelectron Spectroscopy (XPS, Escalab-250Xi).
3. Results and discussion
3.1 Continuous preparation of MG on SiC
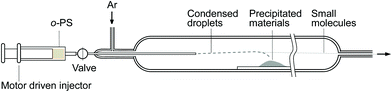
In this paper, the continuous preparation system (Fig. 1) is applied to stably prepare gram-scale MG on SiC. Compared with the one-pot method, the continuous preparation method is a more economical way to obtain large-scale MG on SiC for further chemical modification. After o-PS was pyrolyzed at 1300 °C in an Ar atmosphere, the as-prepared black powder is examined by SEM. As shown in Fig. 2, the black powder actually consisted of a large amount of fluffy microspheres. The pyrolyzed products of the continuous preparation system (Fig. 2a and b) are almost identical to a previously reported structure.27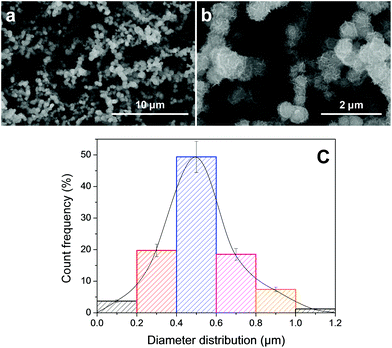 | ||
| Fig. 2 The SEM images of MG on SiC (a and b) and the microsphere size distribution analysis of (b) by the software of Nano Measurer 1.2 (c). | ||
Benefiting from the improved synthesis techniques, especially the reactive atmosphere control using a mass flow meter, the diameter distribution of MG on SiC is relatively narrower than the former report (the average diameter of the reported MG on SiC is ∼0.9 μm27). However, the diameters of nearly 90% microspheres are found to be in the range from 0.2 μm to 0.8 μm after the continuous preparation system was applied (Fig. 2c). More importantly, the average diameter of the as-prepared MG on SiC is ∼0.5 μm as indicated in Fig. 2c, compared with a previously reported MG on SiC (∼0.9 μm27) prepared using a one-pot method.
3.2 Nitrogen doped MG on SiC prepared by chemical modification (NG on SiCm)
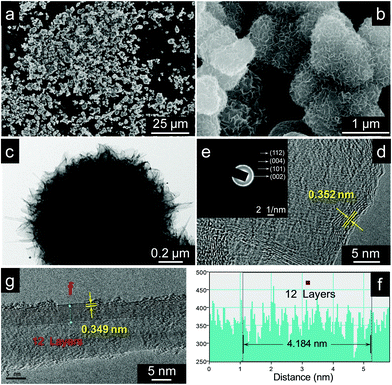
Chemical modification is an efficient way to control heteroatom doping in the field of graphene modification. In this paper, the nitrogen doping route could be simply described as the oxidation process followed by the reduction of hydrazine and annealing in argon. After oxidation (oxidizing in concentrated HNO3 for 96 hours), reduction (reducing by hydrazine) and annealing processes, the basic microstructure (Fig. 3a and b) of MG on SiC does not change in the modification reactions. Compared with the untreated specimen, the unique structure of graphene standing on SiC is quite stable in oxidation treatment.The TEM image (Fig. 3c) confirms that the basic nanostructure of the NG on SiCm is hardly changed in chemical modification reactions. Similar to the MG on SiC, the dimension of the NG on SiCm's fluffy layer is about 200 nm. The high resolution images (Fig. 3d and g) of the NG on SiCm indicate that there are two different microstructures, nanoplatelets and nanotubes, in the fluffy layer. XRD analysis (Fig. S5 in the ESI†) of the NG on SiCm (structure shown in Fig. 3c) reveals the graphene and SiC nature of the NG on SiCm prepared by the reactions between hydrazine and the MG on SiC oxidized in concentrated HNO3 for 96 hours. According to JCPDS Card No. 75-1621, 73-1665 and 73-1662 (standard XRD diffraction data), the graphite 2H, SiC 3C and SiC 15R could be identified from Fig. S5 (ESI†).
More importantly, the SAED pattern of the nanostructure's edge (Fig. 3e) shows four distinct circular rings, which are attributed to the diffraction of the (112), (004), (101), and (002) lattice planes of graphite according to JCPDS Card No. 75-1621. The results from the SAED analysis strongly suggest the graphite-like structure of the fluffy layer of the NG on SiCm.
As shown in Fig. 3d, the interplanar spacing of the nanoplatelets measured by the software “Gatan DigitalMicrograph” is ∼0.352 nm. This is coincident with the results from the measurement of a 12-layer nanotube (Fig. 3g and f). The total length of the 12 layers is 4.184 nm (Fig. 3f), indicating that the average interplanar spacing of the 12-layer nanotube is ∼0.349 nm (Fig. 3g). Owing to the graphite-like-structure nature of the fluffy layer of the NG on SiCm and the proper interplanar spacing of the nanostructures (∼0.35 nm), the nanoplatelets and the nanotube should be a part of graphene and the carbon nanotube. However, the distribution of the graphene and the carbon nanotube is uneven since the nanotube is very sparse in the TEM analysis. The basic nanostructure of the NG on SiCm is tough in the modification reactions, which means that the NG on SiCm could be applied under harsh conditions without agglomeration and nanostructure collapse. This would be one of the excellent properties of the pre-dispersed graphene-based nanostructure.
According to the TEM analysis (shown in Fig. 3g), the chemical modification reactions could produce some defects on the surface of the carbon nanotube. A few layers on the external surface of the carbon nanotube are rearranged curly compared with the inner walls of the nanotube. There should be the same phenomenon happening on the surface on the graphene nanoplatelets that grow on the SiC microspheres. The drawback of chemical modification may affect the mechanical strength of the carbonaceous nanostructures in a negative way.
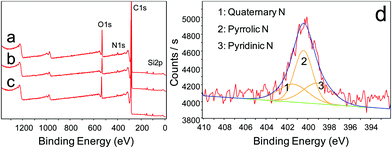
Based on the XPS analysis, the impact on the surface elemental variation of NG on SiCm has been investigated for further understanding of the chemical modification process. During surface activation treatments, the oxidized MG on SiC was reduced in hydrazine solution and then annealed in Ar to synthesize nitrogen doped products (NG on SiCm). As expected, the elemental composition on the surface of MG on SiC is certainly changed according to the examination of XPS analysis (Fig. S1, ESI†). Apparently, oxidation treatment could efficiently promote the oxygen content of MG on SiC, and the oxygen concentrations increase with increasing oxidation time. Based on the XPS investigation, the atomic percent of oxygen in the samples is confirmed to be 8.22, 8.99 and 13.32 at% while the oxidation times are 6, 12 and 96 hours (Fig. S1, ESI†). Compared with the C 1s peaks of untreated MG on SiC,27 two peaks at 289.3 (assigned to –COOH) and 285.4 eV (assigned to –C–O)29 were found in the curves of the oxidized MG on SiC samples (Fig. S2a–c, ESI†). The calculated results show the peak area ratio of –C–O groups is nearly twice than that of –COOH (Fig. 4d), which suggests that more hydroxyl groups were introduced onto the surface of oxidized MG on SiC than carboxyl groups. The oxidized MG on SiC affords facilities for further chemical modification of nitrogen doping.30 Moreover, the three samples also share the peak at 284.5 eV, which could be attributed to the sp2 hybrid carbon. The result of the sp2 hybrid carbon is similar to that of the SAED analysis as shown in Fig. 3e, which indicates the graphite-like-structure of the fluffy layer of the NG on SiCm.
As shown in Fig. 4, XPS analysis suggests that the nitrogen element could be easily introduced into the graphene structure of NG on SiCm by the designed route. Theoretically, the oxidation process would create more active sites on MG on SiC (the high resolution XPS analysis of C 1s in MG on SiCm in Fig. S2 (ESI†) shows the increasing molar ratio of oxygen containing carbonaceous bonds), which thus provide more sites for the subsequent reactions of hydrazine reduction and finally introduced the nitrogen element onto the surface of NG on SiCm. As shown in Table 1, the nitrogen content increases with increasing oxidation time. After the whole chemical modification, the nitrogen content could be promoted to 2.14 at% on the surface of NG on SiCm. On the other hand, the introduction of the nitrogen element or reduction reactions would significantly reduce the oxygen content of the NG on SiCm. Compared with the oxidized MG on SiC (the atomic percent of oxygen is 8.22, 8.99 and 13.32 at% while the oxidation times are 6, 12 and 96 hours shown in Fig. S1 of the ESI†), the atomic percent of oxygen decreases to 6.07, 6.54 and 10.22 at% while the oxidation times of the MG on SiC samples were 6, 12 and 96 hours, respectively. The Si signal detected in the XPS survey reconfirms the results from the XRD analysis (Fig. S5, ESI†), which indicates the SiC nature of the core of NG on SiCm. The signal intensity of Si is relatively low since the XPS detection is a surface analysis method and the SiC core is hardly shown on the surface.
| Oxidation time | Elements at% | |||
|---|---|---|---|---|
| C | Si | O | N | |
| 6 hours | 89.60 | 2.45 | 6.07 | 1.88 |
| 12 hours | 88.59 | 2.86 | 6.54 | 2.01 |
| 96 hours | 85.08 | 2.57 | 10.22 | 2.14 |
High resolution XPS analysis (Fig. 4d) revealed that the nitrogen element in the graphene structure of the NG on SiCm appears as the N–C bonds. The fitting results suggest that quaternary N (∼401.3 eV), pyrrolic N (∼400.1 eV) and pyridinic N (∼399 eV) theoretically exist inside the molecular structure of the NG on SiCm samples. Interestingly, the area ratio of pyrrolic N and pyridinic N is much higher than that of quaternary N, which may indicate that the nitrogen doping reactions of the NG on SiCm tend to occur on the edge of the graphene in the fluffy surface of the NG on SiCm. Compared with Fig. 4d, the N 1s high resolution XPS spectra of other NG on SiCm samples (see Fig. S3, ESI,† the NG on SiCm samples prepared by the reactions between hydrazine and the MG on SiC oxidized in concentrated HNO3 for 6 and 12 hours, respectively) have a relatively lower signal intensity for the nitrogen content. The introduction of nitrogen atoms in the structure of the graphene in the fluffy layer of the NG on SiCm could reduce the ID/IG values (shown in Fig. S4 and Table S1 in ESI†). The modification also results in the highest I2D/IG value (0.615) of the NG on SiCm that was prepared by the reactions between hydrazine and the MG on SiC oxidized in HNO3 for 96 hours. Thus the introduction of nitrogen atoms in the NG on SiCm could promote graphitization, which is similar to the former report.28 However, the oxidation process causes irreversible molecular structure damage, since the smallest ID/IG value of the NG on SiCm is 1.028.
3.3 Selective oxidation activity of NG on SiCm
NG on SiCm is an efficient catalyst in oxidation, which has been proven in the oxidation of benzyl alcohol (Fig. 5). With the help from the NG on SiCm, the benzyl alcohol could be completely oxidized to benzoic acid by TBHP. Otherwise, some benzaldehyde would be found in the products of the oxidation of benzyl alcohol without catalyst. The results from GCMS (Table S2, ESI†) reveal that the NG on SiCm is a powerful oxidation promoter with high selectivity in the oxidation reaction.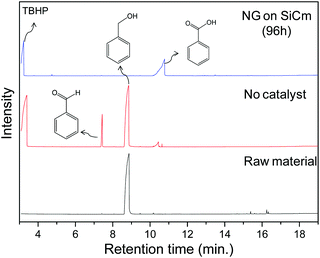 | ||
| Fig. 5 The total ion chromatogram of the oxidation products of benzyl alcohol with NG on SiCm (prepared by the reduction of hydrazine and the MG on SiC oxidized in HNO3 for 96 hours) as the catalyst. | ||
As the product of the selective oxidation reaction of ethyl benzene, acetophenone is a useful plasticizer and parfum. However, phenylacetic acid as a deep oxidation byproduct should be avoided in the oxidation process. In the selective oxidation, NG on SiCm is found to be very effective to synthesize the acetophenone (Table 2). Due to the unique predispersed nanostructure of the NG on SiCm, the novel material is expected to serve as a recyclable high performance catalyst. Based on the analysis of GCMS, the NG on SiCm catalyzed reaction has a relatively high conversion rate and high selectivity (Fig. 6). In the total ion chromatogram of the oxidation products of ethyl benzene with NG on SiCm (prepared by the reduction of hydrazine and the MG on SiC oxidized in HNO3 for 96 hours) as the catalyst, the main product obtained is acetophenone. Meanwhile, the signal of ethyl benzene is hardly seen in the chromatogram.
| Catalyst | N [at%] | Conversion rateb [%] | Selectivityb [%] |
|---|---|---|---|
| a Typical reaction conditions: ethyl benzene (5 mmol), TBHP (30 mmol), catalyst (20 mg), 24 hours, 80 °C. b The conversion rate and selectivity were examined by GC–MS while n-dodecane was used as the internal standard. | |||
| None | 0 | ∼3 | — |
| MG on SiC | 0 | 44 | 86 |
| NG on SiCm | 1.88 | 50 | 86 |
| NG on SiCm | 2.01 | 85 | 87 |
| NG on SiCm | 2.14 | 99 | 91 |
| NG on SiCm 2nd cycle | — | 97 | 91 |
| NG on SiCm 3rd cycle | — | 95 | 90 |
| NG on SiCm 4th cycle | — | 94 | 90 |
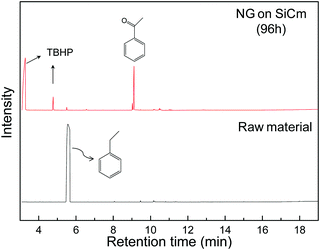 | ||
| Fig. 6 The total ion chromatogram of the oxidation products of ethyl benzene with NG on SiCm (prepared by the reduction of hydrazine and the MG on SiC oxidized in HNO3 for 96 hours) as the catalyst. | ||
The detailed information of the selective oxidation is listed in Table 2. Compared with the reaction without any catalyst, the conversion rate is nearly 3% with reference to the internal standard. The situation is improved since MG on SiC was introduced into the reaction system. Study10 about the selective oxidation mechanism indicates that the carbon atoms in the graphene structure play a key role in the process, thus the MG on SiC has a moderate catalytic reactivity. However, the nitrogen doping of the NG on SiCm significantly improves the catalytic activity since the doped nitrogen atoms could stimulate the catalytic activity of the nearby carbon atoms,10 which give rise to the conversion rate of NG on SiCm in the reactions. Apparently, the conversion rate increases with increasing nitrogen content in the NG on SiCm samples. The conversion rate could reach 99% while the nitrogen content increased to 2.14 at%, and the catalytic reaction shows a high selectivity of up to 91% in the presence of NG on SiCm. The NG on SiCm also demonstrates the potential (the conversion rate of NG on SiCm is only slightly decreased while reused in the repeated same reactions) to serve as a recyclable catalyst.
4. Conclusion
Vertically aligned nitrogen doped graphene standing on SiC microspheres (NG on SiCm) are prepared by the chemical modification of the MG on SiC. The nitrogen doping process hardly changes the nanostructure of NG on SiCm, which is still a core–shell structure and has a diameter in the range from 0.2 μm to 0.8 μm. After oxidation and hydrazine reduction reactions, the nitrogen atoms could be effectively introduced into the nanostructure of NG on SiCm and obtain a nitrogen content as high as 2.14 at%. The unique nanostructure could be applied as an efficient recyclable carbocatalyst in the field of selective oxidation reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for the financial support from the National Natural Science Foundation of China (Granted No. 51402360, 51102281 and 51575525), the Natural Science Foundation of Jiangsu Province (Granted No. BK20180180 and BK20161188), the Xuzhou City Science and Technology Project (Grant No. KH17009) and the Air Force Logistics College Youth Fund (Grant No. KY2018D031C).References
- L. Qu, Y. Liu, J. Baek and L. Dai, ACS Nano, 2010, 4, 1321–1326 CrossRef CAS.
- G. Tian, M. Zhao, D. Yu, X. Kong, J. Huang, Q. Zhang and F. Wei, Small, 2014, 10, 2251–2259 CrossRef CAS.
- P. Zhang, B. B. Xiao, X. L. Hou, Y. F. Zhu and Q. Jiang, Sci. Rep., 2014, 4, 3821 CrossRef CAS.
- X. Kong, C. Chen and Q. Chen, Chem. Soc. Rev., 2014, 43, 2841–2857 RSC.
- G. L. Tian, M. Q. Zhao, D. Yu, X. Y. Kong, J. Q. Huang, Q. Zhang and F. Wei, Small, 2014, 10, 2251–2259 CrossRef CAS.
- D. Li, B. Ren, Q. Jin, H. Cui and C. Wang, J. Mater. Chem. A, 2018, 6, 2176–2183 RSC.
- J. Liang, X. Du, C. Gibson, X. W. Du and S. Z. Qiao, Adv. Mater., 2013, 25, 6226–6231 CrossRef CAS.
- W. Ding, Z. Wei, S. Chen, X. Qi, T. Yang, J. Hu, D. Wang, L. Wan, S. F. Alvi and L. Li, Angew. Chem., 2013, 125, 11971–11975 CrossRef.
- X. Li and M. Antonietti, Angew. Chem., Int. Ed., 2013, 52, 4572–4576 CrossRef CAS.
- Y. Gao, G. Hu, J. Zhong, Z. Shi, Y. Zhu, D. S. Su, J. Wang, X. Bao and D. Ma, Angew. Chem., Int. Ed., 2013, 52, 2109–2113 CrossRef CAS.
- J. Jin, X. Fu, Q. Liu, Y. Liu, Z. Wei, K. Niu and J. Zhang, ACS Nano, 2013, 7, 4764–4773 CrossRef CAS.
- H. Wang, T. Maiyalagan and X. Wang, ACS Catal., 2012, 2, 781–794 CrossRef CAS.
- D. Li, M. B. Müller, S. Gilje, R. B. Kaner and G. G. Wallace, Nat. Nanotechnol., 2008, 3, 101–105 CrossRef CAS.
- Y. Liang, D. Wu, X. Feng and K. Müllen, Adv. Mater., 2009, 21, 1679–1683 CrossRef CAS.
- J. Luo, H. D. Jang, T. Sun, L. Xiao, Z. He, A. P. Katsoulidis, M. G. Kanatzidis, J. M. Gibson and J. Huang, ACS Nano, 2011, 5, 8943–8949 CrossRef CAS.
- A. R. Koltonow, C. Luo, J. Luo and J. Huang, ACS Omega, 2017, 2, 8005–8009 CrossRef CAS.
- S. Deng and V. Berry, Mater. Today, 2016, 19, 197–212 CrossRef CAS.
- H. F. Yen, Y. Y. Horng, M. S. Hu, W. H. Yang, J. R. Wen, A. Ganguly, Y. Tai, K. H. Chen and L. C. Chen, Carbon, 2015, 82, 124–134 CrossRef CAS.
- Q. Liang, X. Yao, W. Wang, Y. Liu and C. P. Wong, ACS Nano, 2011, 5, 2392–2401 CrossRef CAS.
- Y. Wu, B. Yang, G. Han, B. Zong, H. Ni, P. Luo, T. Chong, T. Low and Z. Shen, Adv. Funct. Mater., 2002, 12, 489–494 CrossRef CAS.
- K. Yu, P. Wang, G. Lu, K. Chen, Z. Bo and J. Chen, J. Phys. Chem. Lett., 2011, 2, 537–542 CrossRef CAS.
- X. Dou, A. R. Koltonow, X. He, H. D. Jang, Q. Wang, Y. Chung and J. Huang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 1528–1533 CrossRef CAS.
- Y. S. Yun, Y. Park, S. Chang, B. H. Kim, J. Choi, J. Wang, D. Zhang, P. V. Braun, H. Jin and K. Kang, Carbon, 2016, 99, 658–664 CrossRef CAS.
- X. Cai, B. Y. Xia, J. Franklin, B. Li, X. Wang, Z. Wang, L. Chen, J. Lin, L. Lai and Z. Shen, J. Mater. Chem. A, 2017, 5, 2488–2495 RSC.
- S. Choudhary, H. P. Mungse and O. P. Khatri, J. Mater. Chem., 2012, 22, 21032 RSC.
- S. Liang, Z. Shen, M. Yi, L. Liu, X. Zhang and S. Ma, Carbon, 2016, 96, 1181–1190 CrossRef CAS.
- J. Ma, G. Y. Li, Z. Y. CHu, X. D. Li, Y. H. Li and T. J. Hu, Carbon, 2014, 69, 634–637 CrossRef CAS.
- J. Ma, N. Wu, S. A. Sun, T. Yao, G. Y. Li, T. J. Hu, Y. H. Li and X. D. Li, ChemCatChem, 2018, 10, 825–830 CrossRef CAS.
- P. Chen, T. Xiao, Y. Qian, S. Li and S. Yu, Adv. Mater., 2013, 25, 3192–3196 CrossRef CAS.
- D. Geng, Y. Chen, Y. Chen, Y. Li, R. Li, X. Sun, S. Ye and S. Knights, Energy Environ. Sci., 2011, 4, 760 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The XPS surveys of the oxidized MG on SiC; C 1s high resolution XPS analysis of the oxidized MG on SiC; N 1s high resolution XPS analysis of the NG on SiCm samples; Raman spectra of NG on SiCm samples; the XRD pattern of the NG on SiCm prepared by the reactions between hydrazine and the MG on SiC oxidized in concentrated HNO3 for 96 hours; the ID/IG and I2D/IG values of the NG on SiCm samples based on Raman analysis; the selective oxidation of benzyl alcohol with NG on SiCm as the catalyst. See DOI: 10.1039/c8nj05103h |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2019 |