Recent trends in transition metal dichalcogenide based supercapacitor electrodes
Jayesh
Cherusseri
 a,
Nitin
Choudhary
a,
Kowsik
Sambath Kumar
ab,
Yeonwoong
Jung
a,
Nitin
Choudhary
a,
Kowsik
Sambath Kumar
ab,
Yeonwoong
Jung
 abc and
Jayan
Thomas
abc and
Jayan
Thomas
 *abd
*abd
aNanoScience Technology Center (NSTC), University of Central Florida, FL 32826, USA. E-mail: Jayan.Thomas@ucf.edu
bMaterials Science and Engineering, University of Central Florida, FL 32816, USA
cDepartment of Electrical and Computer Engineering, University of Central Florida, Orlando, Florida 32816, USA
dCollege of Optics and Photonics, University of Central Florida, Orlando, FL 32816, USA
First published on 5th April 2019
Abstract
The 21st century demands the rapid development of energy storage devices and systems that can cater to our daily energy needs of wearable devices in particular and electric vehicles in a large context. The advent of nanostructured materials has urged the scientific community and industry to take a renewed interest in developing electrochemical supercapacitors to nurture the energy needs of wearables and electric vehicles. Transition metal dichalcogenides (TMDs) are proposed to play a key role as active electrode materials in supercapacitors enabled by their large surface area and variable oxidation states. These properties enable them to store significant energy via electrical double layer and pseudocapacitive charge storage mechanisms. Herein, we discuss the recent advances in the development and the electrochemical performances of the TMD based supercapacitor electrodes. These electrodes range from those made in different nanoscale form factors to those exhibiting fascinating structural/electronic properties. The synergistic effects between TMDs and other materials in hybrid electrode designs and asymmetric configurations to meet the demand for high energy density requirements of modern electronic devices have been discussed in detail. Finally, the opportunities, as well as the challenges in TMD based supercapacitor research frontiers are highlighted.
1 Introduction
Electrochemical capacitors or supercapacitors are gradually changing our lives in many ways and revolutionizing a breadth of industries including transport, aerospace, and consumer electronics. Supercapacitors are an inevitable part of the present and future energy industry due to their high-power density and ability to integrate with various energy conversion devices.1 Whether it is about charging electric vehicles in a few minutes or accelerating electric cars to more than 60 mph in a few seconds, supercapacitors are finding niche applications worldwide. The state-of-the-art lithium-ion (Li-ion) batteries are yet to address the aforementioned challenges due to their sluggish charging/discharging nature, limited cycle life, and slow pace of development.2,3 Moreover, serious health hazards associated with Li-ion batteries restrict their widespread applications, especially when the world is witnessing an explosive growth of flexible/wearable electronics which demand commensurate backing up of energy storage systems that are environment-friendly, faster, mechanically robust, and offer wide operational temperatures.4–7 Currently, supercapacitors are mainly used as a complementary aid to batteries primarily due to their inherent weakness of low energy density (i.e., the amount of total stored energy per unit volume) followed by low operation voltages, high self-discharge, and relatively high production cost.8–11 The performance of a supercapacitor critically depends on the quality and electrochemical performance of the electrode materials, which in turn rely on the surface area, electrical conductivity, and wetting behavior of the electrodes and permeability of electrolyte ions.12–15Traditionally used activated carbon-based supercapacitor electrodes often employ binders which are passive components that reduce the effective surface area of electro-active materials. This also adds unnecessary weight to the devices, making them cumbersome.16,17 The ground-breaking discovery of the wonder material ‘graphene’ has led to many significant developments in the realms of energy storage due to its large specific surface area, high conductivity, and unprecedented mechanical strength.18,19 Despite a plethora of research conducted on graphene-based supercapacitors, their energy densities are inferior compared to the Li-ion batteries.20–22 This is because of the fact that graphene stores charges only by the formation of an electrochemical double layer (EDL) at the electrode/electrolyte interface. However, redox materials such as transition metal oxides (for e.g. RuO2 and MnO2, etc.) store charge via Faradaic reactions and electronically conductive polymers (e.g. polypyrrole (PPy), polyaniline (PANI), etc.) are added to obtain better electrochemical performance, but at the cost of poor cycle life.23–27
The revolution in the supercapacitor technology is currently motivated by the renewed interest in the post-graphene layered inorganic materials, known as TMDs. The TMDs are layered inorganic materials composed of transition metals (M) and chalcogens (X: S, Se, and Te) in an X–M–X fashion with a chemical configuration of MX2 which offer a rich set of physio-chemical properties that are highly intriguing for fundamental and technological research.28 Various two-dimensional (2D) TMDs used as supercapacitor electrodes nowadays are molybdenum disulphide (MoS2), molybdenum diselenide (MoSe2), tungsten disulphide (WS2), tungsten diselenide (WSe2), tungsten ditelluride (WTe2), tantalum disulphide (TaS2), tantalum diselenide (TaSe2), titanium disulphide (TiS2), niobium disulphide (NbS2), zirconium disulphide (ZrS2), vanadium disulphide (VS2), vanadium diselenide (VSe2), etc. Among these TMDs, the most popular and studied system is MoS2. But a potential alternative to MoS2 is MoSe2, particularly in supercapacitor applications due to the uniqueness of MoSe2 such as lower size, high electrical conductivity than MoS2, etc. Transition metal selenides exhibit higher electrical conductivity than their sulphide counterparts and are promising candidates as electrode materials in various energy storage devices.29 TMDs exist in two phases namely 2H and 1T where the 2H phase is semiconducting, and the 1T phase is metallic.30 TMDs in their bulk state can also be used as electrodes in supercapacitors, but their low surface area is a major drawback. The large surface area of atomically thin individual sheets and also the presence of multiple oxidation states (e.g., +2 to +4 in MoS2) in 2D TMDs enable them to store charge electrostatically (i.e. by EDL mechanism) as well as via ion intercalation into the interlayer space (i.e. through the Faradaic mechanism) leading to high specific capacitance and energy density.31,32 Moreover, the anisotropic crystal structures in 2D TMDs offer edge planes, which are highly reactive for most favorable electrochemical properties.33,34 Despite several interesting features, the poor intrinsic electrical conductivity in the most stable 2D TMD phase (i.e., hexagonal (2H)) hinders their real potential as state-of-the-art supercapacitor electrode materials.35 In addition, the re-stacking of 2D TMD nanosheets inhibiting large surface area exposed to the electrolyte ions and significant volume change during cycling are potential threats to the use of monolithic TMDs.36,37 Nevertheless, the novel sheet-like morphology, large surface area, sub-atomic thicknesses, and active edge sites in 2D TMDs allow them to easily mix, match and deposit on other electrochemically active functional materials to construct hybrid nanomaterials with enhanced physical and chemical properties. The nanoscale engineering of TMDs in zero-dimensional (0D), one-dimensional (1D) and three-dimensional (3D) rational designs is another fascinating approach to alleviate their inherent weaknesses. In addition, a phase change in TMDs from the 2H semiconducting phase to the metallic (1T) phase has triggered the scientific community to develop a new class of metallic TMDs with tremendous potential in achieving higher energy density.
In this review, we summarize the most recent endeavors towards the development of high energy density supercapacitors using TMD based electrodes. This includes (i) the synthesis of TMDs via various routes, (ii) the preparation of pristine TMD based supercapacitors, (iii) hybrid supercapacitor electrodes composed of TMDs with highly conductive and capacitive materials (e.g. carbon nanomaterials or electronically conducting polymers), which surpass the limited specific capacitance, cyclic stability, and rate performance in pristine TMD electrodes, (iv) rational TMD designs in various form factors such as 1D nanowires and 3D porous structures enabling high porosity, large surface areas, and short diffusion paths for better electrochemical performance, (v) metallic TMDs as one of the emerging classes of supercapacitor materials which are expected to set new paradigms in energy storage owing to their unprecedented electrochemical behavior, and (vi) asymmetric supercapacitor devices based on TMDs as anode materials and various organic/inorganic materials as cathodes to achieve high energy densities. A summary of the review is schematically shown in Fig. 1.
2 Synthesis routes to transition metal dichalcogenides (TMDs)
TMDs have attracted great scientific research interest as they have found applications in the next generation nanoelectronic devices mainly due to the easy fabrication of their complex structures when compared with the 1D nanomaterials. In this section, we review the various synthesis routes available for various 2D and 3D TMDs. In the 2D TMD family, MoS2 is the most commonly used 2D TMD mainly due to its layer dependence of the band structure which is a remarkable feature for nanoelectronic devices. The bulk of MoS2 is an indirect band semiconductor whereas single layered MoS2 is a direct band semiconductor.38,39 Nanostructured MoS2 such as nanotubes and nanowires show a significant difference in their properties due to the quantum confinement effect. Various methodologies are adopted for the synthesis of 2D TMDs including wet-chemical methods,40–47 lithium-based intercalation,48,49 the scotch-tape method,50–53 chemical vapor deposition (CVD),54–57 chemical vapor transport (CVT),58–61 atomic layer deposition (ALD),62–66 pulsed laser deposition (PLD),67,68 the vapor-phase growth method,69–76 and the solvothermal/hydrothermal method,77–89etc. In this section, we briefly discuss these various methodologies used in the synthesis of 2D TMDs with the details such as type of nanostructures formed, their size and shape, peculiar features, etc.2.1 Wet-chemical synthesis
The wet-chemical method is a straightforward and affordable method in which no sophisticated machinery/system is required. This is very important in limiting the cost of the TMDs synthesized. Duphil et al. have reported the chemical solution reaction synthesis of MoS2 nanoparticles at a temperature of 140 °C and the obtained spherical nanoparticles have diameters ranging from 10 to 30 nm.40 They have observed that upon annealing the nanoparticles at 550 °C under vacuum, the nanoparticles not only lost their spherical shape but also slightly crystallized with 2H hexagonal structures. MoS2 nanoclusters ranging from 2.5–7 nm can be prepared via electrochemical routes by refluxing the precursors such as sulfur in degassed para-xylene and molybdenum hexacarbonyl at low temperature.41 1T phase metallic 2D TiS2 hexagonal nanocrystals were synthesized by Muller et al. using titanium tetrachloride (TiCl4), carbon disulfide (CS2), and oleylamine.90 Here, anisotropic growth is observed due to the large difference in surface energy. Chaki et al. reported the room temperature synthesis of SnS2 nanoparticles using stannic chloride pentahydrate and thioacetamide precursors.42 The average diameter of the as-prepared nanoparticles was about 3.87 nm. 2D nanocrystals of TiS2 were synthesized using TiCl4, oleylamine and CS2.43 The as-prepared 2D nanocrystals are composed of the (001) layer with average diameters of ∼50, 100, and 270 nm depending on the reaction conditions and the hexagonal phase was observed for both the bulk and 2D TiS2 nanocrystals. Single layered colloidal TiS2 nanodisks were prepared using TiCl4, oleylamine and sulfur powder at a reaction temperature of 215 °C.44 The concentration of sulfur is found to affect the size of the nanodisks in a manner that the increase in sulfur concentration results in a decrease of the size of the nanodisks. Jeong et al. prepared TiS2 nanocrystals with controlled lateral sizes by altering the concentration of TiCl4 and CS2 at a reaction temperature of 300 °C.45 A tandem intercalation strategy can be adopted to prepare single-layered 2D TMDs such as TiS2, ZrS2, NbS2, WSe2 and MoS2.46 A simple ammonia-assisted exfoliation procedure can be used to synthesize the VS2 nanosheets with metallic behavior derived from the bulk of VS2 flakes using a simple ammonia-assisted exfoliation procedure.47 The thickness of the nanosheets was found to be only 3 nm. Lithium intercalation was used to prepare single layers of MoS2.48,49 Metallic 1T phase MoS2 nanosheets were prepared using this method from bulk MoS2 powders.91 Removal of organolithium contamination was carried out by washing the filtered, exfoliated MoS2 nanosheets with hexane and deionized water. By intercalating organo-lithium into bulk MoS2 and subsequent exfoliation, 1T phase monolayer MoS2 can be synthesized.922.2 Scotch-tape method
The scotch-tape method is the simplest method that can be used to synthesize 2D TMDs in a short period. Monolayers of MoS2 can be prepared using the scotch-tape method.50,51 It is a very simple method as the single-layer MoS2 can be easily transferred to the desired substrate by simple micromechanical exfoliation, which is the versatile method used in the preparation of single-layered graphene.52–54 Tiny crystals of MoS2 are composed of vertically stacked monolayers connected via weak van der Waals interactions as schematically shown in Fig. 2a.93 An optical image of single layered MoS2 with a thickness of 6.5 Å synthesized using the scotch-tape method is shown in Fig. 2b.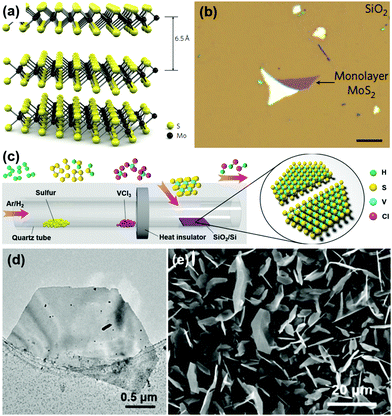 | ||
| Fig. 2 (a) 3D representation of the structure of MoS2 extracted via scotch tape-based micromechanical cleavage, (b) optical image of a single layer of MoS2 (thickness, 6.5 Å) deposited on top of a silicon substrate with a 270 nm-thick SiO2 layer. Reprinted with permission from ref. 93 Copyright (2011) Macmillan Publishers Limited. (c) Schematic diagram of the synthesis route for VS2 by CVD, (d) TEM image of a half-hexagonal VS2 nanosheet, and (e) SEM image of VS2 nanosheets. Reprinted with permission from ref. 57 Copyright (2017) American Chemical Society. | ||
2.3 Chemical vapor deposition method
CVD is a versatile method to prepare high-quality TMDs in terms of high crystallinity and purity. It is feasible to produce large area TMDs using the CVD method and hence it fits very well for industrial applications. The reaction temperature of a CVD process is typically in the range of 600–800 °C.54 An atmospheric pressure CVD was used for the growth of the nanosheets at a temperature of 850 °C for 30 min under an Ar atmosphere. The as-prepared MoS2 nanosheets are vertically aligned with curved edges and are densely populated with a thickness of 80–250 nm and lateral sizes of about 2–20 μm. Due to the higher reaction temperature of the CVD process, this method is incompatible with a variety of flexible substrates, which are currently used for flexible and wearable applications. In order to reduce the reaction temperature, plasma-assisted synthesis of TMDs was developed. Water vapor can be used as the transport agent to prepare MoS2 nanosheets with a monolayer or multi-layers by using a modified vapor deposition method.55 The SiO2/Si substrate with an oxide coating thickness of 300 nm was used for synthesizing the MoS2 flake with a lateral size of 20–40 μm.Lee et al. have prepared large-area MoS2 films on an amorphous SiO2 substrate via the CVD process at a reaction temperature of 650 °C under a nitrogen atmosphere.56 The as-prepared MoS2 films initially exhibited star-shaped morphology and these films further merged to form a single film. The surface pre-treatment of the substrate played a crucial role in determining the morphology of the MoS2 film whereas the untreated substrate could only grow MoS2 nanoparticles instead of the thin film. The CVD growth (Fig. 2c) of VS2 nanosheets on aSiO2/Si substrate was reported recently using precursors such as solid VCl3 and sulfur powder and the obtained nanosheets were of metallic 1T phase with a high electrical conductivity of 3000 S cm−1.57 The growth of the VS2 nanosheets took place at a deposition temperature of 600 °C for a period of 25 min and hexagonal VS2 nanosheets of thickness >100 nm and ultrathin nanosheets with thickness of <10 nm were obtained. The TEM and SEM images of the VS2 nanosheets synthesized are depicted in Fig. 2d and e, respectively. In the CVD process, by changing the concentration and composition of the carrier gas, the size of the VS2 nanosheets can be controlled where the hydrogen gas flow is found mandatory for the growth of the VS2 nanosheets without which no growth was observed on the SiO2/Si substrate. Metallic few-layered 1T VSe2 nanosheets were synthesized by employing the CVD method using vanadium chloride powder and selenium powder as the precursors of V and Se respectively, at a processing temperature of 650 °C.94 Like CVD, CVT is also a feasible method found in the preparation of 2D TiS2 with the possibility of intercalation of various elements.58–61 Semi-metallic 1 Td WTe2 single crystals were synthesized using the CVT method by using iodide (I) as a transporting agent.95 These single crystals had average lengths ranging from 0.5–3 cm with a width of ∼1 mm. Quasi-arrays of 2H-TaSe2 nanobelts were synthesized using the surface-assisted CVT method.96 The vertically grown quasi-arrays had an average height up to 10 μm.
2.4 Atomic layer deposition method
TiS2 nanofilms were synthesized using the ALD method on a variety of substrates such as Si, soda-lime glass, thin films of ruthenium, platinum, rhodium, titanium nitride, zinc sulphide, iridium and palladium using precursors such as TiCl4 and H2S at a temperature of 400–500 °C.65 TiS2 grown on soda-lime glass at 400 °C exhibited random plate-like crystallite morphology but apart from this, some of them exhibited nanotubular morphology. Morphologies such as hexagonal plate-like TiS2 crystallites were observed on rhodium, iridium, and TiN substrates, but such a morphology is not observed on palladium and platinum substrates and cube-shaped TiS2 nanocrystallites were formed on the ruthenium thin film substrate (Fig. 3). WS2 thin films were synthesized using the ALD method using WF6 and H2S precursors at a temperature of 300 °C using nitrogen as a carrier gas.66 Here, the ZnS-coated SiO2/Si substrate was used where ZnS acted as a catalyst for the growth and the thickness of the as-grown WS2 nanosheets was about 250 nm. Another method of preparing high-quality 2D TMDs is PLD. Atomically thin WS2 nanosheets were prepared using the PLD method on silver and quartz substrates at a low temperature of 450 °C.67 The interplanar spacing of the as-synthesized highly crystalline few-layered WS2 nanosheets was found to be 0.62 nm for the (002) plane. Recently, Campbell et al. prepared MoS2 nanosheets by using two types of plasma treatments such as remote plasma and direct plasma at a temperature of 400 or 450 °C.68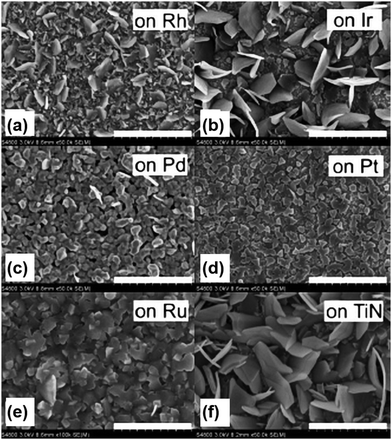 | ||
| Fig. 3 FESEM images of TiS2 films grown via ALD at 400 °C on (a) rhodium, (b) iridium, (c) palladium, (d) platinum, (e) ruthenium, and (f) TiN. Scale bars: (a–d) 1 μm; (e) 500 nm; (f) 1 μm. Reprinted with permission from ref. 65 copyright (2007) John Wiley and Sons. | ||
2.5 Vapor-phase reaction synthesis
Vapor-phase reaction synthesis is another method to synthesize layered TMDs. MoS2 nanotubes have been prepared using this method using precursors such as molybdenum oxide and hydrogen sulfide heat-treated under an argon atmosphere.69 But the reaction product contains not only MoS2 nanotubes but also some of the polyhedral particles. During the synthesis of MoS2 nanotubes, a trisulfide intermediate such as MoS3 was formed and it then decomposed to form MoS2.70 Nath et al. synthesized MoS2 nanotubes from amorphous MoS3 where MoS3 was initially prepared by treating ammonium thiomolybdate with hydrogen.71 MoS2 films were synthesized on a variety of substrates including oxidized Si (300 nm SiO2/Si), glassy carbon and quartz via thermal annealing using a horizontal tube furnace followed by sulfurization.72 A similar method was also used in the preparation of MoSe2 films where instead of sulfur powder, the selenium powder was used during the growth. Lin et al. have prepared MoS2 triangular monolayers on the SiO2/Si substrate at a low reaction temperature of 700 °C under an argon environment and at atmospheric pressure.73 Recently, hierarchical bamboo-like CoSe2 arrays on a carbon cloth substrate were synthesized by thermal annealing.74 The 2H-WS2 nanoladder was synthesized using precursors such as tungstic acid and thiourea by heating the mixture at a temperature of 600 °C for 5 h.75 WS2 nanosheets prepared hydrothermally using WCl6 and thioacetamide at a reaction temperature of 265 °C were single-crystalline in nature with an interplanar spacing of 0.62 nm.762.6 Solvothermal/hydrothermal method
The solvothermal/hydrothermal method is a viable method to synthesize TMDs on a large scale.78–80 Jiang et al. have reported the synthesis of in-plane 1T–2H phase hybridized MoS2 monolayers via the solvothermal route.97 It was observed that the hybridization helps in increasing the stability of the monolayers when compared with their pure 1T counter-parts and helps in obtaining highly stable 2D MoS2. MoS2 nanowires were synthesized hydrothermally using precursors such as MoO3 and Na2S in HCl solution at 260 °C.80 These MoS2 nanowires with a diameter of about 4 nm contain 1–10 sulfide layers. The concentration of HCl solution played a critical role in the synthesis of pure MoS2 nanowires. Geng et al. have prepared pure metallic multilayered MoS2via a hydrothermal route using MoO3, thioacetamide, and urea.98 The layer thickness of the as-synthesized pure metallic MoS2 nanosheets was 6.2 Å. Ammonia-ion intercalated 1T-WS2 was synthesized using the hydrothermal method.99 Ammonia-ion intercalated 1T-WS2 exhibited a ribbon-like morphology with a width of 50–200 nm and a thickness of 3–5 nm. The 2D MoSe2 nanosheets with microsphere morphology were prepared using the solvothermal method by chemically treating precursors such as molybdenum acetylacetonate and selenium powder. The size of the MoSe2 nanosheets so prepared varied from 70 to 200 nm.81 Huang et al. have hydrothermally prepared MoSe2 nanosheets with laminated structures by using sodium molybdate, hydrazine hydrate and selenium powder.82 The same strategy was adopted to prepare MoSe2 nanosheets with layered crystal structures where they used sodium molybdate, selenium powder and sodium borohydride and the nanosheets exhibit a porous structure with lattice fringes of 0.62 ± 0.03 nm.83 2D MoSe2 was prepared by employing the solvothermal method using precursors such as sodium molybdate/ammonium para-molybdate and selenium powder.84 2D SnS2 nanosheets prepared hydrothermally using tin(IV)bis(acetylacetonate) dichloride and Na2S have a thickness of about 30–40 nm.85 Carnation flower-like SnS2 nanosheets prepared solvothermally using tin chloride and thioacetamide have a petal-thickness of ∼38–41 nm.86 Masikhwa synthesized VS2 nanosheets hydrothermally using precursors such as ammonium metavanadate, thioacetamide and ammonia at a reaction temperature of 180 °C for a period of 20 h.87 Ultrathin VS2 nanoplates composed of 4–5 layers with a thickness of about 2.64 nm were synthesized from vanadyl(III) acetylacetonate and oleylamine precursors.88 The hydrothermal method was used to synthesize WS2 nanoparticles using Na2WO4·2H2O, urea and thiourea as precursors and the average diameter of the nanoparticles ranged from 11.6–26.7 nm.78 Liu et al. have hydrothermally synthesized 2H-WS2 nanosheets on the carbon cloth substrate using sodium tungstate dehydrate, WCl3, thioacetamide, hydrogen peroxide and hydrochloric acid at a reaction temperature of 300 °C for 2 h.89 Few-layered ultrathin VS2 nanosheets were synthesized using the hydrothermal method in which the as-prepared nanosheets were metallic in nature.473 Pristine TMD based supercapacitor electrodes
TMDs are layered materials having van der Waals crystal structures akin to graphite and show great promise as supercapacitor electrode materials owing to the remarkably wide range of oxidation states associated with transition metals. This renders excellent Faradaic charge storage in these materials. Edge-oriented TMDs are more intriguing as compared to basal plane dominated nanostructures as they expose a large density of electrochemically active sites and open van der Waals gaps for large inter-layer diffusion/intercalation of the electrolyte ions. The very first report on monolithic TMD based supercapacitors is the CVD grown edge-oriented MoS2 films which exhibited excellent electrochemical properties and achieved a very high capacitance of 70 mF cm−2 (with a gravimetric capacitance of 100 F g−1) at a scan rate of 1 mV s−1 in the 0.5 M H2SO4 electrolyte.100 The electrochemical performance of these MoS2 films exceeded many of the carbon nanomaterial-based supercapacitor electrodes as their capacitance was mainly attributed to Faradaic processes rather than the EDL mechanism. Microsupercapacitors fabricated by spray painting of MoS2 followed by laser patterning exhibited a high area capacitance of 8 mF cm−2.101 Edge-oriented MoS2 obtained by electrochemical anodization of the Mo metal in the presence of sulfur vapor exhibits a capacitance up to 14.5 mF cm−2 using galvanostatic charge–discharge (GCD) measurements at a current density of 1 mA cm−2 and 12.5 mF cm−2 obtained from CV measurements at a scan rate of 50 mV s−1.102 MoS2 has common drawbacks of poor electrical conductivity due to a larger band gap and it undergoes volume change during cycling. To overcome these drawbacks, Sun et al. hydrothermally synthesized oxygen incorporated MoS2 (O-MoS2) microspheres with a tunable interior structure.103 Among the different microsphere structures synthesized by them, hollow microspheres exhibited a large surface area of 143.9 m2 g−1, which is essential for large charge storage. Also, the incorporation of oxygen into MoS2 increased the intrinsic conductivity by lowering the bandgap from 1.8 to 1.3 eV and increased the interlayer spacing up to 9.8 Å favoring faster electrolyte diffusion. These optimizations synergistically made O-MoS2 deliver a specific capacitance of 744.2 F g−1 at a current density of 1 A g−1 with over 77% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
WS2 is another interesting supercapacitor electrode material with a layered structure and high capacitance. However, it suffers from poor conductivity and shorter life. To improve the durability of WS2, hydrothermally grown WS2 nanosheets on carbon fibers were calcined at 300 °C.104 The calcination step improved the crystallinity of the nanosheets developed, as evident from HRTEM and XRD data. Calcined WS2 exhibited a specific capacitance of 211 F g−1 at a current density of 4 mA cm−2 with enhanced cycling stability (85.6% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) compared to uncalcined WS2. WS2 nanoplates well dispersed on carbon fiber cloth (CFC) were synthesized using the solvothermal method by Shang et al.105 The 3D framework of the CFC prevents aggregation of nanoplates and offers a very low charge transfer resistance (Rct) of 0.1 Ω which helps in a faster ion transfer. WS2/CFC delivers a high specific capacitance of 399 F g−1 at a current density of 1 A g−1 retaining 99% capacitance after 500 cycles. Developing the TMDs on such a substrate would also allow us to develop wearable supercapacitors which are in demand for wearable electronics. As one of the key factors for an ideal supercapacitor electrode is a large surface area and high density of active sites, synthesizing these TMDs as quantum dots (QDs) will be a smart approach.106,107 Following this idea, WS2 quantum dots (QD) with a size of ∼2.2 nm were synthesized using the hot injection method to expose more edge atoms for enhanced electrochemical activity.107 Restacking issues associated with WS2 can be resolved by capping them with organic groups using ethanedithiol. The CV measurements done on the QDs showed redox behavior with possible conversion between W6+ and W4+ states of WS2. The specific capacitance of capped QDs showed a high value of 457 F g−1 compared to only 151 F g−1 obtained for uncapped QDs. The capping agent improved the cycle life of the WS2 QDs with increased capacitance at the end of 8000 cycles with no capacitance decay.
000 cycles) compared to uncalcined WS2. WS2 nanoplates well dispersed on carbon fiber cloth (CFC) were synthesized using the solvothermal method by Shang et al.105 The 3D framework of the CFC prevents aggregation of nanoplates and offers a very low charge transfer resistance (Rct) of 0.1 Ω which helps in a faster ion transfer. WS2/CFC delivers a high specific capacitance of 399 F g−1 at a current density of 1 A g−1 retaining 99% capacitance after 500 cycles. Developing the TMDs on such a substrate would also allow us to develop wearable supercapacitors which are in demand for wearable electronics. As one of the key factors for an ideal supercapacitor electrode is a large surface area and high density of active sites, synthesizing these TMDs as quantum dots (QDs) will be a smart approach.106,107 Following this idea, WS2 quantum dots (QD) with a size of ∼2.2 nm were synthesized using the hot injection method to expose more edge atoms for enhanced electrochemical activity.107 Restacking issues associated with WS2 can be resolved by capping them with organic groups using ethanedithiol. The CV measurements done on the QDs showed redox behavior with possible conversion between W6+ and W4+ states of WS2. The specific capacitance of capped QDs showed a high value of 457 F g−1 compared to only 151 F g−1 obtained for uncapped QDs. The capping agent improved the cycle life of the WS2 QDs with increased capacitance at the end of 8000 cycles with no capacitance decay.
High redox activity and multiple valence states make CoS2 a promising electrode candidate for energy storage. Uniform and octahedron-shaped single crystalline CoS2 synthesized via the hydrothermal process offered a specific capacitance of 236.5 F g−1 at a current density of 1 A g−1.108 The CoS2 electrodes had a capacitance loss of 7.4% after 2000 charge–discharge cycles. CoS2 ellipsoids with tube-like cavities were developed via thermal decomposition followed by sulfidation.109 The ellipsoids are formed with an opening on both ends along with rough and porous structures as shown in Fig. 4a and b offering a high surface area for charge storage. The CV measurement on CoS2 ellipsoids showed a typical pseudocapacitive behavior which comes from the Co2+/Co3+ redox couple. The GCD curves were symmetrical showing a good capacitive behavior (Fig. 4c) and the pseudocapacitive charge storage provides a good capacitance of 1040 F g−1 at a current density of 0.5 A g−1.
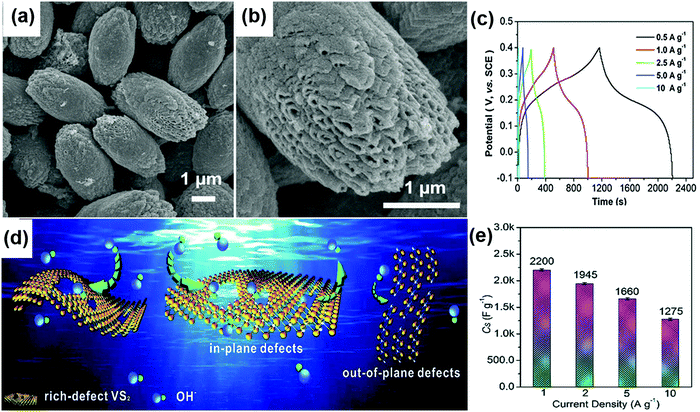 | ||
| Fig. 4 (a and b) FESEM images of CoS2 ellipsoids with anisotropic tube-like cavities and (c) GCD curves at various current densities. Reproduced with permission from ref. 109 Copyright (2012) Royal Society of Chemistry. (d) Schematic illustrating the redox reactions occurred on rich-defect VS2 nanoplates. (e) Specific capacitance of VS2 nanoplates at different current densities. Reproduced with permission from ref. 88 Copyright (2012) Royal Society of Chemistry. | ||
Carnation flower-like SnS2 electrode-based supercapacitors showed excellent electrochemical properties with a specific capacitance of 524.5 F g−1 and a power density of 12.3 W kg−1 at a current density of 0.08 A g−1.86 At a high current density of 0.38 A g−1, the said supercapacitor exhibited a specific capacitance of 215.9 F g−1 with a power density of 61.4 W kg−1. Supercapacitors assembled with 2D TiS2 nanocrystal-based electrodes exhibited a specific capacitance of 320 F g−1.43 TiS2 nanodisc electrode-based supercapacitors provided a specific capacitance of 70 F g−1 when tested in a 6 M KOH electrolyte. Guo et al. prepared ultrathin VS2 nanoplates with in-plane and out-of-plane defects via colloidal chemical synthesis. The presence of so many defects enhances the specific surface area and exposes more active sites for redox reactions to occur (Fig. 4d). They can deliver up to a high capacitance value of 2200 F g−1 as shown in Fig. 4e.88
Selenide based TMDs are electrically more conductive than sulfide-based ones. Molybdenum diselenide (MoSe2) is a TMD similar in structure to MoS2 with Se–Mo–Se stacked atom layers bonded together by van der Waals forces. Their structure is similar to graphene with very high inherent electrical conductivity. This makes them a promising candidate for energy storage.110 Hydrothermally prepared MoSe2 nanosheet electrodes exhibited a specific capacitance of 198.9 F g−1. The symmetric device assembled using these electrodes exhibited a specific capacitance of 49.7 F g−1.83 MoSe2 has a 2D sheet-like structure, and this provides a high surface area along with more active sites for the faster reversible redox reaction. The MoSe2 nanosheet electrode-based supercapacitor showed good capacitance retention of about 75% even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles when cycled at a current density of 5 A g−1. Hierarchical MoSe2 spheres prepared via a simple hydrothermal route were interlaced together making interconnected channels for faster charge transfer.111 This kind of hierarchical structure is essential for attaining faster ion diffusion from the electrolyte to all electrode surfaces. These MoSe2 spheres delivered a capacitance of 243 F g−1 at a current density of 0.5 A g−1 with a rate capability of 60% even at 15 A g−1. Another work involving hydrothermal preparation of the MoSe2 microsphere hierarchical structure composed of 2D nanosheets exhibited a specific capacitance of 272 F g−1 at a current density of 1 A g−1. The MoSe2 nanosheets prepared on the Ni-foam substrate using the hydrothermal method showed excellent electrochemical performance with a specific capacitance of 1114.3 F g−1 and the capacitance retained is about 104.7% after 1500 cycles.82 This high value of capacitance achieved would be because of the sieve-like feature of MoSe2 grown on the Ni foam creating pores for easy access of ions to the entire surface.
000 cycles when cycled at a current density of 5 A g−1. Hierarchical MoSe2 spheres prepared via a simple hydrothermal route were interlaced together making interconnected channels for faster charge transfer.111 This kind of hierarchical structure is essential for attaining faster ion diffusion from the electrolyte to all electrode surfaces. These MoSe2 spheres delivered a capacitance of 243 F g−1 at a current density of 0.5 A g−1 with a rate capability of 60% even at 15 A g−1. Another work involving hydrothermal preparation of the MoSe2 microsphere hierarchical structure composed of 2D nanosheets exhibited a specific capacitance of 272 F g−1 at a current density of 1 A g−1. The MoSe2 nanosheets prepared on the Ni-foam substrate using the hydrothermal method showed excellent electrochemical performance with a specific capacitance of 1114.3 F g−1 and the capacitance retained is about 104.7% after 1500 cycles.82 This high value of capacitance achieved would be because of the sieve-like feature of MoSe2 grown on the Ni foam creating pores for easy access of ions to the entire surface.
Nickel diselenide (NiSe2), another emerging and promising TMD electrode material with multiple oxidation states and tunable electronic configuration has recently been used in supercapacitors.112,113 The first instance of employing NiSe2 as a supercapacitor electrode material is the hydrothermal synthesis of hexapod like NiSe2 made up of nanoparticles with a size of ∼30 nm.114 NiSe2 nanoparticles delivered a maximum specific capacitance of 75 F g−1 at a scan rate of 2 mV s−1 with charges being stored via both EDLC and pseudocapacitance as evident from their quasi-rectangular shaped CV curves. They also possessed excellent cycling stability retaining 94% capacitance even after cycling at 100 mV s−1 for 5000 times. In another work, single crystal NiSe2 cubes were synthesized using a solvothermal method.115 Structural characterizations showed that NiSe2 was formed as truncated cubes with smooth surfaces and an edge length of 100 to 400 nm. The polyhedral structure enhances the electrochemical performance by providing abundant active sites for charge storage and also helping in easy access of electrolyte ions. CV studies showed a pseudocapacitive nature of NiSe2 crystals and GCD curves exhibited symmetric behavior indicating good chemical reversibility. The NiSe2 electrode exhibited a good specific capacitance of 1044 F g−1 at a current density of 2 A g−1 with ∼60% rate capability even at a higher current density of 30 A g−1. Though the NiSe2 crystals showed a promising capacitance value, they suffered from stability as they lost 33% of the initial capacitance after 2000 cycles because of the change in their morphology from cubes to agglomerated particles. This change in morphology can be controlled by forming a composite with carbon-based materials which can effectively improve the capacitance and also enhance the cycle life. An interesting approach of developing NiSe2 as a flexible electrode was accomplished by Bao et al.116 NiSe2 nanosheet arrays were deposited electrochemically on a 3D carbon fiber cloth, and these nanosheets were well connected offering a continuous charge transfer. The NiSe2 electrode with a mass loading of 1.1 mg delivered a maximum capacitance of 1058.5 F g−1 at a current density of 2 A g−1 with a rate capability of ∼94% at 10 A g−1. This remarkable rate capability was due to the low internal and charge transfer resistance of 1.5 Ω and 2.2 Ω respectively.
4 TMD-based hybrid electrodes
Pristine TMD based electrodes have disadvantages such as poor electronic conductivity, poor cycling stability, and low surface area, etc. In order to overcome these limitations, hybrid electrodes are prepared with one or more different electrode-active materials along with pristine TMDs during the electrode fabrication.117–119 Rationally designing TMD nanostructures in various form factors such as nanowires, nanorods, nanobelts, core/shell nanostructures or 3D nanoflower type structures has been a successful strategy to maximize their effective surface area. However, to attain good electronic conductivity and stability, it is mandatory to add secondary electrode-active materials. Combining 2D TMDs with a variety of electrochemically active organic or inorganic materials to synthesize hybrid materials is one of the prudent approaches to mitigate the intrinsically low electrical conductivity and low surface area of pristine TMDs.120–124 The secondary electrode-active material used in the preparation of hybrid electrodes helps to maximize their surface area utilization which is prevented due to the restacking of TMD layers when used in their monolithic form. These hybrids have been accomplished using a variety of top-down (mechanical exfoliation, liquid exfoliation) and bottom-up (hydrothermal, solvothermal, CVD) synthetic routes as well as a combination of these methods.125–129Nanostructured carbonaceous materials are the most promising electrode candidates in supercapacitors due to their unique features such as high electrical conductivity, large surface area, good chemical and electrochemical stability, environment-friendliness, etc.130–132 Hence, they are widely used as conductive additives providing large-surface area for the preparation of composite electrodes.133–137 Therefore, carbon nanomaterials are suitable candidates to increase the electrical conductivity as well as the electrochemical performance while using them to prepare hybrid electrodes with 2D TMDs.138–142
Undoubtedly, MoS2 is the most widely studied TMD for supercapacitors, but other TMDs based on different transition metals and other chalcogens are gaining significant attention. For example, WS2 shows even higher intrinsic conductivity compared with MoS2. To increase the active surface area and the density of the edge sites of MoS2, several hydrothermal procedures have been adopted to synthesize different nanostructures of MoS2 and utilize them as electrode materials in supercapacitors. To fully magnify this edge, 2D MoS2 is often synthesized with amorphous carbon, conductive polymers, and metal oxide decoration to do the surface-treatment. Their geometrical likeliness to the graphene structure suggests possible performance enhancements by forming stable composites with graphene. TMDs as semiconductors could then benefit from the excellent electronic conductivity of carbon nanomaterials such as carbon nanotubes (CNTs) and graphene.120,143–146 For example, MoS2/multi-walled CNT (MWNT) composites fabricated using a facile hydrothermal method resulted in flowerlike MoS2 nanosheets wrapping around MWNTs to form a 3D nanoarchitecture.147 These electrodes exhibited a high specific capacitance of 452.7 F g−1 and maintained ∼95% of the initial capacitance after 1000 cycles.
Recently, a facile and cost-effective successive ionic layer adsorption and reaction method has been reported to synthesize a hierarchical core/shell nanostructure. In this structure, the VS2 nanoparticles were decorated onto the MWNT matrix core.148 These electrodes demonstrate a very high capacitance of 830 F g−1 and excellent cycle life with 95.9% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The extraordinary performance of these electrodes is attributed to the modified surface architecture of MWCNTs using VS2 which not only promotes a fast Faradic charge transfer but also establishes a highly conductive VS2/MWCNT interface. The remarkable performance in these composites is attributed to the 3D design with a conductive network which promotes fast and efficient charge transport and prevents the volume expansion during charging and discharging cycles. However, in several instances, 2D TMDs have been found to easily detach from the CNT scaffold network during the fast charge/discharge process, resulting in poor cycling stability.149,150 In this regard, reduced graphene oxide (rGO) has been considered as a better carbon host because of its 2D structural similarity with TMDs providing better stability due to intimate 2D/2D atomic hetero-interfaces. In addition, rGO offers a large surface area, excellent electronic conductivity, and porosity for better electrochemical performance in TMD/RGO composites.76,151,152 A binder-free 3D flexible supercapacitor electrode was constructed by coating a few layers of rGO nanosheets via vacuum filtration onto a hydrothermally grown MoS2@CNT composite network template (Fig. 5a).143Fig. 5b shows the representative SEM image of the MoS2@CNT/rGO hybrid showing a uniform distribution of MoS2 nanoflowers onto CNTs. This hybrid electrode yields a high areal capacitance of 129 mF cm−2 at a current density of 0.1 mA cm−2. The cyclic voltammetry (CV) as shown in Fig. 5c indicates a significant enhancement in the capacitive performance of the MoS2@CNT/rGO hybrid as compared to pristine CNT, MoS2@CNT or MoS2/rGO electrodes. The observed electrochemical behavior of the MoS2@CNT/rGO electrodes is mainly attributed to the interconnected 3D porous network structure and high toughness/stability induced by the rGO conductive coating. Moreover, this electrode endures 10
000 cycles. The extraordinary performance of these electrodes is attributed to the modified surface architecture of MWCNTs using VS2 which not only promotes a fast Faradic charge transfer but also establishes a highly conductive VS2/MWCNT interface. The remarkable performance in these composites is attributed to the 3D design with a conductive network which promotes fast and efficient charge transport and prevents the volume expansion during charging and discharging cycles. However, in several instances, 2D TMDs have been found to easily detach from the CNT scaffold network during the fast charge/discharge process, resulting in poor cycling stability.149,150 In this regard, reduced graphene oxide (rGO) has been considered as a better carbon host because of its 2D structural similarity with TMDs providing better stability due to intimate 2D/2D atomic hetero-interfaces. In addition, rGO offers a large surface area, excellent electronic conductivity, and porosity for better electrochemical performance in TMD/RGO composites.76,151,152 A binder-free 3D flexible supercapacitor electrode was constructed by coating a few layers of rGO nanosheets via vacuum filtration onto a hydrothermally grown MoS2@CNT composite network template (Fig. 5a).143Fig. 5b shows the representative SEM image of the MoS2@CNT/rGO hybrid showing a uniform distribution of MoS2 nanoflowers onto CNTs. This hybrid electrode yields a high areal capacitance of 129 mF cm−2 at a current density of 0.1 mA cm−2. The cyclic voltammetry (CV) as shown in Fig. 5c indicates a significant enhancement in the capacitive performance of the MoS2@CNT/rGO hybrid as compared to pristine CNT, MoS2@CNT or MoS2/rGO electrodes. The observed electrochemical behavior of the MoS2@CNT/rGO electrodes is mainly attributed to the interconnected 3D porous network structure and high toughness/stability induced by the rGO conductive coating. Moreover, this electrode endures 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles with 97.7% capacitance retention. In another report, highly capacitive WS2 nanosheets were combined with highly conducting RGO by using a molten salt method to construct paper electrodes.153 The perfect 2D/2D hybrid nanostructure represents the high synergy of the large Faradaic charge storage in WS2 active sites and fast charge transferability in rGO nanosheets, accomplishing a high specific capacitance of 2508.07 F g−1 at a scan rate of 1 mV s−1. Moreover, this hybrid electrode provided an excellent cyclic stability of 98.6% capacitance after 5000 cycles without any loss in the coulombic efficiency.
000 charge/discharge cycles with 97.7% capacitance retention. In another report, highly capacitive WS2 nanosheets were combined with highly conducting RGO by using a molten salt method to construct paper electrodes.153 The perfect 2D/2D hybrid nanostructure represents the high synergy of the large Faradaic charge storage in WS2 active sites and fast charge transferability in rGO nanosheets, accomplishing a high specific capacitance of 2508.07 F g−1 at a scan rate of 1 mV s−1. Moreover, this hybrid electrode provided an excellent cyclic stability of 98.6% capacitance after 5000 cycles without any loss in the coulombic efficiency.
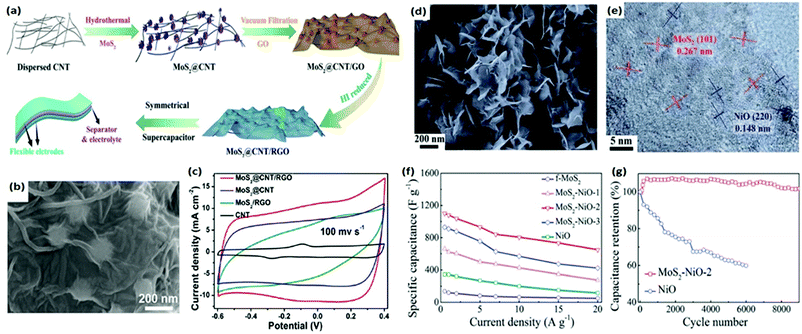 | ||
| Fig. 5 (a) Schematic showing the synthesis steps for MoS2@CNT/rGO electrodes, (b) SEM images of the as-synthesized 3D MoS2@CNT/rGO hybrid, and (c) CV results comparing the electrochemical performance of CNT, MoS2/rGO, MoS2@CNT, and MoS2@CNT/rGO electrodes. Reprinted with permission from ref. 143 Copyright (2017) John Wiley and Sons. (d) SEM and (e) TEM images of the MoS2–NiO hybrid, (f) specific capacitance comparison of MoS2–NiO with pure NiO and MoS2 at various current densities and (g) cyclic stability of MoS2–NiO with pristine NiO at 2 A g−1. Reprinted with permission from ref. 154 Copyright (2017) the Royal Society of Chemistry. | ||
Transition metal oxides (MOs) and hydroxides are other promising additives which substantially enhance the capacitive performance of TMDs via the Faradaic charge storage mechanism in which fast and reversible redox reactions occur in the electrodes.154 Ni, Co, and Fe-based oxides have been preferred as emerging supercapacitor electrode materials replacing traditional SnO2 and RuO2 due to their fast-redox kinetics, environment friendliness, and cost-effectiveness.155–157 For example, 2D MoS2 nanosheets were recently integrated with various porous MOs such as NiO, Co3O4, and Fe2O3 to form hybrids using a simple, scalable solvent-exchange method.158–161Fig. 5d and e show the SEM and TEM images of the as-prepared MoS2–NiO hybrids clearly showing uniform confinement of the NiO nanosheets on MoS2 nanosheets. The MoS2–NiO electrode exhibits good electrochemical performance due to its Faradaic charge storage with a specific capacitance of 1080 F g−1 (Fig. 5f) and good cycle life of >100% capacitance retention after 9000 cycles at a current density of 2 A g−1 (Fig. 5g).158
Another TMD of great interest due to its low cost, high chemical stability, and environmental friendliness is tin disulfide (SnS2). When SnS2 is made into a composite with SnO2, the energy storage is enhanced by better electrochemical properties. A SnS2–SnO2 nanostructure obtained using the solvothermal method achieved a capacitance of 149 F g−1 at a current density of 2 A g−1, which was higher than pristine SnS2.162 Fang et al. synthesized a novel cauliflower like ZnO/VS2 nanocomposite via a wet chemical method for a supercapacitor electrode.163 The ZnO which was formed in situ as nanospheres on the VS2 nanosheets effectively prevented the restacking. This helped in achieving a very high specific capacitance of 2695.7 F g−1 at a current density of 1 A g−1. This nanocomposite had a 3D structure which accommodates the volume change during the cycling due to which good cycle stability of 92.6% retention was achieved after 5000 cycles.
Electronically conductive polymers are pseudocapacitive materials with good electronic conductivity and large surface area that provide short diffusion paths for ions/electrons when mixed with TMD layers.164–167 2D TMDs, in turn, offer high mechanical stability to electronically conducting polymers and significantly mitigate the poor cycle life endurance inherent in the pristine electronically conducting polymer-based supercapacitor electrodes. In situ polymerization of electronically conducting polymers with 2D TMDs has commonly been pursued to develop these hybrids.168,169 For example, 2D MoS2/PPy hybrids prepared using this method showed a very high capacitance of ∼700 F g−1 at 10 mV s−1, which is unprecedented in any of the previously reported supercapacitors using pristine PPy electrodes.170 In addition, these hybrids yield a very high energy density of 83.3 W h kg−1. Moreover, the MoS2/PPy nanocomposite electrode maintains 85% of its initial capacitance whereas the pristine PPy electrode decays to 50% after 4000 continuous charge/discharge cycles. In another study,171 2D MoS2/PANI nanoneedle arrays presented remarkable cycling stability showing 91% of the initial capacitance after 4000 cycles, while delivering a high energy density of 106 W h kg−1 and a capacitance of 669 F g−1 at 1 A g−1. The excellent electrochemical performance in this hybrid is attributed to the MoS2/PANI architecture in which atomically thin sheets of MoS2 act as a charge reservoir allowing the access of significant electrolyte ions into its 2D layers, while the PANI nanoneedle architecture facilitates the electron transport and accommodates the strain in MoS2 caused by the insertion/extraction of electrolyte ions. This results in high capacitance, rate capability, and stability.
A direct deposition of 3D porous MoS2 films on the flexible Cu foil and polyimide substrates using the sputtering technique has recently provided a novel electrode architecture possessing a large surface area and high density of active edge sites.172 It exhibits an excellent capacitance of 330 F cm−3 and retained over 97% capacitance after 5000 cycles. The high capacitance and stability in this electrode are attributed to the 3D open pore structure and good adhesion and contact of MoS2 with the current collector. Rational carbon materials and polymer architectures act as excellent backbone structures for the growth of novel TMD designs while providing highly conductive networks/pathways. For example, aligned CNTs used as templates enabled the growth of few-layered MoS2 uniformly wrapped around them.150 The hybrid design leverages the high electrical conductivity in CNTs and high energy storage capacity in MoS2 to exhibit an overall specific capacitance of 135 F cm−3. Moreover, the CNT/MoS2 hybrid sheet-based supercapacitor electrodes were twisted in the form of a fiber which retained 95% capacitance even after 1000 bending cycles, which is an indication of high mechanical stability and strong interaction between the constituent materials of the hybrid. Flower-like MoS2 nanostructures fabricated on the 3D graphene skeleton exhibited a high capacitance of 410 F g−1 and excellent cycling stability, i.e. 80.3% capacitance retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous cycles.173
000 continuous cycles.173
Singh et al. reported the synthesis of graphene/MoS2 nanoflower hybrids used as electrodes in supercapacitors.174 The solid-state supercapacitor assembled using the graphene/MoS2 nanoflower hybrids achieved a specific capacitance of 58 F g−1 with an energy density of 24.59 W h kg−1 and a power density of 8.8 W kg−1. A rational 3D tubular MoS2/PANI hybrid electrode in which hydrothermally prepared 3D MoS2 suspensions were uniformly covered by PANI nanowire arrays via in situ oxidative polymerization not only showed a high specific capacitance of 552 F g−1 but also exhibited an improved rate capacitance of 82% at 30 A g−1 and a capacitance retention of 88% after 1000 cycles.175 The excellent capacitive performance and stability in these nanostructures are mainly attributed to the novel 3D construction of tubular electrodes which provides better insertion/extraction pathways to the electrolyte ions as well as accommodates the expansion/contraction of the PANI nanowire during the charge–discharge process. It has been observed that novel supercapacitor electrode architectures made of amorphous TMDs show unexpectedly high capacitive performances as compared to crystalline TMDs as they are capable of accommodating high volume change along with exhibiting better redox activity.176 A core/shell nanosphere architecture fabricated using a highly conductive Ni3S4 core and an amorphous MoS2 shell via one-pot synthesis demonstrated an impressive specific capacitance of 1440.9 F g−1 at 2 A g−1 and retained 90.7% capacitance after 3000 cycles at a scan rate of 10 A g−1.177 In a recent report,178 a high areal capacitance of 83.9 mF cm−2 was achieved in 3D electrodes made of amorphous MoSx thin films coated on carbon nanofiber papers using the facile hydrothermal method.
Core–shell nanostructures with a highly conducting core and large surface area/porous shells are intriguing for high-performance supercapacitors as they offer fast conduction of charge carriers, high mechanical stability during volume expansion, and enhanced electrode/electrolyte interfaces. Choudhary et al. reported a novel strategy of sequential oxidation/sulfurization in which a highly conducting tungsten foil was chemically converted to a tungsten trioxide (WO3) nanorod core with few-layered WS2 shells forming chemically self-assembled core/shell nanowire structures.179Fig. 6a represents the schematic of a one-body array of a h-WO3/WS2 core/shell nanowire supercapacitor. A digital image of the WO3/WS2 core/shell nanowire on a tungsten foil under bending conditions (Fig. 6b) shows its high flexibility. The SEM images of the nanowires (Fig. 6c) reveal a high density of nanowires with atomically sharp interfaces. The near rectangular nature of the CV curves (Fig. 6d) with a large area under the curve and symmetric GCD curves (Fig. 6e) of the solid-state supercapacitor show its excellent charge storage capability. The high mechanical stability in these core/shell nanowire electrodes leads to unprecedented cycling stability with zero loss of capacitance even after 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. Moreover, these electrodes could be bent at different bending angles without any significant change in their electrochemical performance (Fig. 6f), suggesting their ability to be used in flexible devices. Another interesting approach uses a highly conducting carbon/nickel template to grow a hierarchically double core/shell architecture of C@Ni3S2@MoS2 nanorods.180 The C@Ni3S2@MoS2 supercapacitor electrodes show a specific capacitance of 1544 F g−1 at a current density of 2 A g−1 and a capacitance retention of 92.8% after 2000 cycles at a high current density of 20 A g−1.
000 cycles. Moreover, these electrodes could be bent at different bending angles without any significant change in their electrochemical performance (Fig. 6f), suggesting their ability to be used in flexible devices. Another interesting approach uses a highly conducting carbon/nickel template to grow a hierarchically double core/shell architecture of C@Ni3S2@MoS2 nanorods.180 The C@Ni3S2@MoS2 supercapacitor electrodes show a specific capacitance of 1544 F g−1 at a current density of 2 A g−1 and a capacitance retention of 92.8% after 2000 cycles at a high current density of 20 A g−1.
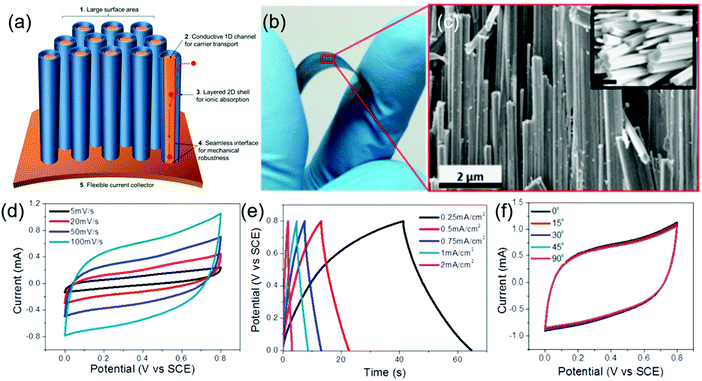 | ||
| Fig. 6 (a) Schematic of one-body array of h-WO3/WS2 core/shell nanowire supercapacitors, (b) digital image of h-WO3/WS2 core/shell nanowires on tungsten foil under bending conditions, and (c) SEM image of well-aligned core/shell nanowires (the inset shows the SEM image of nanowires with their faceted surface, scale bar: 500 nm); (d) CV curves at different scan rates, (e) GCD curves at different current densities, and (f) CV curves at different bending angles of the solid-state core/shell supercapacitor prepared with the Na2SO4 gel electrolyte. Reprinted with permission from ref. 179 Copyright (2016) American Chemical Society. | ||
5 Futuristic metallic 2D TMDs
Most of the 2D TMDs exist in their thermodynamically stable hexagonal (2H) phase which is semiconducting and exhibits poor electronic conductivity, and is thus detrimental to their use as first-choice supercapacitor electrodes. This urged scientists to add conductive carbon materials or CPs to TMDs to make them efficient nanocomposite electrode structures. However, the complexities in fabricating good quality composites such as weak interfacial adhesion, poor connectivity between the constituent materials, and use of binders make these electrodes/devices cumbersome and incompetent. Very recently, with the use of metallic TMDs, a metastable 1T phase has emerged as an alternative high-performance material due to its unprecedented electronic and electrochemical properties.181–184The Chhowalla group reported 107 higher electronic conductivity in 1T MoS2 phases compared with their 2H semiconducting counterparts.91 They prepared binder free 1T MoS2 film electrodes by chemical exfoliation of MoS2 nanosheets via lithium intercalation and tested their electrochemical performance in various aqueous and organic electrolytes. Fig. 7a shows the CV curves of the 1T phase of the MoS2 nanosheet paper in different electrolyte solutions at a scan rate of 20 mV s−1. The GCD curves (Fig. 7b) show good rate capability and exhibit high volumetric capacitances (Fig. 7c) in sulfate-ion based electrolyte solutions. The cycle life of 1T MoS2 nanosheet electrodes also showed a long cycle life with nearly 100% capacitance retention (Fig. 7d). The stacked 1T MoS2 nanosheet electrodes could electrochemically intercalate a wide range of electrolyte ions such as H+, Li+, Na+, and K+ and a schematic representing the same is depicted in Fig. 7e. When used in organic media, the 1T MoS2 electrode shows a high operation voltage of 3.5 V with a high coulombic efficiency of 95% over 5000 cycles. The unprecedented electrochemical performance exhibited by 1T MoS2 is attributed to its high hydrophilicity and superior electrical conductivity. Despite superior electrochemical performance shown by 1T-MoS2, the use of hazardous Li metal and the presence of the impurity phase during synthesis as well as the reversibility of the 1T to 2H phase cannot be denied. In this regard, a simple and safe hydrothermal method has been employed to produce few-layered metallic MoS2 nanosheets using water as a solvent.98 The as-prepared MoS2 electrodes demonstrate a high specific capacitance of 380 F g−1 at 5 mV s−1. Interestingly, the symmetric supercapacitor based on these electrodes yields a high energy and a power density of 51 W h kg−1 and 1000 W kg−1, respectively. Using a similar synthesis method, metallic 1T WS2 nanoribbon electrodes have been shown to exhibit 12 times higher capacitance (2813 mF cm−2) compared to semiconducting 2H-phase WS2.99 Metallic ultrathin VS2 nanosheet based electrodes are found to be potential candidates for in-plane supercapacitor devices and a high area specific capacitance of 4760 μF cm−2 was obtained without any degradation even after 1000 charge–discharge cycles.47
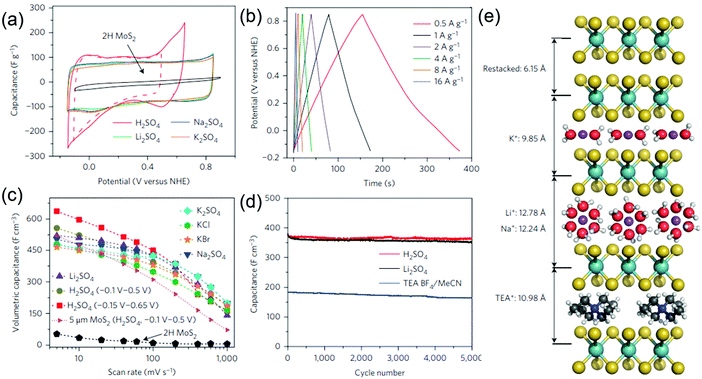 | ||
| Fig. 7 (a) CV curves of 1T phase MoS2 nanosheet paper in different electrolyte solutions at 20 mV s−1, (b) GCD curves at different current densities in K2SO4, (c) volumetric capacitance of 1T MoS2 as a function of scan rate in different electrolytes, (d) cycling stability in various electrolytes, and (e) schematics showing the intercalated and non-intercalated 1T MoS2 restacked nanosheets. Reprinted with permission from ref. 91 Copyright (2015) Macmillan Publishers Limited. | ||
Motivated by the excellent electrochemical performance in metallic 2D TMDs, several studies have been carried out by making them as a composite with other capacitive materials.92,185,186 A coin cell supercapacitor constructed from a 1T-MoS2 and graphene composite structure achieved a volumetric capacitance of 560 F cm−3 in an aqueous electrolyte and a capacitance retention of about 90% after 5000 cycles.92 Supercapacitor electrodes composed of mixed 2D TMD phases such as electrically conducting 1T with a highly stable 2H phase are expected to show interesting electrochemical properties due to combined effects of a large surface area as well as high electron/ion transport, which are the two pre-requisites for any high-performance supercapacitor electrode. Jiang et al. reported about 100 times enhancement in capacitance of MoS2 based supercapacitors fabricated by optimizing in-plane 1T–2H phase hybridization of the monolayers.97 In another report,185 mixed phase MoS2 nanosheets were assembled with rGO to make a hybrid aerogel electrode with a specific capacitance of 416 F g−1 at 1 A g−1. Moreover, these electrodes in a planar symmetric supercapacitor configuration showed no loss in capacitance up to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
VS2 is another promising supercapacitor electrode candidate due to its metallic nature and easy exfoliation of its 2D layers stacked up via weak van der Waals forces. Feng et al. assembled the VS2 nanosheets to fabricate in-plane supercapacitors which exhibited a specific capacitance of 4760 μF cm−2 with no loss in capacitance after 1000 charge/discharge cycles.187 The VS2 nanosheets have also manifested impressive performance as supercapacitor electrodes for energy-related applications. The high capacitive performance in the VS2 nanostructures is believed to have originated from their ultrahigh quantum capacitance. Supercapacitors assembled with 1T VS2 nanosheets exhibited a maximum specific capacitance of 860 F g−1 and the supercapacitors utilizing different electrolytes such as H2SO4, Li2SO4, Na2SO4 and K2SO4 showed superior cycling stabilities when tested for 1000 cycles.57 Thus, the high electrical conductivity with a significant electrochemical intercalation process (as shown schematically in Fig. 8a) leads to high Faradaic charge storage in VS2. Highly crystalline VS2 nanosheets of thickness below 10 nm with domain sizes of tens of micrometers were prepared using the CVD method.57 These nanoflakes were dispersed on glassy carbon substrates to test their electrochemical performance in 0.5 M sulfate electrolyte solutions. Moreover, the VS2/glassy carbon electrodes exhibited a high rate performance by retaining 200 F g−1 of initial capacitance even at a high scan rate of 200 mV s−1 as shown in Fig. 8b. A specific capacitance as high as 860 F g−1 was recorded at a scan rate of 5 mV s−1 (Fig. 8c), which is comparable to high-performance metallic nanosheets of 1T MoS2.91 The VS2/glassy carbon electrodes exhibited good cycling stability with good rate capability in different sulfate based electrolytes as shown in Fig. 8d. Metallic 1T-VSe2 is another interesting emerging electrode material with high conductivity and huge surface area for charge storage. High conductivity emerges from the atom plane with strong electron coupling of the V–V atom network. The CVD method was followed to develop 1T VSe2 nanosheets for a flexible, in-plane solid-state supercapacitor.188 The solid-state supercapacitor built on the PET substrate with PVA/KNO3 retained its rectangular shaped CV curve up to 20 V s−1 revealing its dominant EDLC behavior. Also, the linear relationship of the discharge current density showed a high-power capability of the device. A maximum specific capacitance of 4.17 mF cm−2 was achieved at a current density of 1 mA cm−2 with 78% capacitance retention after 2000 cycles. The bending test performed at an angle of 40° showed that 90% of its initial capacitance was retained. Hybridizing VSe2 with rGO would enhance the total electrochemical performance due to the synergistic effect of redox and EDLC charge storage. The VSe2/rGO supercapacitor developed via a hydrothermal route with 0.3 wt% of rGO delivered a good capacitance of 680 F g−1 at a mass normalized current density of 1 A g−1.186 This value is ∼7 times higher than the VSe2 supercapacitor alone. The device also delivered a very high energy density of 212 W h kg−1 and showed good cycling stability of 81% retention after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
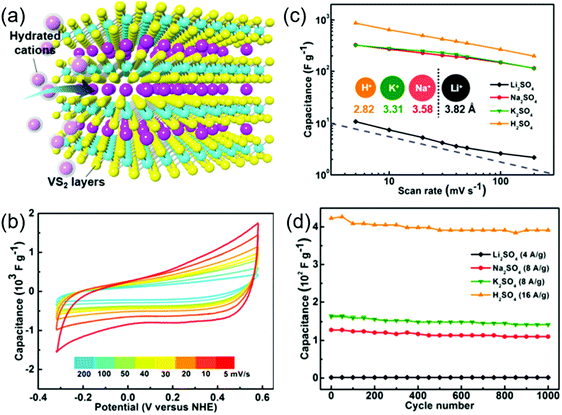 | ||
| Fig. 8 (a) Schematic showing the intercalation process for charge-storage in 1T VS2 nanosheets, (b) CV curves of VS2/glassy carbon electrodes in 0.5 M H2SO4 at different scan rates, (c) scan rate dependence of the capacitance of VS2/glassy carbon electrodes and (d) cycling performance in sulfate electrolyte solutions. Reprinted with permission from ref. 57 Copyright (2017) American Chemical Society. | ||
Most of the 2D TMD electrodes are sulfide and selenide-based, while a few works are based on telluride-based TMDs as the energy storage material. They have a better electrical conductivity as they are metallic in nature. Yu et al. performed liquid exfoliation of CVT grown 1T WTe2 single crystals to develop 1T WTe2 nanosheets.95 These nanosheets had a lateral size in the range of several hundred nanometers to micrometers with a thickness of 1.3–5.31 nm. An all-solid-state supercapacitor assembled with these nanosheets and PVA/H3PO4 electrolytes delivered a specific capacitance of 221 F g−1 with an energy density of 31 W h kg−1. The 1T WTe2 phase also showed stability by retaining 91% capacitance after 5500 cycles. Nevertheless, 2D TiS2 nanocrystals have also emerged as semimetal TMDs that have recently demonstrated good electrochemical performance for Li-ion batteries90 and are expected to open opportunities in the area of energy storage, particularly in supercapacitors.
6 Asymmetric supercapacitor designs
Rationally designing 2D TMD nanostructures in various form factors has certainly enhanced the energy storage capacity in these materials. However, the conventional use of two identical electrodes in a symmetric configuration still limits their energy density due to the limited operational voltage window. For practical applications, supercapacitors with high operating voltages are needed as energy density depends on the voltage (E = 1/2CV2).8 An asymmetric supercapacitor configuration is one in which two different electrode materials are used to achieve a high cell voltage. 2D TMD based asymmetric electrodes have attracted great interest in developing high voltage supercapacitors in the recent past.115,116,189,190 For brevity, we will discuss only the asymmetric supercapacitors using TMDs as positive or negative electrode materials in this review. Recently, a solid-state asymmetric supercapacitor assembled with flower-like MoS2 on graphene nanosheets (Fig. 9a) as a negative electrode, and a MnO2/graphene nanosheet nanocomposite as a positive electrode exhibited a specific capacitance of 320 F g−1 at a scan rate of 2 A g−1. This device worked up to 2 V (Fig. 9b) and delivered a maximum energy density of 78.9 W h kg−1 at a power density of 284.1 W kg−1.140 It is used to light up different colored commercial LEDs as shown in Fig. 9c. In yet another work, the MoS2–rGO/MWNT hybrid positive electrode was prepared by incorporating MoS2 and rGO nanosheets into aligned MWNT fibers. The negative electrode was prepared with rGO/MWNT hybrid fibers. These two electrodes were used to assemble an asymmetric supercapacitor which can be operated at a potential of 1.4 V and exhibited a 100% coulombic efficiency even after 7000 cycles.191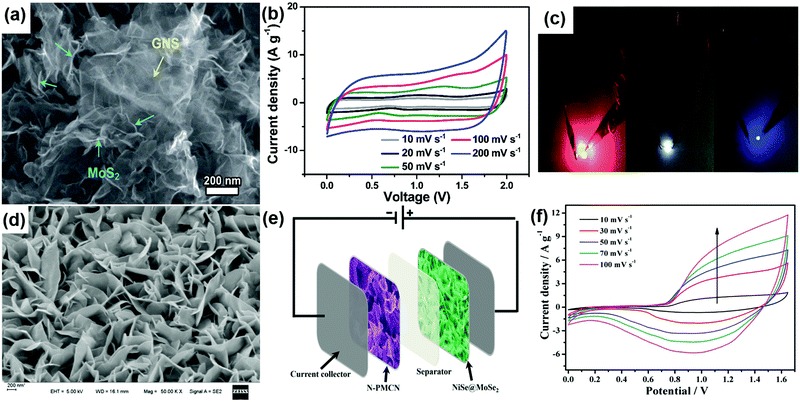 | ||
| Fig. 9 (a) SEM image of the MoS2/GNS hybrid, (b) CV curves of the fabricated MnO2/GNS//MoS2/GNS ASC device measured at various scan rates in the voltage range of 0 to 2.0 V and (c) two assembled all-solid-state ASC devices connected in series to simultaneously light up the commercial LEDs. Reproduced with permission from ref. 140 copyright (2016) Royal Society of Chemistry. (d) FESEM images of NiSe@MoSe2 nanosheet arrays (e) schematic illustration of the NiSe@MoSe2//N-PMCN ASC and (f) CV curves of the NiSe@MoSe2//N-PMCN ASC at different scan rates. Reproduced with permission from ref. 192 copyright (2017) American Chemical Society. | ||
To achieve a high energy density, an advanced hierarchical NiS/MoS2 hybrid anode structure was prepared in which NiS nanoparticles were loaded into MoS2 using the glucose assisted hydrothermal method in the presence of the CNT backbone.192 The entangled 1D hierarchical structures intertwined with each other construct 3D porous conducting networks which facilitate the fast diffusion of electrolyte ions and conducting electron pathways. The asymmetric supercapacitor device assembled using the NiS/MoS2/CNT hybrid anode with activated carbon manifests a high energy density of 40 W h kg−1. A novel NiSe@MoSe2 nanosheet array (Fig. 9d) prepared using the one-step hydrothermal method directly from nickel foam was assembled with nitrogen-doped carbon nanosheets. A supercapacitor assembled in an asymmetric configuration (Fig. 9e) was able to operate at 1.65 V (Fig. 9f) and demonstrated an energy density of 32.6 W h kg−1. The device showed outstanding cycling stability with 91.4% capacitance retention after 5000 cycles.193 Metallic VS2 shows extraordinary performance as a positive electrode with various negative electrodes based on carbon materials, polymers and their composites. For example, VS2 nanosheets have been used with activated carbon as positive and negative electrodes, respectively, to construct an asymmetric supercapacitor device which yields a maximum energy density of 42 W h kg−1 and a power density of 700 W kg−1 within an operational voltage window of 0–1.4 V.87 Moreover, a capacitance retention of 99% was observed after 5000 cycles at a current density of 2 A g−1. In a recent report, an asymmetric supercapacitor was assembled with the VS2 nanosheets as a positive electrode and carbonized iron (C-Fe)/PANI nanocomposite as a negative electrode.194 The as-fabricated supercapacitor exhibits a high operating voltage of 1.7 V in KOH electrolyte at a current density of 2 A g−1. Notably, this device endures 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles with only 5% loss in capacitance and demonstrated high energy and power densities of 27.8 W h kg−1 and 2991.5 W kg−1, respectively. An asymmetric Li-ion supercapacitor assembled with 2D TiS2 and activated carbon electrodes exhibited an energy density of 49 W h kg−1 and retained 76% of its initial capacitance after 2000 cycles.60
000 charge/discharge cycles with only 5% loss in capacitance and demonstrated high energy and power densities of 27.8 W h kg−1 and 2991.5 W kg−1, respectively. An asymmetric Li-ion supercapacitor assembled with 2D TiS2 and activated carbon electrodes exhibited an energy density of 49 W h kg−1 and retained 76% of its initial capacitance after 2000 cycles.60
7 Conclusions and outlook
2D TMDs have been rapidly transforming the global interest and attention of energy storage systems from conventional Li-ion batteries to high-performance supercapacitors. The entry of 2D TMDs in the field of supercapacitors is endowed by their unique 2D layered structure, large surface areas, non-toxic nature, and high mechanical stability. Despite significant advances, TMDs’ electrochemical performance (i.e., capacitance or energy density) is currently limited by their low electronic conductivity, which results in poor capacitive properties as well as improper utilization of the complete surface areas by the electrolyte ions. Nevertheless, a plethora of research has been conducted to overcome the limitations of 2D TMDs and significant enhancement in the specific capacitance, energy density, and cyclic stability was achieved. One effective strategy to achieve high-performance in TMD-based supercapacitors is to make hybrid nanostructures by mixing, wrapping, or depositing TMDs with a range of organic/inorganic conductive yet capacitive materials. Carbonaceous materials such as graphene show great potential as a suitable additive to TMDs due to their unique 2D structural features along with excellent electrical conductivity. The hybridization of 2D TMDs with highly conductive polymers shows high synergistic effects due to their Faradaic charge storage mechanism and high porosity which enables highly active electrode/electrolyte interfaces. Despite significant enhancement in electrochemical performance in these hybrid composites, the re-stacking of ultrathin 2D TMDs via van der Waals interactions and the presence of polymer binders used during electrode preparation impede their real performance by limiting their surface area as well as interfacial contacts.Despite several approaches towards achieving high energy and power densities for storage applications using 2D TMDs, their commercial success towards large scale industrial production is meager because:
(i) The current synthesis methods for most of the 2D materials mainly rely on the mechanical/chemical exfoliation of the nano/micro size 2D flakes from their bulk counterparts. This method is non-scalable, uncontrollable and unreliable, and therefore not practical for commercial production. Hence, viable methods for the direct integration of large area 2D TMD active materials and their hybrids must be developed.
(ii) Significant efforts have been invested in achieving large specific capacitance or energy density by scaling 2D TMDs with an array of organic components in various morphologies. Unfortunately, the success dictated by these endeavors is merely experimental. Theoretical aspects of the TMD-based hybrids must be simultaneously established to unveil the fundamental mechanism of the structure–property relationship, synergistic effects, and adaptability of 2D TMDs with distinct host materials.
(iii) The success of the metallic TMD phase is remarkable and shows great promise towards solving energy storage problems. But, the phase change in TMDs from an unstable 1T phase to a stable 2H phase or the presence of mixed phases (1T–2H) currently limits their true potential. Primarily, the production of metallic phase 2D TMDs other than MoS2 must be achieved using other methods rather than chemical methods.
(iv) Like electrode materials, the electrolyte plays a vital role in deciding the electrochemical performance of supercapacitors. However, the repeated use of available aqueous/non-aqueous electrolytes has made them incompetent/obsolete with tremendous research invested in the development of electrode materials. It is imperative to simultaneously research novel electrolytes that reinforce the functioning of newly available TMDs to achieve higher operating voltages/energy densities.
(v) Other 2D materials such as MXenes and phosphorene have recently been explored to exhibit extraordinary performances due to their high electronic conductivity, large surface areas, and high chemical stability. A combination of these materials with 2D TMDs in an asymmetric device configuration is expected to show unprecedented electrochemical performance for practical applications.
Hence, the continued efforts towards desirable electronic/mechanical properties in 2D TMDs are of great significance to overcome their inherent limitations. We strongly believe that the days are nearer to use a 2D material-based storage device in wearable devices.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. T. acknowledges the NASA-Florida Space Consortium grant (NASA Training Grant#NX15AI10H) and the UCF “Reach for the stars award” for the financial support. This work is also supported by the P3 Pre-Eminent Postdoctoral Research Fellowship awarded to J. C. by the University of Central Florida, Florida, USA.References
- C. Li, M. M. Islam, J. Moore, J. Sleppy, C. Morrison, K. Konstantinov, S. X. Dou, C. Renduchintala and J. Thomas, Nat. Commun., 2016, 7, 13319 CrossRef CAS PubMed.
- A. Patil, V. Patil, D. W. Shin, J. W. Choi, D. S. Paik and S. J. Yoon, Mater. Res. Bull., 2008, 43, 1913–1942 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- L. B. Dong, C. J. Xu, Y. Li, Z. H. Huang, F. Y. Kang, Q. H. Yang and X. Zhao, J. Mater. Chem. A, 2016, 4, 4659–4685 RSC.
- Q. S. Wang, P. Ping, X. J. Zhao, G. Q. Chu, J. H. Sun and C. H. Chen, J. Power Sources, 2012, 208, 210–224 CrossRef CAS.
- X. H. Xia, J. Y. Zhan, Y. Zhong, X. L. Wang, J. P. Tu and H. J. Fan, Small, 2017, 13, 1602742 CrossRef PubMed.
- S. J. Varma, K. Sambath Kumar, S. Seal, S. Rajaraman and J. Thomas, Adv. Sci., 2018, 5, 1800340 CrossRef PubMed.
- N. Choudhary, C. Li, J. Moore, N. Nagaiah, L. Zhai, Y. Jung and J. Thomas, Adv. Mater., 2017, 29, 1605336 CrossRef PubMed.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- L. Weinstein and R. Dash, Mater. Today, 2013, 16, 356–357 CrossRef.
- K. S. Kumar, N. Choudhary, Y. Jung and J. Thomas, ACS Energy Lett., 2018, 3, 482–495 CrossRef CAS.
- J. Cherusseri, R. Sharma and K. K. Kar, Carbon, 2016, 105, 113–125 CrossRef CAS.
- Z. Yang, J. Tian, Z. Yin, C. Cui, W. Qian and F. Wei, Carbon, 2019, 141, 467–480 CrossRef CAS.
- J. Cherusseri, R. Sharma and K. K. Kar, Handbook of Polymer Nanocomposites. Processing, Performance and Application, Springer, 2015, pp. 479–510 Search PubMed.
- J. Cherusseri and K. K. Kar, Phys. Chem. Chem. Phys., 2016, 18, 8587–8597 RSC.
- J. Gamby, P. L. Taberna, P. Simon, J. F. Fauvarque and M. Chesneau, J. Power Sources, 2001, 101, 109–116 CrossRef CAS.
- W. T. Gu and G. Yushin, Wires Energy Environ., 2014, 3, 424–473 CrossRef CAS.
- C. G. Liu, Z. N. Yu, D. Neff, A. Zhamu and B. Z. Jang, Nano Lett., 2010, 10, 4863–4868 CrossRef CAS PubMed.
- Y. Wang, Z. Q. Shi, Y. Huang, Y. F. Ma, C. Y. Wang, M. M. Chen and Y. S. Chen, J. Phys. Chem. C, 2009, 113, 13103–13107 CrossRef CAS.
- H. Yang, S. Kannappan, A. S. Pandian, J. H. Jang, Y. S. Lee and W. Lu, Nanotechnology, 2017, 28, 445401 CrossRef PubMed.
- S. L. Chiam, H. N. Lim, S. M. Hafiz, A. Pandikumar and N. M. Huang, Sci. Rep., 2018, 8, 3093 CrossRef CAS PubMed.
- G. P. Xiong, P. G. He, B. Y. Huang, T. F. Chen, Z. Bo and T. S. Fisher, Nano Energy, 2017, 38, 127–136 CrossRef CAS.
- Z. Y. Lin, Y. Liu, Y. G. Yao, O. J. Hildreth, Z. Li, K. Moon and C. P. Wong, J. Phys. Chem. C, 2011, 115, 7120–7125 CrossRef CAS.
- G. X. Qu, J. L. Cheng, X. D. Li, D. M. Yuan, P. N. Chen, X. L. Chen, B. Wang and H. S. Peng, Adv. Mater., 2016, 28, 3646–3652 CrossRef CAS PubMed.
- K. Subramani, S. Kowsik and M. Sathish, ChemistrySelect, 2016, 1, 3455–3467 CrossRef CAS.
- K. Sambath Kumar, J. Cherusseri and J. Thomas, ACS Omega, 2019, 4, 4472–4480 CrossRef CAS.
- J. Cherusseri, K. S. Kumar, N. Choudhary, N. Nagaiah, Y. Jung, T. Roy and J. Thomas, Nanotechnology, 2019, 30, 202001 CrossRef PubMed.
- M. Chhowalla, H. S. Shin, G. Eda, L. J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- A. Eftelthari, Appl. Mater. Today, 2017, 8, 1–17 CrossRef.
- A. Eftekhari, J. Mater. Chem. A, 2017, 5, 18299–18325 RSC.
- W. Choi, N. Choudhary, G. H. Han, J. Park, D. Akinwande and Y. H. Lee, Mater. Today, 2017, 20, 116–130 CrossRef CAS.
- M. S. Nam, U. Patil, B. Park, H. B. Sim and S. C. Jun, RSC Adv., 2016, 6, 101592 RSC.
- M. Pumera, Z. Sofer and A. Ambrosi, J. Mater. Chem. A, 2014, 2, 8981–8987 RSC.
- Y. Yang, H. L. Fei, G. D. Ruan, C. S. Xiang and J. M. Tour, Adv. Mater., 2014, 26, 8163–8168 CrossRef CAS PubMed.
- J. M. Soon and K. P. Loh, Electrochem. Solid-State Lett., 2007, 10, A250–A254 CrossRef CAS.
- J. Wang, Z. C. Wu, K. H. Hu, X. Y. Chen and H. B. Yin, J. Alloys Compd., 2015, 619, 38–43 CrossRef CAS.
- Y. Ge, R. Jalili, C. Y. Wang, T. Zheng, Y. F. Chao and G. G. Wallace, Electrochim. Acta, 2017, 235, 348–355 CrossRef CAS.
- K. F. Mak, C. Lee, J. Hone, J. Shan and T. F. Heinz, Phys. Rev. Lett., 2010, 105, 136805 CrossRef PubMed.
- K. Kam and B. Parkinson, J. Phys. Chem., 1982, 86, 463–467 CrossRef CAS.
- D. Duphil, S. Bastide and C. Levy-Clement, J. Mater. Chem., 2002, 12, 2430–2432 RSC.
- B. C. Helmly, W. E. Lynch and D. A. Nivens, Spectrosc. Lett., 2007, 40, 483–492 CrossRef CAS.
- S. H. Chaki, M. P. Deshpande, D. P. Trivedi, J. P. Tailor, M. D. Chaudhary and K. Mahato, Appl. Nanosci., 2013, 3, 189–195 CrossRef CAS.
- G. A. Muller, J. B. Cook, H. S. Kim, S. H. Tolbert and B. Dunn, Nano Lett., 2015, 15, 1911–1917 CrossRef CAS PubMed.
- K. H. Park, J. Choi, H. J. Kim, D. H. Oh, J. R. Ahn and S. U. Son, Small, 2008, 4, 945–950 CrossRef CAS PubMed.
- S. Jeong, D. Yoo, J. T. Jang, M. Kim and J. Cheon, J. Am. Chem. Soc., 2012, 134, 18233–18236 CrossRef CAS PubMed.
- S. Jeong, D. Yoo, M. Ahn, P. Miro, T. Heine and J. Cheon, Nat. Commun., 2015, 6, 5763 CrossRef CAS PubMed.
- J. Feng, X. Sun, C. Z. Wu, L. L. Peng, C. W. Lin, S. L. Hu, J. L. Yang and Y. Xie, J. Am. Chem. Soc., 2011, 133, 17832–17838 CrossRef CAS PubMed.
- P. Joensen, R. F. Frindt and S. R. Morrison, Mater. Res. Bull., 1986, 21, 457–461 CrossRef CAS.
- A. Schumacher, L. Scandella, N. Kruse and R. Prins, Surf. Sci., 1993, 289, L595–L598 CrossRef CAS.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- R. F. Frindt, J. Appl. Phys., 1966, 37, 1928–1929 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Y. Son, Q. H. Wang, J. A. Paulson, C. J. Shih, A. G. Rajan, K. Tvrdy, S. Kim, B. Alfeeli, R. D. Braatz and M. S. Strano, ACS Nano, 2015, 9, 2843–2855 CrossRef CAS PubMed.
- G. Deokar, D. Vignaud, R. Arenal, P. Louette and J. F. Colomer, Nanotechnology, 2016, 27, 075604 CrossRef CAS PubMed.
- S. C. Zhao, J. X. Weng, S. Z. Jin, Y. F. Lv and Z. G. Ji, Coatings, 2018, 8, 78 CrossRef.
- Y. H. Lee, X. Q. Zhang, W. Zhang, M. T. Chang, C. T. Lin, K. D. Chang, Y. C. Yu, J. T. W. Wang, C. S. Chang and L. J. Li, Adv. Mater., 2012, 24, 2320–2325 CrossRef CAS PubMed.
- Q. Q. Ji, C. Li, J. L. Wang, J. J. Niu, Y. Gong, Z. P. Zhang, Q. Y. Fang, Y. Zhang, J. P. Shi, L. Liao, X. S. Wu, L. Gu, Z. F. Liu and Y. F. Zhang, Nano Lett., 2017, 17, 4908–4916 CrossRef CAS PubMed.
- M. Inoue and H. Negishi, J. Phys. Soc. Jpn., 1985, 54, 380–388 CrossRef CAS.
- E. Amzallag, H. Martinez, I. Baraille, M. Rerat, M. Loudet and D. Gonbeau, Solid State Sci., 2007, 9, 594–599 CrossRef CAS.
- A. Chaturvedi, P. Hu, V. Aravindan, C. Kloc and S. Madhavi, J. Mater. Chem. A, 2017, 5, 9177–9181 RSC.
- M. Parvaz, S. Ahmed, M. B. Khan, Rahul, S. Ahmad and Z. H. Khan, AIP Conf. Proc., 2018, 1953, 030121 CrossRef.
- A. Rautiainen, Y. Koskinen, J. Skarp and S. Lindfors, MRS Online Proc. Libr., 1991, 222, 263 CrossRef CAS.
- L. Reijnen, B. Meester, A. Goossens and J. Schoonman, Chem. Vap. Deposition, 2003, 9, 15–20 CrossRef CAS.
- E. Nykanen, J. Laineylijoki, P. Soininen, L. Niinisto, M. Leskela and L. G. Hubertpfalzgraf, J. Mater. Chem., 1994, 4, 1409–1412 RSC.
- V. Pore, M. Ritala and M. Leskela, Chem. Vap. Deposition, 2007, 13, 163–168 CrossRef CAS.
- T. W. Scharf, S. V. Prasad, T. M. Mayer, R. S. Goeke and M. T. Dugger, J. Mater. Res., 2004, 19, 3443–3446 CrossRef CAS.
- T. A. J. Loh, D. H. C. Chua and A. T. S. Wee, Sci. Rep., 2015, 5, 18116 CrossRef CAS PubMed.
- P. M. Campbell, C. J. Perini, J. Chiu, A. Gupta, H. S. Ray, H. Chen, K. Wenzel, E. Snyder, B. K. Wagner, J. Ready and E. M. Vogel, 2D Mater., 2018, 5, 015005 CrossRef.
- Y. Feldman, E. Wasserman, D. J. Srolovitz and R. Tenne, Science, 1995, 267, 222–225 CrossRef CAS PubMed.
- R. Tenne, M. Homyonfer and Y. Feldman, Chem. Mater., 1998, 10, 3225–3238 CrossRef CAS.
- M. Nath, A. Govindaraj and C. N. R. Rao, Adv. Mater., 2001, 13, 283–286 CrossRef CAS.
- D. S. Kong, H. T. Wang, J. J. Cha, M. Pasta, K. J. Koski, J. Yao and Y. Cui, Nano Lett., 2013, 13, 1341–1347 CrossRef CAS PubMed.
- Z. Lin, M. T. Thee, A. L. Elias, S. M. Feng, C. J. Zhou, K. Fujisawa, N. Perea-Lopez, V. Carozo, H. Terrones and M. Terrones, APL Mater., 2014, 2, 092514 CrossRef.
- T. Chen, S. Z. Li, P. B. Gui, J. Wen, X. M. Fu and G. J. Fang, Nanotechnology, 2018, 29, 205401 CrossRef PubMed.
- C. C. Tu, L. Y. Lin, B. C. Xiao and Y. S. Chen, J. Power Sources, 2016, 320, 78–85 CrossRef CAS.
- S. Ratha and C. S. Rout, ACS Appl. Mater. Interfaces, 2013, 5, 11427–11433 CrossRef CAS PubMed.
- M. Yi and C. Zhang, RSC Adv., 2018, 8, 9564–9573 RSC.
- C. Nagaraju, C. V. V. M. Gopi, J. W. Ahn and H. J. Kim, New J. Chem., 2018, 42, 12357–12360 RSC.
- L. J. Ye, H. Y. Xu, D. K. Zhang and S. J. Chen, Mater. Res. Bull., 2014, 55, 221–228 CrossRef CAS.
- W. J. Li, E. W. Shi, J. M. Ko, Z. Z. Chen, H. Ogino and T. Fukuda, J. Cryst. Growth, 2003, 250, 418–422 CrossRef CAS.
- J. B. Jia, J. H. Wu, J. Dong, Y. G. Tu, Z. Lan, L. Q. Fan and Y. L. Wei, IEEE J. Photovolt., 2016, 6, 1196–1202 Search PubMed.
- K. J. Huang, J. Z. Zhang and Y. Fan, Mater. Lett., 2015, 152, 244–247 CrossRef CAS.
- S. K. Balasingam, J. S. Lee and Y. Jun, Dalton Trans., 2015, 44, 15491–15498 RSC.
- V. Ghritlahre, J. Kumari and P. Agarwal, AIP Conf. Proc., 2018, 1953, 050048 CrossRef.
- H. Chauhan, M. K. Singh, P. Kumar, S. A. Hashmi and S. Deka, Nanotechnology, 2017, 28, 025401 CrossRef PubMed.
- R. K. Mishra, G. W. Baek, K. Kim, H. I. Kwon and S. H. Jin, Appl. Surf. Sci., 2017, 425, 923–931 CrossRef CAS.
- T. M. Masikhwa, F. Barzegar, J. K. Dangbegnon, A. Bello, M. J. Madito, D. Momodu and N. Manyala, RSC Adv., 2016, 6, 38990–39000 RSC.
- Z. Guo, L. Yang, W. Wang, L. Cao and B. Dong, J. Mater. Chem. A, 2018, 6, 14681–14688 RSC.
- S. Liu, Y. Zeng, M. Zhang, S. Xie, Y. Tong, F. Cheng and X. Lu, J. Mater. Chem. A, 2017, 5, 21460–21466 RSC.
- G. A. Muller, J. B. Cook, H.-S. Kim, S. H. Tolbert and B. Dunn, Nano Lett., 2015, 15, 1911–1917 CrossRef CAS PubMed.
- M. Acerce, D. Voiry and M. Chhowalla, Nat. Nanotechnol., 2015, 10, 313–318 CrossRef CAS PubMed.
- A. Ejigu, I. A. Kinloch, E. Prestat and R. A. W. Dryfe, J. Mater. Chem. A, 2017, 5, 11316–11330 RSC.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- C. Wang, X. Wu, Y. Ma, G. Mu, Y. Li, C. Luo, H. Xu, Y. Zhang, J. Yang and X. Tang, J. Mater. Chem. A, 2018, 6, 8299–8306 RSC.
- P. Yu, W. Fu, Q. Zeng, J. Lin, C. Yan, Z. Lai, B. Tang, K. Suenaga, H. Zhang and Z. Liu, Adv. Mater., 2017, 29, 1701909 CrossRef PubMed.
- M. Wang, L. Zhang, Y. J. Zhong, M. R. Huang, Z. Zhen and H. W. Zhu, Nanoscale, 2018, 10, 17341–17346 RSC.
- L. Jiang, S. Zhang, S. A. Kulinich, X. Song, J. Zhu, X. Wang and H. Zeng, Mater. Res. Lett., 2015, 3, 177–183 CrossRef CAS.
- X. Geng, Y. Zhang, Y. Han, J. Li, L. Yang, M. Benamara, L. Chen and H. Zhu, Nano Lett., 2017, 17, 1825–1832 CrossRef CAS PubMed.
- A. Khalil, Q. Liu, Q. He, T. Xiang, D. Liu, C. Wang, Q. Fang and L. Song, RSC Adv., 2016, 6, 48788–48791 RSC.
- J. M. Soon and K. P. Loh, Electrochem. Solid-State Lett., 2007, 10, A250–A254 CrossRef CAS.
- L. Cao, S. Yang, W. Gao, Z. Liu, Y. Gong, L. Ma, G. Shi, S. Lei, Y. Zhang and S. Zhang, Small, 2013, 9, 2905–2910 CrossRef CAS PubMed.
- Y. Yang, H. Fei, G. Ruan, C. Xiang and J. M. Tour, Adv. Mater., 2014, 26, 8163–8168 CrossRef CAS PubMed.
- T. H. Sun, Z. P. Li, X. H. Liu, L. M. Ma, J. Q. Wang and S. R. Yang, J. Power Sources, 2017, 352, 135–142 CrossRef CAS.
- S. Liu, Y. X. Zeng, M. Zhang, S. L. Xie, Y. X. Tong, F. L. Cheng and X. H. Lu, J. Mater. Chem. A, 2017, 5, 21460–21466 RSC.
- X. Shang, J. Q. Chi, S. S. Lu, J. X. Gou, B. Dong, X. Li, Y. R. Liu, K. L. Yan, Y. M. Chai and C. G. Liu, Appl. Surf. Sci., 2017, 392, 708–714 CrossRef CAS.
- A. Ghorai, A. Midya and S. K. Ray, New J. Chem., 2018, 42, 3609–3613 RSC.
- W. Yin, D. He, X. Bai and W. Y. William, J. Alloys Compd., 2019, 786, 764–769 CrossRef CAS.
- J. C. Xing, Y. L. Zhu, Q. W. Zhou, X. D. Zheng and Q. J. Jiao, Electrochim. Acta, 2014, 136, 550–556 CrossRef CAS.
- L. Zhang, H. B. Wu and X. W. Lou, Chem. Commun., 2012, 48, 6912–6914 RSC.
- V. K. Mariappan, K. Krishnamoorthy, P. Pazhamalai, S. Sahoo and S. J. Kim, Electrochim. Acta, 2018, 265, 514–522 CrossRef CAS.
- Y. P. Gao, K. J. Huang, H. L. Shuai and L. Liu, Mater. Lett., 2017, 209, 319–322 CrossRef CAS.
- K. L. Guo, F. F. Yang, S. Z. Cui, W. H. Chen and L. W. Mi, RSC Adv., 2016, 6, 46523–46530 RSC.
- S. Jiang, J. H. Wu, B. R. Ye, Y. Y. Fan, J. H. Ge, Q. Y. Guo and M. L. Huang, J. Mater. Sci.: Mater. Electron., 2018, 29, 4649–4657 CrossRef CAS.
- N. S. Arul and J. I. Han, Mater. Lett., 2016, 181, 345–349 CrossRef CAS.
- S. L. Wang, W. Li, L. P. Xin, M. Wu, Y. Long, H. T. Huang and X. J. Lou, Chem. Eng. J., 2017, 330, 1334–1341 CrossRef CAS.
- Q. L. Bao, J. H. Wu, L. Q. Fan, J. H. Ge, J. Dong, J. B. Jia, J. L. Zeng and J. M. Lin, J. Energy Chem., 2017, 26, 1252–1259 CrossRef.
- X. Liu, J.-Z. Zhang, K.-J. Huang and P. Hao, Chem. Eng. J., 2016, 302, 437–445 CrossRef CAS.
- K.-J. Huang, J.-Z. Zhang and J.-L. Cai, Electrochim. Acta, 2015, 180, 770–777 CrossRef CAS.
- B. Hu, X. Qin, A. M. Asiri, K. A. Alamry, A. O. Al-Youbi and X. Sun, Electrochem. Commun., 2013, 28, 75–78 CrossRef CAS.
- E. G. da Silveira Firmiano, A. C. Rabelo, C. J. Dalmaschio, A. N. Pinheiro, E. C. Pereira, W. H. Schreiner and E. R. Leite, Adv. Energy Mater., 2014, 4, 1301380 CrossRef.
- C. S. Dai, P. Y. Chien, J. Y. Lin, S. W. Chou, W. K. Wu, P. H. Li, K. Y. Wu and T. W. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 12168–12174 CrossRef CAS PubMed.
- G. Sun, J. Liu, X. Zhang, X. Wang, H. Li, Y. Yu, W. Huang, H. Zhang and P. Chen, Angew. Chem., 2014, 126, 12784–12788 CrossRef.
- C. L. Wang, X. Wu, H. J. Xu, Y. J. Zhu, F. Liang, C. Luo, Y. Xia, X. Y. Xie, J. Zhang and C. G. Duan, Appl. Phys. Lett., 2019, 114, 023902 CrossRef.
- J. L. Gao, Y. Ma, J. B. Li, J. C. Fan, P. H. Shi, Q. J. Xu and Y. L. Min, J. Nanopart. Res., 2018, 20, 298 CrossRef.
- M. A. Bissett, I. A. Kinloch and R. A. W. Dryfe, ACS Appl. Mater. Interfaces, 2015, 7, 17388–17398 CrossRef CAS PubMed.
- M. J. Crane, M. B. Lim, X. Z. Zhou and P. J. Pauzauskie, Microsyst. Nanoeng., 2017, 3, 17032 CrossRef.
- G. F. Ma, H. Peng, J. J. Mu, H. H. Huang, X. Z. Zhou and Z. Q. Lei, J. Power Sources, 2013, 229, 72–78 CrossRef CAS.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- Y. M. Shi, W. Zhou, A. Y. Lu, W. J. Fang, Y. H. Lee, A. L. Hsu, S. M. Kim, K. K. Kim, H. Y. Yang, L. J. Li, J. C. Idrobo and J. Kong, Nano Lett., 2012, 12, 2784–2791 CrossRef CAS PubMed.
- J. Cherusseri and K. K. Kar, RSC Adv., 2015, 5, 34335–34341 RSC.
- H. Hu, Z. B. Zhao, W. B. Wan, Y. Gogotsi and J. S. Qiu, Adv. Mater., 2013, 25, 2219–2223 CrossRef CAS PubMed.
- J. Cherusseri and K. K. Kar, J. Mater. Chem. A, 2015, 3, 21586–21598 RSC.
- J. Cherusseri and K. K. Kar, RSC Adv., 2016, 6, 60454–60466 RSC.
- D. Pech, M. Brunet, H. Durou, P. H. Huang, V. Mochalin, Y. Gogotsi, P. L. Taberna and P. Simon, Nat. Nanotechnol., 2010, 5, 651–654 CrossRef CAS PubMed.
- J. Cherusseri and K. K. Kar, J. Mater. Chem. A, 2016, 4, 9910–9922 RSC.
- J. Cherusseri and K. K. Kar, Polymer Nanocomposites Based on Inorganic and Organic Nanomaterials, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2015, pp. 229–256 Search PubMed.
- X. Zhang, Y. Yang, Z. Li, X. Wang, W. Wang, Z. Yi, L. Qiang, Q. Wang and Z. Hu, J. Phys. Chem. Solids, 2019, 130, 84–92 CrossRef CAS.
- K. J. Huang, L. Wang, Y. J. Liu, Y. M. Liu, H. B. Wang, T. Gan and L. L. Wang, Int. J. Hydrogen Energy, 2013, 38, 14027–14034 CrossRef CAS.
- S. Byun, D. M. Sim, J. Yu and J. J. Yoo, ChemElectroChem, 2015, 2, 1938–1946 CrossRef CAS.
- X. Yang, H. Niu, H. Jiang, Q. Wang and F. Y. Qu, J. Mater. Chem. A, 2016, 4, 11264–11275 RSC.
- F. Zhang, Y. B. Tang, H. Liu, H. Y. Ji, C. L. Jiang, J. Zhang, X. L. Zhang and C. S. Lee, ACS Appl. Mater. Interfaces, 2016, 8, 4691–4699 CrossRef CAS PubMed.
- T. H. Sun, X. H. Liu, Z. P. Li, L. M. Ma, J. Q. Wang and S. R. Yang, New J. Chem., 2017, 41, 7142–7150 RSC.
- S. Wang, J. Zhu, Y. Shao, W. Li, Y. Wu, L. Zhang and X. Hao, Chem. – Eur. J., 2017, 23, 3438–3446 CrossRef CAS PubMed.
- Y. Liu, W. Wang, H. B. Huang, L. Gu, Y. W. Wang and X. S. Peng, Chem. Commun., 2014, 50, 4485–4488 RSC.
- F. Clerici, M. Fontana, S. Bianco, M. Serrapede, F. Perrucci, S. Ferrero, E. Tresso and A. Lamberti, ACS Appl. Mater. Interfaces, 2016, 8, 10459–10465 CrossRef CAS PubMed.
- T. H. Sun, Z. P. Li, X. H. Liu, L. M. Ma, J. Q. Wang and S. R. Yang, J. Power Sources, 2016, 331, 180–188 CrossRef CAS.
- K.-J. Huang, L. Wang, J.-Z. Zhang, L.-L. Wang and Y.-P. Mo, Energy, 2014, 67, 234–240 CrossRef CAS.
- B. Pandit, S. S. Karade and B. R. Sankapal, ACS Appl. Mater. Interfaces, 2017, 9, 44880–44891 CrossRef CAS PubMed.
- S. Zhang, X. Yu, H. Yu, Y. Chen, P. Gao, C. Li and C. Zhu, ACS Appl. Mater. Interfaces, 2014, 6, 21880–21885 CrossRef CAS PubMed.
- Y. Luo, Y. Zhang, Y. Zhao, X. Fang, J. Ren, W. Weng, Y. Jiang, H. Sun, B. Wang and X. Cheng, J. Mater. Chem. A, 2015, 3, 17553–17557 RSC.
- M. Yang, J. M. Jeong, Y. S. Huh and B. G. Choi, Compos. Sci. Technol., 2015, 121, 123–128 CrossRef CAS.
- E. G. D. Firmiano, A. C. Rabelo, C. J. Dalmaschio, A. N. Pinheiro, E. C. Pereira, W. H. Schreiner and E. R. Leite, Adv. Energy Mater., 2014, 4, 1301380 CrossRef.
- C.-C. Tu, L.-Y. Lin, B.-C. Xiao and Y.-S. Chen, J. Power Sources, 2016, 320, 78–85 CrossRef CAS.
- C. X. Hao, F. S. Wen, J. Y. Xiang, L. M. Wang, H. Hou, Z. B. Su, W. T. Hu and Z. Y. Liu, Adv. Funct. Mater., 2014, 24, 6700–6707 CrossRef CAS.
- H. W. Wang, H. Yi, X. Chen and X. F. Wang, J. Mater. Chem. A, 2014, 2, 3223–3230 RSC.
- R. R. Salunkhe, J. Tang, Y. Kamachi, T. Nakato, J. H. Kim and Y. Yamauchi, ACS Nano, 2015, 9, 6288–6296 CrossRef CAS PubMed.
- C. Guan, J. L. Liu, Y. D. Wang, L. Mao, Z. X. Fan, Z. X. Shen, H. Zhang and J. Wang, ACS Nano, 2015, 9, 5198–5207 CrossRef CAS PubMed.
- K. Wang, J. Yang, J. X. Zhu, L. Li, Y. Liu, C. Zhang and T. X. Liu, J. Mater. Chem. A, 2017, 5, 11236–11245 RSC.
- D. W. Liang, Z. F. Tian, J. Liu, Y. X. Ye, S. L. Wu, Y. Y. Cai and C. H. Liang, Electrochim. Acta, 2015, 182, 376–382 CrossRef CAS.
- F. Ghasemi, M. Jalali, A. Abdollahi, S. Mohammadi, Z. Sanaee and S. Mohajerzadeh, RSC Adv., 2017, 7, 52772–52781 RSC.
- X. J. Yang, H. M. Sun, L. S. Zhang, L. J. Zhao, J. S. Lian and Q. Jiang, Sci. Rep., 2016, 6, 31591 CrossRef CAS PubMed.
- P. Asen, M. Haghighi, S. Shahrokhian and N. Taghavinia, J. Alloys Compd., 2019, 782, 38–50 CrossRef CAS.
- L. Fang, Z. Zhang, X. Li, H. Zhou, K. Ma, L. Ge and K. Huang, Colloids Surf., A, 2016, 501, 42–48 CrossRef CAS.
- A. Liang, D. Li, W. Zhou, Y. Wu, G. Ye, J. Wu, Y. Chang, R. Wang, J. Xu and G. Nie, J. Electroanal. Chem., 2018, 824, 136–146 CrossRef CAS.
- M. S. Nam, U. Patil, B. Park, H. B. Sim and S. C. Jun, RSC Adv., 2016, 6, 101592 RSC.
- H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117–1123 CrossRef CAS PubMed.
- K. Gopalakrishnan, S. Sultan, A. Govindaraj and C. Rao, Nano Energy, 2015, 12, 52–58 CrossRef CAS.
- K. J. Huang, L. Wang, Y. J. Liu, H. B. Wang, Y. M. Liu and L. L. Wang, Electrochim. Acta, 2013, 109, 587–594 CrossRef CAS.
- A. K. Thakur, A. B. Deshmukh, R. B. Choudhary, I. Karbhal, M. Majumder and M. V. Shelke, Mater. Sci. Eng., B, 2017, 223, 24–34 CrossRef CAS.
- H. J. Tang, J. Y. Wang, H. J. Yin, H. J. Zhao, D. Wang and Z. Y. Tang, Adv. Mater., 2015, 27, 1117–1123 CrossRef CAS PubMed.
- J. X. Zhu, W. P. Sun, D. Yang, Y. Zhang, H. H. Hoon, H. Zhang and Q. Y. Yan, Small, 2015, 11, 4123–4129 CrossRef CAS PubMed.
- N. Choudhary, M. Patel, Y. H. Ho, N. B. Dahotre, W. Lee, J. Y. Hwang and W. Choi, J. Mater. Chem. A, 2015, 3, 24049–24054 RSC.
- T. Sun, Z. Li, X. Liu, L. Ma, J. Wang and S. Yang, J. Power Sources, 2016, 331, 180–188 CrossRef CAS.
- K. Singh, S. Kumar, K. Agarwal, K. Soni, V. R. Gedela and K. Ghosh, Sci. Rep., 2017, 7, 9458 CrossRef PubMed.
- L. Ren, G. Zhang, Z. Yan, L. Kang, H. Xu, F. Shi, Z. Lei and Z.-H. Liu, ACS Appl. Mater. Interfaces, 2015, 7, 28294–28302 CrossRef CAS PubMed.
- Q. Lu, J. G. Chen and J. Q. Xiao, Angew. Chem., Int. Ed., 2013, 52, 1882–1889 CrossRef CAS PubMed.
- Y. Zhang, W. Sun, X. Rui, B. Li, H. T. Tan, G. Guo, S. Madhavi, Y. Zong and Q. Yan, Small, 2015, 11, 3694–3702 CrossRef CAS PubMed.
- S. K. Balasingam, A. Thirumurugan, J. S. Lee and Y. Jun, Nanoscale, 2016, 8, 11787–11791 RSC.
- N. Choudhary, C. Li, H.-S. Chung, J. Moore, J. Thomas and Y. Jung, ACS Nano, 2016, 10, 10726–10735 CrossRef CAS PubMed.
- L. Li, H. Yang, J. Yang, L. Zhang, J. Miao, Y. Zhang, C. Sun, W. Huang, X. Dong and B. Liu, J. Mater. Chem. A, 2016, 4, 1319–1325 RSC.
- M. Liu, Z. Wang, J. Liu, G. Wei, J. Du, Y. Li, C. An and J. Zhang, J. Mater. Chem. A, 2017, 5, 1035–1042 RSC.
- D. Z. Wang, Y. Y. Xiao, X. N. Luo, Z. Z. Wu, Y. J. Wang and B. Z. Fang, ACS Sustainable Chem. Eng., 2017, 5, 2509–2515 CrossRef CAS.
- J. Wu, J. Peng, Z. Yu, Y. Zhou, Y. Guo, Z. Li, Y. Lin, K. Ruan, C. Wu and Y. Xie, J. Am. Chem. Soc., 2017, 140, 493–498 CrossRef PubMed.
- Y. C. Jiao, A. M. Hafez, D. X. Cao, A. Mukhopadhyay, Y. Ma and H. L. Zhu, Small, 2018, 14, 1800640 CrossRef PubMed.
- A. Gigot, M. Fontana, M. Serrapede, M. Castellino, S. Bianco, M. Armandi, B. Bonelli, C. F. Pirri, E. Tresso and P. Rivolo, ACS Appl. Mater. Interfaces, 2016, 8, 32842–32852 CrossRef CAS PubMed.
- S. R. Marri, S. Ratha, C. S. Rout and J. N. Behera, Chem. Commun., 2017, 53, 228–231 RSC.
- J. Feng, X. Sun, C. Wu, L. Peng, C. Lin, S. Hu, J. Yang and Y. Xie, J. Am. Chem. Soc., 2011, 133, 17832–17838 CrossRef CAS PubMed.
- C. L. Wang, X. Wu, Y. H. Ma, G. Mu, Y. Y. Li, C. Luo, H. J. Xu, Y. Y. Zhang, J. Yang, X. D. Tang, J. Zhang, W. Z. Bao and C. G. Duan, J. Mater. Chem. A, 2018, 6, 8299–8306 RSC.
- T. Chen, S. Li, J. Wen, P. Gui, Y. Guo, C. Guan, J. Liu and G. Fang, Small, 2018, 14, 1700979 CrossRef PubMed.
- N. Yu, M. Q. Zhu and D. Chen, J. Mater. Chem. A, 2015, 3, 7910–7918 RSC.
- G. Sun, X. Zhang, R. Lin, J. Yang, H. Zhang and P. Chen, Angew. Chem., Int. Ed., 2015, 54, 4651–4656 CrossRef CAS PubMed.
- X. J. Yang, L. J. Zhao and J. S. Lian, J. Power Sources, 2017, 343, 373–382 CrossRef CAS.
- H. Peng, J. Z. Zhou, K. J. Sun, G. F. Ma, Z. G. Zhang, E. Feng and Z. Q. Lei, ACS Sustainable Chem. Eng., 2017, 5, 5951–5963 CrossRef CAS.
- M. N. Rantho, M. J. Madito, F. O. Ochai-Ejeh and N. Manyala, Electrochim. Acta, 2018, 260, 11–23 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |