Atomic-scale dynamic observation reveals temperature-dependent multistep nucleation pathways in crystallization†
Junjie
Li
 *ab,
Yunping
Li
c,
Qiang
Li
d,
Zhongchang
Wang
*ab,
Yunping
Li
c,
Qiang
Li
d,
Zhongchang
Wang
 e and
Francis
Leonard Deepak
e and
Francis
Leonard Deepak
 *a
*a
aNanostructured Materials Group, Department of Advanced Electron Microscopy, Imaging and Spectroscopy, International Iberian Nanotechnology Laboratory (INL), Avenida Mestre Jose Veiga, Braga 4715-330, Portugal. E-mail: junjie.li@inl.int; leonard.francis@inl.int
bCAS Key Laboratory of Functional Materials and Devices for Special Environments, Xinjiang Technical Institute of Physics & Chemistry, CAS, Xinjiang Key Laboratory of Electronic Information Materials and Devices, 40-1 South Beijing Road, Urumqi 830011, China
cState Key Lab for Powder Metallurgy, Central South University, Changsha 410083, China
dSchool of Mechanical Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China
eDepartment of Quantum and Energy Materials, International Iberian Nanotechnology Laboratory (INL), Avenida Mestre Jose Veiga, 4715-330 Braga, Portugal
First published on 15th July 2019
Abstract
Uncovering kinetic pathways of non-classical multistep nucleation at the atomic-scale is critical for understanding the complex microscopic mechanism of heterogeneous nucleation and crystallization. However, due to the intricacies in tackling such challenging topics experimentally, the structure of intermediate states and the temperature dependent effect on multistep nucleation at the atomic-scale are still not fully understood. Here, we conduct direct in situ atomic-scale observations of kinetic processes of interfacial multistep nucleation pathways using an aberration-corrected TEM. We provide direct evidence for temperature-dependent multistep nucleation pathways in a supported bismuth system at the atomic-scale: a droplet-crystal two-step nucleation pathway at high temperature close to the melting point of bulk bismuth, a droplet-local ordered structure-crystal three-step nucleation pathway at medium temperature between a critical temperature Tc1 and the lowest size-dependent melting temperature of bismuth nanoparticles, and a cluster-crystal two-step nucleation pathway at a temperature lower than the lowest melting point (Tl). The related critical temperature Tc1 and the lowest melting point in the three pathways are confirmed based on the size-dependent melting phase diagram of Bi nanoparticles. Statistical analyses imply that the nucleation pathway plays an important role in deciding the nucleation and crystallinity kinetics, and the critical size of crystal nucleation is controlled by temperature. The understanding of multiple intermediate steps in nucleation and the temperature effect on nucleation pathways has important implications for the synthesis and growth of clusters, amorphous and crystalline materials, and these findings may further enrich the nucleation theory.
New conceptsExperimentally, uncovering kinetic pathways of multistep nucleation at the atomic scale is critical for understanding complex microscopic mechanisms of crystal nucleation and growth. In this study direct atomic scale experimental results are provided for temperature-dependent multistep nucleation pathways: (i) droplet-crystal two-step pathway at high temperature, (ii) droplet-local ordered structure-crystal three-step pathway at medium temperature, (iii) cluster-crystal classical two-step pathway at low temperature using an aberration-corrected transmission electron microscope. These results provide rare insights into temperature effected non-classical and classical multistep nucleation at the atomic-scale and confirm the existence of temperature-dependent critical size and intermediate states during different multistep nucleation pathways in heterogeneous nucleation, which should be helpful in guiding the synthesis and growth of clusters, amorphous and crystalline materials; and the findings further enrich the nucleation theory. |
Introduction
Understanding the phenomena of crystallization is important for both fundamental science as well as for practical importance in many research fields such as chemistry, biology, condensed-matter physics and materials science.1–7 In such a process, nucleation, an initial transformation of a disordered phase into an ordered one, represents a key step in tuning the desired size and manipulating the properties of crystalline solids.8–11 Based on the classical homogeneous nucleation and growth model, a crystal nucleus is first generated by spontaneous random aggregation of species from liquid or solution, followed by atomic or molecular attachment to form a stable crystal structure, providing a framework for understanding crystallization.12–15 However, such a mechanism cannot address all aspects of the nucleation and growth process properly,16–19 which has resulted in the recent development of non-classical nucleation mechanisms, such as particle coalescence or attachment mediated nucleation,20–24 phase transformations induced two-step nucleation,25–31 spinodal decomposition and phase transformation co-affected three-step nucleation.32,33To develop exact models for multistep nucleation which often deviate from classical nucleation theory, direct experimental observation of nucleation dynamics is timely and important, yet extremely challenging. With recent developments of the liquid cell and electrochemical cell in a transmission electron microscope (TEM), in situ dynamic observations of multistep homogeneous nucleation and interfacial heterogeneous nucleation have indeed been fulfilled at the nanoscale or atomic scale.18–20,24–27,31–45 For example, Alivisatos et al. observed the growth trajectories of a single colloidal platinum nanocrystal in solution using an in situ liquid cell in TEM and showed that the platinum nanocrystal can grow by either monomer attachment from solution or particle coalescence.20 Yoreo et al. conducted real-time TEM observation of CaCO3 nucleation in solution and revealed that two nucleation pathways are simultaneously co-operative, (i) direct nucleation from solution and (ii) an indirect pathway, through the transformation from amorphous to crystalline precursors.18 Mirsaidov et al. reported in situ observations of a three-step nucleation mechanism of Au (Ag) nanocrystals in solution, involving spinodal decomposition into liquid phases, nucleation of amorphous nanoclusters within the metal-rich liquid phase, and crystallization of the amorphous clusters.33 Moreover, Neumann et al. reported a direct imaging of mineralized agent-dependent classical and non-classical nucleation processes of polyfluoroxometallate crystal in solutions using cryo-TEM at the molecular scale.38 Nevertheless, such observations on solid nucleation are often conducted at the nanoscale mainly due to the strong interference of the window layer with the thickness of the solution in the liquid cell and also for the lack of consideration of temperature effects on nucleation and crystallization because of the intricacies in tackling such challenging topics at the atomic scale.46
Here, we report in situ atomic-scale dynamic observations of temperature-dependent multistep nucleation pathways of crystallization in an aberration-corrected TEM. Bismuth is adopted as a model material due to its low melting point of 544.4 K. Bi nanoparticles can be generated within the TEM since they tend to segregate from SrBi2Ta2O9 nanoparticles under electron beam irradiation.40,47–49 As such, the temperature can be precisely controlled by manipulating electron dose induced local heating in the TEM.50,51 We uncover unambiguously three temperature-dependent multistep pathways in the heterogeneous nucleation at the atomic scale: (i) a droplet-crystal two-step pathway at high temperature, (ii) a droplet-local ordered structure-crystal three-step pathway at medium temperature, and (iii) a cluster-crystal two-step pathway at low temperature. Interestingly, the nucleation and crystallization dynamics can be controlled by the temperature-dependent nucleation pathways.
Results and discussion
The SrBi2Ta2O9 (SBTO) nanoplatelets were prepared by a molten salt method, as shown in the experimental sections (ESI†). X-ray diffraction (XRD) analysis (Fig. S1, ESI†), TEM and high-angle annular-dark-field scanning TEM (HAADF-STEM) imaging, and electron diffraction patterns (Fig. S2, ESI†) show the successful fabrication of SBTO platelets with an orthorhombic structure (JCPDS card no. 49-0649). It is known that Bi tends to diffuse out of SBTO platelets under electron or ultraviolet light irradiation to form Bi nanoparticles.40,48,52 Hence this unique system is used to probe the effects of temperature on nucleation pathways at the atomic scale by manipulating electron doses in an aberration corrected TEM at 200 kV, which offers an unprecedented opportunity to probe ultra-small structures with sub-Ångström resolution.53–55 The chemical composition and the atomic structure of segregated Bi are confirmed by energy-dispersive X-ray spectroscopy (EDS) (Fig. S3, ESI†) and high resolution TEM (HRTEM) (Fig. S4, ESI†).To probe the dynamic nucleation process, we show in Fig. 1 sequential HRTEM images obtained under an electron dose of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 e Å−2 s−1, revealing a two-step nucleation pathway by which a surface and interface ordered liquid droplet transforms to the crystal nucleus (see also Video S1, ESI†). The segregated Bi tends to form liquid droplets at the initial stage, which is attributed to the low melting point in Bi nanoparticles induced by the size effect. The small Bi droplets (indicated by arrows in Fig. 1a–d and f) show a surface and interface ordered structure, which prefer to grow up and migrate on the surface of the support. Such an ordered droplet structure is further confirmed in an enlarged HRTEM image and the corresponding intensity profile analyses (Fig. S5, ESI†). Subsequently, a crystalline structure (marked by a red arrow in Fig. 1e) appears at 51.2 s immediately from the droplet (marked by a white arrow in Fig. 1d at 50.4 s) when the droplet is close in size to a critical diameter of ∼13 nm. The newly formed nanocrystal then undergoes a structural fluctuation between droplet and crystal. The structural fluctuations can be understood in light of the critical size governed by the excess free energy, which can be expressed as follows for a spherical embryo formed on a support:27,56,57
000 e Å−2 s−1, revealing a two-step nucleation pathway by which a surface and interface ordered liquid droplet transforms to the crystal nucleus (see also Video S1, ESI†). The segregated Bi tends to form liquid droplets at the initial stage, which is attributed to the low melting point in Bi nanoparticles induced by the size effect. The small Bi droplets (indicated by arrows in Fig. 1a–d and f) show a surface and interface ordered structure, which prefer to grow up and migrate on the surface of the support. Such an ordered droplet structure is further confirmed in an enlarged HRTEM image and the corresponding intensity profile analyses (Fig. S5, ESI†). Subsequently, a crystalline structure (marked by a red arrow in Fig. 1e) appears at 51.2 s immediately from the droplet (marked by a white arrow in Fig. 1d at 50.4 s) when the droplet is close in size to a critical diameter of ∼13 nm. The newly formed nanocrystal then undergoes a structural fluctuation between droplet and crystal. The structural fluctuations can be understood in light of the critical size governed by the excess free energy, which can be expressed as follows for a spherical embryo formed on a support:27,56,57
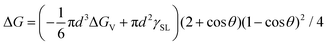 | (1) |
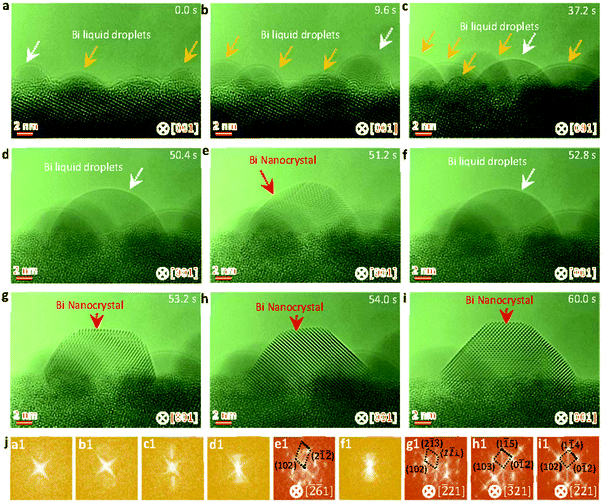 | ||
| Fig. 1 Sequential HRTEM images showing the liquid droplet-crystal two step nucleation pathway. (a–d) HRTEM images showing the growth of Bi droplets under electron beam irradiation. (e) HRTEM image showing the formation of a crystal nucleus through liquid to crystal phase transformation, as indicated by the red arrow. (f) HRTEM image showing the structural fluctuations of the newly formed crystal between crystal and droplet. (g–i) HRTEM images showing the formation of a stable crystal nucleus and the atomic rearrangements resulting in the energy favorable crystal nucleus. (j) Corresponding fast Fourier transformation (FFT) analyses of the marked nanoparticles by white and red arrows in Fig. 1a–i. The electron dose rate is 2.5 × 104 e Å−2 s−1. | ||
Fig. 2 shows sequential HRTEM images taken under an electron dose of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 e Å−2 s−1, which reveals a local structure ordering assisted three-step nucleation pathway (see also Video S2, ESI†). At the low electron dose, the Bi droplets with surface and interface ordering are first formed and grow up on the oxide surface under electron beam irradiation (as shown in Fig. 2a–e), similar to the initial stages in the two-step nucleation pathway in Fig. 1. It is worth mentioning that the interfacial ordering in the Bi droplet can be attributed to the template effect of the substrate and that the surface ordering should be related to the surface tension induced surface pre-crystallinity in the liquid.59–61 Subsequently, such Bi droplets are transformed to the interior local ordered structure at 52.4 s (Fig. 2f), followed by further crystallization in the nanoparticle (Fig. 2f–i). At 65.2 s, the interior local ordered structure changes to a perfect crystal nucleus with a diameter of ∼12 nm, followed by further growth enabled by merging with surrounding nanoparticles.62,63 Such three-step structural transformation pathways are further verified by the FFT patterns of the Bi nanoparticles in Fig. 2m(c1–l1) (marked by white and red arrows).
000 e Å−2 s−1, which reveals a local structure ordering assisted three-step nucleation pathway (see also Video S2, ESI†). At the low electron dose, the Bi droplets with surface and interface ordering are first formed and grow up on the oxide surface under electron beam irradiation (as shown in Fig. 2a–e), similar to the initial stages in the two-step nucleation pathway in Fig. 1. It is worth mentioning that the interfacial ordering in the Bi droplet can be attributed to the template effect of the substrate and that the surface ordering should be related to the surface tension induced surface pre-crystallinity in the liquid.59–61 Subsequently, such Bi droplets are transformed to the interior local ordered structure at 52.4 s (Fig. 2f), followed by further crystallization in the nanoparticle (Fig. 2f–i). At 65.2 s, the interior local ordered structure changes to a perfect crystal nucleus with a diameter of ∼12 nm, followed by further growth enabled by merging with surrounding nanoparticles.62,63 Such three-step structural transformation pathways are further verified by the FFT patterns of the Bi nanoparticles in Fig. 2m(c1–l1) (marked by white and red arrows).
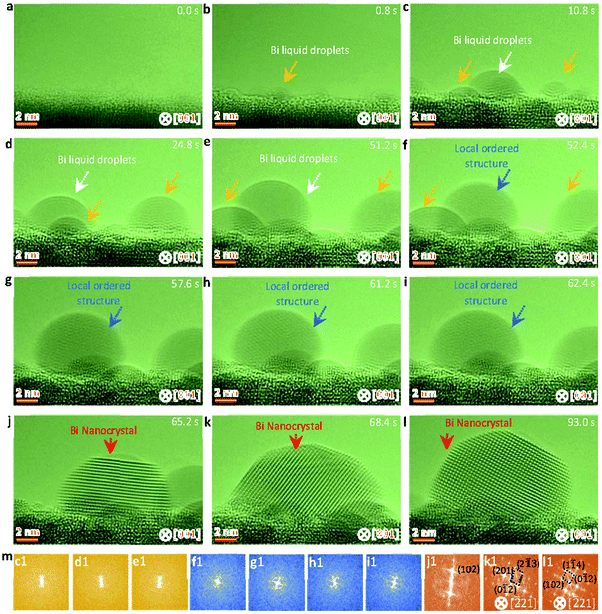 | ||
| Fig. 2 Sequential HRTEM images showing the liquid droplet-interior local ordered structure-crystal three step nucleation pathway. (a–e) HRTEM images showing the growth and formation of surface and interface ordered Bi droplets under electron beam irradiation. (f–i) HRTEM images showing the interior local ordered structure formed from the surface and interface ordered Bi droplet, as indicated by the blue arrow. (j–l) HRTEM images showing the formation and growth of a stable crystal nucleus from the previous interior local ordered structure. (m) Corresponding FFT analyses of the nanoparticles marked by white, blue and red arrows in Fig. 2c–l. The electron dose rate is 1.8 × 104 e Å−2 s−1. | ||
A two-step classical nucleation pathway from a local ordering cluster structure to a crystal nucleus is further uncovered in the sequential HRTEM images (Fig. 3) taken under an electron dose of 5000 e Å−2 s−1 (see also Video S3, ESI†). Since the induced temperature at the low electron dose is lower than the lowest melting point of the Bi nanoparticles (see discussions related to Fig. 4), there forms a local ordered cluster structure rather than the liquid droplets at the initial stage (Fig. 3a–c). When the length of the local ordered solid structure is close to a critical size of ∼12 nm, a rapid structural transformation from local ordered solid structure to crystal is clearly visible (Fig. 3d). Along with the volume expansion, the crystal nucleus becomes stable and show a faceted structure to minimize the surface and total energy (Fig. 3d–i). Further FFT patterns of the nanoparticles in Fig. 3c–i (marked as blue and red arrows) confirm the structural transformation from a local ordered cluster structure to a crystal nucleus in the classical two-step pathway. The orientation relationship between Bi and SBTO is [![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 21]Bi//[001]SBTO.
21]Bi//[001]SBTO.
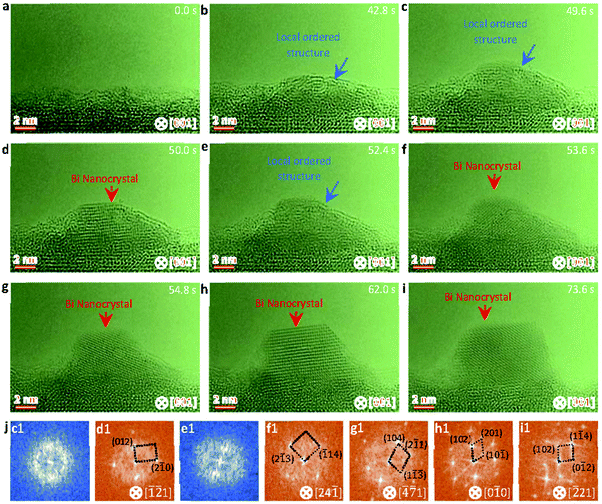 | ||
| Fig. 3 Sequential HRTEM images showing the cluster-crystal two step nucleation pathway. (a–c) HRTEM images showing the formation and growth of a local ordered Bi cluster structure under electron beam irradiation. (d) HRTEM image showing the formation of a crystal nucleus from the local ordered Bi cluster structure, as indicated by a red arrow. (e) HRTEM image showing the structure of the newly formed crystal transforming back to the ordered Bi cluster structure. (f–i) HRTEM images showing the formation of a stable crystal nucleus and the atomic rearrangement resulting in the energy favorable crystal nucleus. (j) Corresponding FFT analyses of the nanoparticles marked by blue and red arrows in Fig. 3c–i. The electron dose rate is 5.0 × 103 e Å−2 s−1. | ||
To uncover the temperature-induced heterogeneous nucleation on supported nanoparticles, we measure the size dependent melting point of Bi nanoparticles under direct heating in TEM, as shown in Fig. S6 (ESI†). In situ heating experiments are carried out in the TEM equipped with a NanoEx-i/v-Single-Tilt TEM heating holder, which allows a very rapid heating rate and exhibits negligible thermal drift. The SBTO is first irradiated by electron beam to form Bi nanoparticles with different sizes. To accurately measure the melting temperatures and to minimize the electron beam effect, a low heating (cooling) rate of ∼0.1 °C s−1 and an electron dose rate of ∼2.5 × 103 e Å−2 s−1 are adapted. Fig. 4a shows the fitted size-dependent melting point curve of Bi, which matches Shi's model,64–67
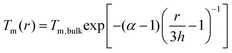 | (2) |
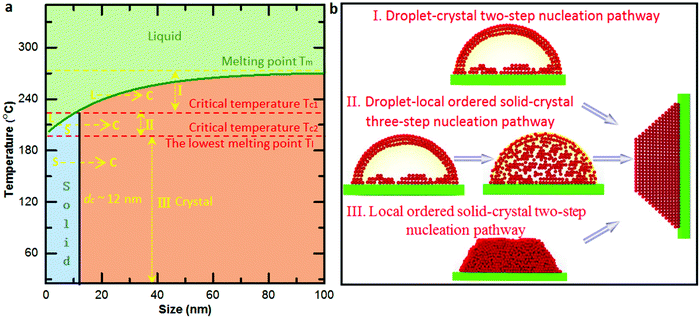
Based on the size-dependent melting phase diagram, one can find that there are two critical temperatures (marked by red dash lines in Fig. 4a) below the melting point of bulk Bi: one is the critical temperature Tc1 induced by phase transformation from solid cluster or local ordered structure to crystal (as also confirmed by the observed critical size during the phase transformation), and the other is the size-dependent lowest melting point Tc2, as confirmed by the fitted size-dependent melting point curve (green curve in Fig. 4a). In terms of the bulk melting point and the two critical temperatures, the size-dependent melting phase diagram can be divided into four parts (Fig. 4a). When the temperature is higher than the melting point of bulk Bi, there is no solid nucleation. The crystallization from liquid to crystal two-step nucleation takes place in the region between the bulk melting point and Tc1 (named region I). There occurs a liquid–solid local ordered structure-crystal three-step nucleation pathway in the region between Tc1 and Tc2 (named II) and a solid local ordered cluster structure-crystal two-step nucleation pathway in the region below Tc2 (named III). To explain the electron beam induced Bi nanocrystal growth with different pathways (Fig. S7, ESI†) from Fig. 1–3, we calculate the electron dose induced maximum temperature increase based on the Fisher's model as 208 K, 187 K and 41 K (ESI†). The temperature of the Bi nanoparticles from Fig. 1–3 is estimated to be about 228 °C, 207 °C, and 61 °C, respectively. The temperatures are located in the region I, II and III, showing the temperature-dependent multistep nucleation pathways in the supported nanoparticles.
To further gain insights into the disordered and ordered states in the temperature-dependent multistep nucleation pathways, we quantify the involved phase transitions of the Bi nanoparticle by calculating crystalline order of the nanoparticle as a function of time by measuring peak intensity of the Fourier spectrum from the FFT pattern (Fig. S8–S10, ESI†), as shown in Fig. 5a–c. One can notice that the multistep nucleation pathways have a profound impact on the nucleation intermediate states and the nucleation and crystalline dynamics: (a) a rapid crystallization from liquid to crystal via a two-step nucleation pathway at high temperature; (b) an eventual crystallization for local structure ordering assisted three-step nucleation pathway at medium temperature; (c) a cluster structure to crystal two-step nucleation pathway at low temperature. To shed light on the structural evolution of Bi nanoparticles in the three nucleation pathways, we further investigate the changes in phase, length, height and length–height aspect ratio with time (see Video S1–S3, ESI†) and find that the nucleation pathways affect the nonlinear nucleation dynamics (Fig. 5d–i and Fig. S11, ESI†), and the length–height aspect ratio of the formed crystal nucleus varies from 1.9 to 1.5 (Fig. 5d–f). Interestingly, based on the statistical analyses of the length as a function of time, it is evident that the critical size of crystal nucleation is controlled by the temperature, indicated by red dash lines with three steps in Fig. 5g–i. The results should have an implication on the growth of ultra-small nanoparticles.
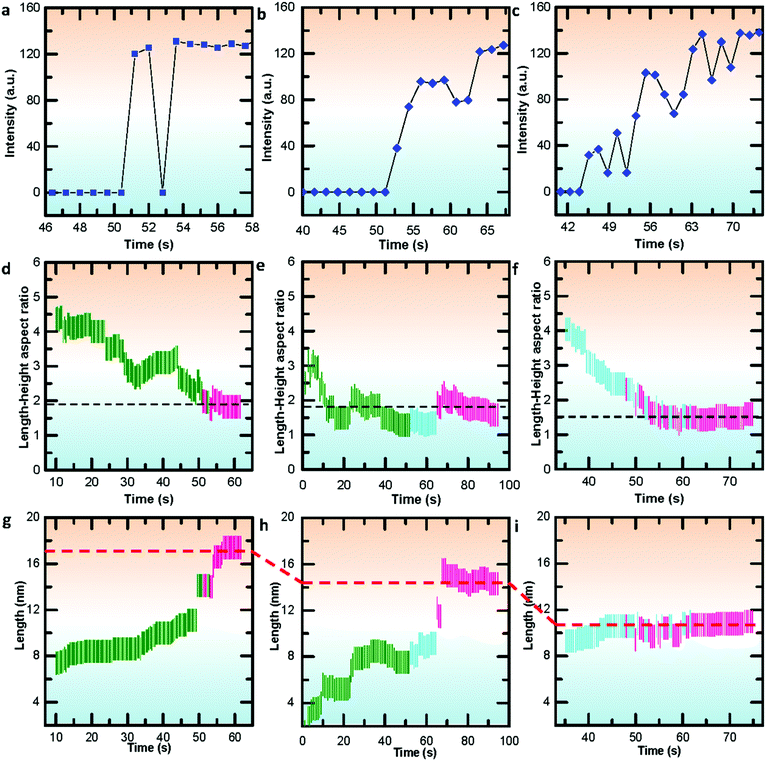 | ||
| Fig. 5 Quantifying the crystallizations and statistical analyses of nucleation dynamics during the multistep nucleation pathways in Videos S1–S3 (ESI†). (a–c) Quantifying the crystallization process during the multistep nucleation pathways involving phase transformations. (d–f) Statistical analyses of the length–height aspect ratio as a function of time. (g–i) Statistical analyses of the length as a function of time. The green, blue and red lines in (d–f) represent liquid, local ordered solid and crystal phase, respectively. The black dashed lines indicate the energy stabilized length–height ratio. | ||
Conclusions
The ability to conduct direct atomic scale observations of kinetic pathways of multistep nucleation represents a significant step forward in understanding non-classical nucleation mechanisms at the atomic scale. We reveal three temperature-dependent multistep pathways in heterogeneous nucleation at the atomic scale: a droplet-crystal two-step pathway at high temperature close to the melting point of bulk Bi, a droplet-local ordered solid structure-crystal three-step pathway at medium temperature between Tc1 and the lowest size-dependent melting temperature of Bi nanoparticle, and a cluster-crystal two-step classical nucleation pathway at lower temperature than the lowest melting point. The Tc1 and the lowest melting in the three pathways are further confirmed based on the size-dependent melting phase diagram of Bi nanoparticles obtained by experimental statistical analyses. Further crystalline order and nucleation dynamics analyses show that the nucleation pathway and temperature play important roles in deciding the nucleation intermediate states, as well as the nucleation and crystalline dynamics. These results highlight the importance of temperature on heterogeneous nucleation and crystallization dynamics, which advances our understanding of non-classical multistep heterogeneous nucleation and helps to clarify the atomic origin of temperature effected behaviour in nanomaterials and hetero-structures.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
JJL acknowledges the support from the “One Hundred Talents Project Foundation Program” of Chinese Academy of Sciences. JJL and FLD acknowledge the financial support by the N2020: Nanotechnology based functional solutions (NORTE-45-2015-02). ZCW acknowledges the support from the National Science Foundation of China (NSFC) under grant no. 51728202 and the state key laboratory of powder metallurgy in Central South University.References
- D. Erdemir, A. Y. Lee and A. S. Myerson, Acc. Chem. Res., 2009, 42, 621–629 CrossRef CAS PubMed.
- S. E. Wolf, J. Leiterer, M. Kappl, F. Emmerling and W. Tremel, J. Am. Chem. Soc., 2008, 130, 12342–12347 CrossRef CAS PubMed.
- J. Pang, R. G. Mendes, A. Bachmatiuk, L. Zhao, H. Q. Ta, T. Gemming, H. Liu, Z. Liu and M. H. Rummeli, Chem. Soc. Rev., 2019, 48, 72–133 RSC.
- H. Ta, L. Zhao, D. Pohl, J. Pang, B. Trzebicka, B. Rellinghaus, D. Pribat, T. Gemming, Z. Liu and A. Bachmatiuk, Crystals, 2016, 6, 100 CrossRef.
- X. Zhang, X. Sun, S.-X. Guo, A. Bond and J. Zhang, Energy Environ. Sci., 2019, 12, 1334–1340 RSC.
- E. Wu, K. Feng, R. Shi, R. Lv, F. Ouyang, S. S. Li, J. Zhong and J. Liu, Chem. Sci., 2019, 10, 257–267 RSC.
- H. Gu, W. Fan and T. Liu, Nanoscale Horiz., 2017, 2, 277–283 RSC.
- N. E. Chayen, E. Saridakis and R. Sear, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 597–601 CrossRef CAS PubMed.
- J. De Yoreo, Nat. Mater., 2013, 12, 284–285 CrossRef CAS PubMed.
- A. S. Myerson and B. L. Trout, Science, 2013, 341, 855–856 CrossRef CAS PubMed.
- T. Liu, E. Diemann, H. Li, A. W. Dress and A. Müller, Nature, 2003, 426, 59 CrossRef CAS PubMed.
- D. W. Oxtoby, Acc. Chem. Res., 1998, 31, 91–97 CrossRef CAS.
- M. Kulmala, Science, 2003, 302, 1000–1001 CrossRef CAS PubMed.
- W. J. Habraken, et al. , Nat. Commun., 2013, 4, 1507 CrossRef PubMed.
- M. Sleutel, J. Lutsko, A. E. Van Driessche, M. A. Durán-Olivencia and D. Maes, Nat. Commun., 2014, 5, 5598 CrossRef CAS PubMed.
- D. Gebauer, A. Völkel and H. Cölfen, Science, 2008, 322, 1819–1822 CrossRef CAS PubMed.
- M. Sleutel and A. E. Van Driessche, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, E546–E553 CrossRef CAS PubMed.
- M. H. Nielsen, S. Aloni and J. J. De Yoreo, Science, 2014, 345, 1158–1162 CrossRef CAS PubMed.
- V. M. Yuwono, N. D. Burrows, J. A. Soltis and R. L. Penn, J. Am. Chem. Soc., 2010, 132, 2163–2165 CrossRef CAS PubMed.
- H. Zheng, et al. , Science, 2009, 324, 1309–1312 CrossRef CAS PubMed.
- Y. Jiang, et al. , Nat. Commun., 2017, 8, 15933 CrossRef CAS PubMed.
- J. Li, et al. , Adv. Sci., 2018, 5, 1700992 CrossRef PubMed.
- J. F. Banfield, S. A. Welch, H. Zhang, T. T. Ebert and R. L. Penn, Science, 2000, 289, 751–754 CrossRef CAS PubMed.
- J. J. De Yoreo, et al. , Science, 2016, 349, aaa6760 CrossRef PubMed.
- S.-Y. Chung, Y.-M. Kim, J.-G. Kim and Y.-J. Kim, Nat. Phys., 2009, 5, 68 Search PubMed.
- J. M. Walker, B. Marzec and F. Nudelman, Angew. Chem., Int. Ed., 2017, 129, 11902–11905 CrossRef.
- J. Li, Z. Wang and F. L. Deepak, ACS Nano, 2017, 11, 5590–5597 CrossRef CAS PubMed.
- C. Guo, J. Wang, J. Li, Z. Wang and S. Tang, J. Phys. Chem. Lett., 2016, 7, 5008–5014 CrossRef CAS PubMed.
- S. Lee, et al. , Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13618–13623 CrossRef CAS PubMed.
- J. Baumgartner, et al. , Nat. Mater., 2013, 12, 310 CrossRef CAS PubMed.
- A. Van Driessche, et al. , Science, 2012, 336, 69–72 CrossRef CAS PubMed.
- J. T. McKeown, Y. Wu, J. D. Fowlkes, P. D. Rack and G. H. Campbell, Adv. Mater., 2015, 27, 1060–1065 CrossRef CAS PubMed.
- N. D. Loh, et al. , Nat. Chem., 2017, 9, 77 CrossRef CAS PubMed.
- A. F. Wallace, et al. , Science, 2013, 341, 885–889 CrossRef CAS PubMed.
- H.-G. Liao, L. Cui, S. Whitelam and H. Zheng, Science, 2012, 336, 1011–1014 CrossRef CAS PubMed.
- P. Tan, N. Xu and L. Xu, Nat. Phys., 2014, 10, 73–79 Search PubMed.
- P. J. Smeets, K. R. Cho, R. G. Kempen, N. A. Sommerdijk and J. J. De Yoreo, Nat. Mater., 2015, 14, 394 CrossRef CAS PubMed.
- R. E. Schreiber, et al. , Nat. Chem., 2017, 9, 369 CrossRef CAS PubMed.
- S.-Y. Chung, Y.-M. Kim, J.-G. Kim and Y.-J. Kim, Nat. Phys., 2009, 5, 68–73 Search PubMed.
- Y. Li, et al. , ACS Nano, 2016, 10, 2386–2391 CrossRef CAS PubMed.
- J. Liu, P. Zhou, W. Zhang, X. Chen, J. Huang, J. Li, M. Chi and J. Niu, Chem. Commun., 2019, 55, 529–532 RSC.
- R. G. Mendes, J. Pang, A. Bachmatiuk, H. Q. Ta, L. Zhao, T. Gemming, L. Fu, Z. Liu and M. H. Rümmeli, ACS Nano, 2019, 13, 978–995 CAS.
- A. M. Tripathi, W.-N. Su and B. J. Hwang, Chem. Soc. Rev., 2018, 47, 736–851 RSC.
- F.-C. Chen, J.-Y. Chen, Y.-H. Lin, M.-Y. Kuo, Y.-J. Hsu and W.-W. Wu, Nanoscale, 2019, 11, 10486–10492 RSC.
- K. Wu, F. Chen, Z. Ma, B. Guo, Y. Lyu, P. Wang, H. Yang, Q. Li, H. Wang and A. Nie, Chem. Commun., 2019, 55, 5611–5614 RSC.
- D. J. Kelly, et al. , Nano Lett., 2018, 18, 1168–1174 CrossRef CAS PubMed.
- E. Olson, M. Y. Efremov, M. Zhang, Z. Zhang and L. Allen, J. Appl. Phys., 2005, 97, 034304 CrossRef.
- Y. Li, et al. , Chem. Mater., 2013, 25, 2045–2050 CrossRef CAS.
- R. He, D. Xu, B. Cheng, J. Yu and W. Ho, Nanoscale Horiz., 2018, 3, 464–504 RSC.
- S. Fisher, Radiat. Eff., 1970, 5, 239–243 CrossRef CAS.
- R. Egerton, P. Li and M. Malac, Micron, 2004, 35, 399–409 CrossRef CAS PubMed.
- J. Li, Z. Wang and F. L. Deepak, J. Phys. Chem. Lett., 2018, 9, 961–969 CrossRef CAS PubMed.
- C.-L. Jia, K. W. Urban, M. Alexe, D. Hesse and I. Vrejoiu, Science, 2011, 331, 1420–1423 CrossRef CAS PubMed.
- J. Li, D. Yin, C. Chen, Q. Li, L. Lin, R. Sun, S. Huang and Z. Wang, J. Appl. Phys., 2015, 117, 085303 CrossRef.
- J. Li, Z. Wang, Y. Li and F. L. Deepak, Adv. Sci., 2019, 6, 1802131 CrossRef PubMed.
- D. A. Porter, K. E. Easterling and M. Sherif, Phase Transformations in Metals and Alloys, (Revised Reprint), CRC Press, 2009 Search PubMed.
- H. Fredriksson and U. Akerlind, Solidification and crystallization processing in metals and alloys, John Wiley & Sons, 2012 Search PubMed.
- J. Van Duijneveldt and D. Frenkel, J. Chem. Phys., 1992, 96, 4655–4668 CrossRef CAS.
- S. H. Oh, Y. Kauffmann, C. Scheu, W. D. Kaplan and M. Rühle, Science, 2005, 310, 661–663 CrossRef CAS PubMed.
- O. G. Shpyrko, R. Streitel, V. S. Balagurusamy, A. Y. Grigoriev, M. Deutsch, B. M. Ocko, M. Meron, B. Lin and P. S. Pershan, Science, 2006, 313, 77–80 CrossRef CAS PubMed.
- X. Wu, B. Ocko, E. Sirota, S. Sinha, M. Deutsch, B. Cao and M. Kim, Science, 1993, 261, 1018–1021 CrossRef CAS PubMed.
- D. Li, et al. , Science, 2012, 336, 1014–1018 CrossRef CAS PubMed.
- B. Jin, M. L. Sushko, Z. Liu, C. Jin and R. Tang, Nano Lett., 2018, 18, 6551–6556 CrossRef CAS PubMed.
- Y. Li, et al. , Nat. Commun., 2017, 8, 14462 CrossRef CAS PubMed.
- F. G. Shi, J. Mater. Res., 1994, 9, 1307–1314 CrossRef CAS.
- W. Qi, Acc. Chem. Res., 2016, 49, 1587–1595 CrossRef CAS PubMed.
- Q. Mei and K. Lu, Prog. Mater. Sci., 2007, 52, 1175–1262 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00308h |
| This journal is © The Royal Society of Chemistry 2019 |