Multifunctional hollow sandwich structure with many active sites for electronic transfer modulation and its application in energy storage and conversion†
Wen
Wang
a,
Jixin
Yao
*a,
Xueqin
Zuo
a,
Qun
Yang
a,
Mingzai
Wu
ab,
Huaibao
Tang
ab,
Shaowei
Jin
ab and
Guang
Li
 *abc
*abc
aSchool of Physics and Materials Science, Anhui University, Hefei 230601, China. E-mail: liguang1971@ahu.edu.cn; liguang64@163.com; Fax: +86 0551 63861992; Tel: +86 0551 63861867
bAnhui Key Laboratory of Information Materials and Devices, Hefei 230601, China
cInstitute of Physical Science and Information Technology, Anhui University, Hefei 230601, China. E-mail: b16201045@stu.ahu.edu.cn
First published on 25th May 2019
Abstract
Revealing the fundamental mechanism of effective, low-cost multifunctional electrocatalysts based on a hollow sandwich structure is desirable for energy storage and conversion. Transition metal sulfides (TMS) and carbon-based heteroatom-doped materials are designed to achieve hollow structures with large specific surface areas. These materials require the well-organized integration of dual carbon layers with different morphologies and active Ni3S2 nanosheets to form a multifunctional electrocatalyst. The interactions among the three components also require elucidation. In this work, we synthesized N-doped hollow carbon spheres (NHC) to obtain a large specific surface area and many active sites. Then, hierarchical Ni3S2 nanosheets were adhered to the NHC surfaces to form an NHC/Ni3S2 nanocomposite. The nanocomposite was encapsulated in reduced graphene oxide (RGO) to form an NHC/Ni3S2/RGO sandwich structure. The NHC/Ni3S2/RGO nanocomposite showed outstanding performance as a multifunctional catalyst in dye-sensitized solar cells (DSSCs), in the acidic hydrogen evolution reaction (HER), and in the energy storage systems of supercapacitors (pseudocapacitance). A DSSC containing the nanocomposite as a counter electrode achieved a photoelectric conversion efficiency (PCE) of up to 9.03%. The specific capacitance of the supercapacitor containing the nanocomposite was 990.6 F g−1. When the composite was used as a catalytic electrode for the HER, a current density of 10 mA cm−2 was achieved with an overpotential of only −142 mV. In addition, the Tafel slope (98.1 mV dec−1) in 0.5 M H2SO4 solution was small. This work contributes a strategy for the development of multifunctional catalytic materials for energy storage and conversion.
New conceptsCatalytic materials in many catalytic fields have single characteristics, so they cannot be used in other fields. To develop and use materials more economically, we have studied common material performance requirements across many catalytic fields, and rationally designed multifunctional materials that can be used in many catalytic fields. In this work, we report a hollow sandwich composite consisting of nitrogen-doped hollow carbon spheres, nickel disulfide flakes, and a conductive reduced graphene oxide protecting layer (NHC/Ni3S2/RGO). The large specific surface area contributes to the rapid ion transport in the electrochemical reaction, and the hollow structure provides space for ion storage. The composite provides support for catalysis and energy storage and conversion, and achieves the goal of developing one material for multiple purposes. A dye-sensitized solar cell based on a NHC/Ni3S2/RGO counter electrode achieved a power conversion efficiency of 9.03%, a supercapacitor containing the nanocomposite had a specific capacitance of 990.6 mF g−1, and as a catalyst for the hydrogen evolution reaction the nanocomposite achieved a current density of 10 mA cm−2 with an overpotential of only −142 mV and a small Tafel slope (98.1 mV dec−1) in 0.5 M H2SO4 solution. |
1. Introduction
The development of human society is closely linked with the development and use of energy. The demand for energy is increasing rapidly with economic development, which has depleted reserves of non-renewable energy resources.1–5 In addition, the large-scale use of fossil fuel presents severe problems with pollution.6–8 Currently, in response to global initiatives to protect energy resources and the environment, research is focusing on developing energy sources, devices, and products.9–11 Compared with fossil fuel, new energy sources have larger reserves and produce less pollution. Solar energy is both a primary energy and renewable energy source.12–14 It is a rich resource that is free to use and does not require transportation. Most importantly, it does not pollute the environment and has the potential to improve people's lives. The non-renewable resources we are currently using were created by the photosynthesis of ancient plankton and other organisms. Dye-sensitized solar cells (DSSCs) are photoelectric devices that simulate photosynthesis to produce energy.15–17 In addition, hydrogen is a clean and renewable energy source.18–20 At present, due to their low-cost, sustainability, excellent electrochemical properties, electrochemical clean energy systems consisting of DSSCs and the hydrogen evolution reaction (HER) are an important area of research in energy conversion. These systems require the development of effective electrocatalysts and the improvement of their electrochemical performance. In addition, supercapacitors are an important method of energy storage.21–24Typically, research into electrocatalysts has focused on developing synthesis strategies with precise structure control and design; however, assembling highly efficient multifunctional catalyst electrodes remains a major challenge. To improve the electrochemical performance of an advanced catalytic material for energy storage and conversion, multifunctional catalysts with the following properties are required. (1) The catalyst should form homogeneous nanostructures and exhibit good electron transport. (2) The catalyst should have excellent electrocatalytic activity. (3) The catalyst should have high surface area and absorb the maximum number of electrons in the electrolyte. (4) The catalyst should have high stability in electrolytes. (5) The catalyst should be cheap.
Materials with hollow structures are frequently used in energy storage and conversion,25–27 owing to their large specific surface area for reactions and high capacity for storing electrons, large number of defects, high electrical conductivity, and large number of active sites. Hollow carbon materials have been used in many fields, such as solar cells,28 supercapacitors,29 the oxygen reduction reaction,30 the oxygen evolution reaction,31 and the HER,32 and their electrochemical properties are excellent. Transition metal sulfides (TMS) have also attracted attention owing to their excellent properties and extensive applications in electrochemical energy conversion and storage. Nevertheless, there are few examples of the efficient integration of the carbon/metal–sulfide/carbon constituents into a multifunctional catalyst material.
In this work, we designed N-doped hollow carbon spheres (NHC)/Ni3S2/reduced graphene oxide (RGO) nanocomposites with unique ternary interfacial structures. First, Ni3S2 nanosheets were embedded on the surface of NHCs, forming interconnected networks to ensure the smooth routes for electrolyte ion diffusion in different directions. Introducing RGO had three advantages. First, the electrons diffusion rate was increased by the two-dimensional layered structure of RGO. Second, RGO introduced more surface defects, which took up more electrons and provided a constant supply of electrons for electrochemical reactions. Third, RGO is a flexible carbon material that protects NHC/Ni3S2 from electrolyte corrosion. The NHC/Ni3S2/RGO nanocomposites were used as a counter electrode (CE) for DSSCs, and photoelectric conversion efficiency (PCE) of 9.03% was achieved, which was higher than that of Pt (7.75%). The NHC/Ni3S2/RGO nanocomposite was also as a catalyst in the HER and showed a respectable electrocatalytic performance; it required an overpotential of only −142 mV in 0.5 M H2SO4 solution to provide a current density of 10 mA cm−2. In addition, our multifunctional material could also be used as an active material for supercapacitors and had a good electrochemical performance with a specific capacity of 990.6 F g−1 at a current density of 1 A g−1.
2. Results and discussion
2.1 Morphology and composition
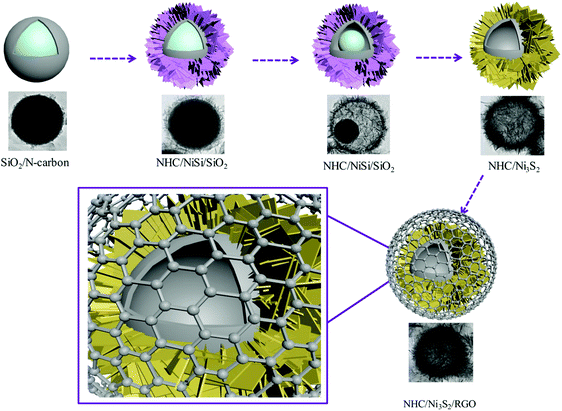
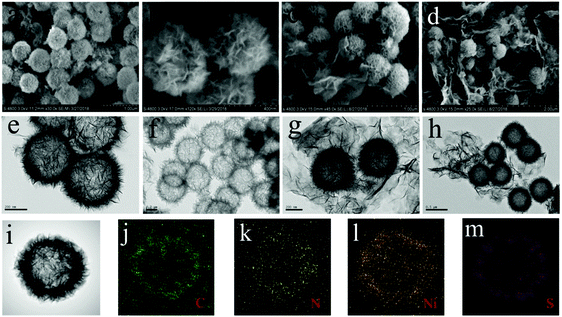
The synthesis of the NHC/Ni3S2/RGO composite with a hollow sandwich structure is shown in Fig. 1. First, SiO2 nanospheres were coated with nitrogen-doped carbon to form SiO2/N-carbon, and then nickel silicate sheets were anchored to the surface of the N-carbon spheres to form N-carbon/NiSi/SiO2. The SiO2 and N-carbon spheres were used as sacrificial templates. The SiO2 was etched away, leaving N-doped hollow carbon spheres (NHC), which were vulcanized to form NHC coated with Ni3S2 nanosheets (NHC/Ni3S2) and finally sandwiched with RGO to form a multilayer composite structure (NHC/Ni3S2/RGO).SEM and TEM were used to characterize the morphology of the nanocomposites. Fig. 2 shows SEM images at different magnification of NHC/Ni3S2 (Fig. 2a and b) and NHC/Ni3S2/RGO (Fig. 2c and d). In Fig. 2a, many uniform nanospheres are visible (magnification of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). Fig. 2b shows that the nanospheres are about 400 nm in diameter (high magnification of 120
000×). Fig. 2b shows that the nanospheres are about 400 nm in diameter (high magnification of 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×). Criss-crossing nanosheets are attached to the surface of the nanospheres, and the gaps between the nanosheets are clearly visible. These loose sheets increase the specific surface area of the material, and thus increase the contact area between the electrolyte and the material to minimize dead volume. Fig. 2c and d show images of NHC/Ni3S2/RGO, and it is clear that the RGO encloses the complex. The introduction of RGO increases the electronic transport network and protects the material from corrosion and collapse in the acid–base electrolyte. The TEM images (Fig. 2e–h) show that the hollow structure of the Ni3S2 nanosheet shell combined with NHC provides abundant electrochemical active sites and a large contact area for the electrolytic electrode for electrochemical reactions.33 The NHC promotes electron transfer and improves the speed of electrochemical reactions.34 Element mapping by TEM-EDS of the NHC/Ni3S2/RGO nanocomposite is shown in Fig. 2i–m. C, N, S, and Ni are uniformly distributed over the hollow sphere shell structure without other impurities.
000×). Criss-crossing nanosheets are attached to the surface of the nanospheres, and the gaps between the nanosheets are clearly visible. These loose sheets increase the specific surface area of the material, and thus increase the contact area between the electrolyte and the material to minimize dead volume. Fig. 2c and d show images of NHC/Ni3S2/RGO, and it is clear that the RGO encloses the complex. The introduction of RGO increases the electronic transport network and protects the material from corrosion and collapse in the acid–base electrolyte. The TEM images (Fig. 2e–h) show that the hollow structure of the Ni3S2 nanosheet shell combined with NHC provides abundant electrochemical active sites and a large contact area for the electrolytic electrode for electrochemical reactions.33 The NHC promotes electron transfer and improves the speed of electrochemical reactions.34 Element mapping by TEM-EDS of the NHC/Ni3S2/RGO nanocomposite is shown in Fig. 2i–m. C, N, S, and Ni are uniformly distributed over the hollow sphere shell structure without other impurities.
2.2 Crystal structure and composition
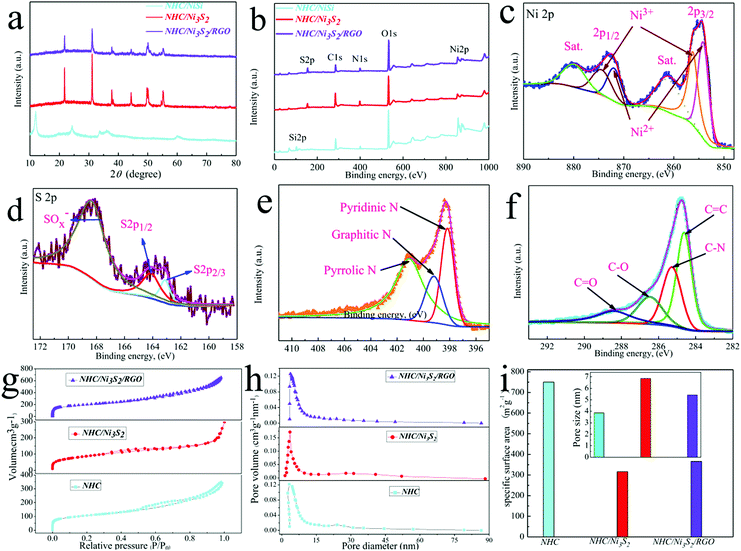
The crystal structures of NHC/NiSi, NHC/Ni3S2 and NHC/Ni3S2/RGO were characterized by XRD (Fig. 3a). The characteristic peaks at 21.7°, 31.1°, 38.3°, 44.3°, 55.1° and 77.6° corresponded to the (101), (110), (021), (202), (122) and (223) crystal planes of Ni3S2 (JCPSD 44-1418) in the NHC/Ni3S2/RGO composite, respectively.35 The XRD pattern of NHC/Ni3S2 was similar to that of NHC/Ni3S2/RGO, except that the peak strengths at 21.7° or 31.1° were decreased by the surface coverage of RGO, which confirmed the introduction of RGO.The surface chemical states of the samples were characterized by XPS (Fig. 3b). Only the S 2p (153.9 eV), C 1s (285.3 eV), N 1s (399.6 eV), O 1s (533.5 eV), and Ni 2p (854.7 eV) peaks were observed. There were no impurities, which was in good agreement with the element mapping results. The Ni 2p region (Fig. 3c) contained two peaks at 854.5 and 872.6 eV for Ni 2p2/3 and Ni 2p1/2, respectively. The peaks at 874.7 and 856.2 eV corresponded to Ni3+, and the peaks at 872.1 and 854.3 eV corresponded to Ni2+. The S 2p (Fig. 3d) region contained two peaks at 163.1 and 164.1 eV for S 2p2/3 and S 2p1/2, respectively, and the peak at 168.4 eV corresponded to SOx−. The N 1s region is shown in Fig. 3e. The N 1s peak was fitted to the following three components according to the binding energies: pyridinic N (401.1 eV), graphitic N (399.2 eV), and pyrrolic N (398.2 eV).36 The C 1s spectra of NHC/Ni3S2/RGO showed typical peaks for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.6 eV), C–N (285.3 eV), C–O (286.5 eV) and C
C (284.6 eV), C–N (285.3 eV), C–O (286.5 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.4 eV).37 These results indicated N was incorporated into the hollow carbon sphere.
O (288.4 eV).37 These results indicated N was incorporated into the hollow carbon sphere.
2.3 Brunauer–Emmett–Teller analysis
The specific surface area and porosity of NHC, NHC/Ni3S2, and NHC/Ni3S2/RGO were determined by nitrogen adsorption and desorption isotherms (Fig. 3g). NHC had a large specific surface area (750.27 m2 g−1), owing to the hollow spherical frame. Adding Ni3S2 sheets to the surface of NHC to form NHC/Ni3S2 decreased the specific surface area (316.74 m2 g−1). However, the introduction of RGO increased the specific surface area to 365.84 m2 g−1 for the NHC/Ni3S2/RGO composite. This increase was attributed to the large internal space, rough surface structure and the introduction of RGO. The large specific surface area increased the contact area, avoided excessive dead volume, and introduced more active sites, which improved the electrochemical properties. The pore size distribution was evaluated using the Barrett–Joyner–Halenda method (Fig. 3h). The pore size distributions of NHC, NHC/Ni3S2 and NHC/Ni3S2/RGO were evaluated as 3.89, 6.85, and 5.40 nm, respectively.2.4 Performance of DSSCs
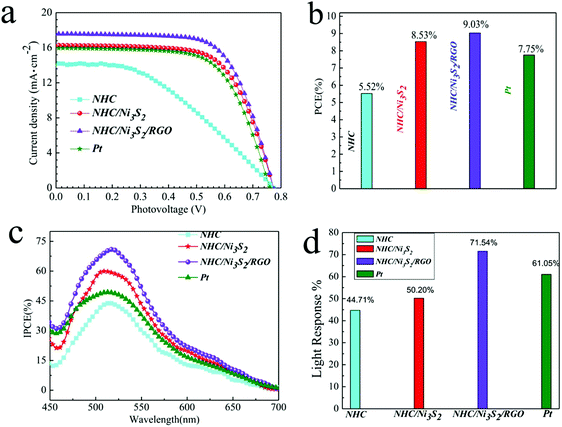
To demonstrate the application of the as-prepared materials as the CE in DSSCs and to measure the photovoltaic performance, the photocurrent density–voltage (J–V) curves of NHC, NHC/Ni3S2, and NHC/Ni3S2/RGO (Fig. 4a) were tested under AM 1.5 irradiation (100 mW cm−2),38 and the results are shown in Fig. 4b. The PCE of NHC, NHC/Ni3S2, NHC/Ni3S2/RGO, and Pt as DSSC CEs were 5.52%, 8.53%, 9.03% and 7.75%, respectively. NHC/Ni3S2/RGO had the best PCE (9.03%), which was almost twice that of NHC (5.52%), and higher than that of Pt (7.75%). All parameters are listed in Table 1. The photoelectric transformation performance results show that in the optimized structure, the hollow carbon spheres provide huge ion storage, which rapidly provides electrolyte ions for the electrochemical reaction, and the conductivity of the carbon shell also provides a better channel for electron transfer. However, the relatively smooth surface structure of the carbon shell only provides a limited number of active sites for taking up electrons.8,39–41 In contrast, the increased surface roughness NHC/Ni3S2 and NHC/Ni3S2/RGO increases the number of active sites, which allows greater take up of electrons, and thus increases the speed of the transformation of the iodine ions.42| CEs | R s (Ω cm2) | R ct (Ω cm2) | lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J0 (mA cm−2) J0 (mA cm−2) |
lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Jlim (mA cm−2) Jlim (mA cm−2) |
J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|---|---|
| NHC | 19.1 ± 0.01 | 1.88 ± 0.01 | 0.44 ± 0.01 | 1.51 ± 0.01 | 14.1 | 0.773 | 50.57 | 5.52 |
| NHC/Ni3S2 | 13.8 ± 0.01 | 0.25 ± 0.01 | 0.73 ± 0.01 | 1.67 ± 0.01 | 16.3 | 0.768 | 67.97 | 8.53 |
| NHC/Ni3S2/RGO | 13.7 ± 0.01 | 0.14 ± 0.01 | 0.87 ± 0.01 | 1.69 ± 0.01 | 17.7 | 0.773 | 66.44 | 9.03 |
| Pt | 13.9 ± 0.01 | 0.27 ± 0.01 | 0.56 ± 0.01 | 1.54 ± 0.01 | 14.1 | 0.759 | 64.14 | 7.75 |
Fig. 4c shows the incident photoelectron conversion efficiency (IPCE) curve for DSSCs containing each material as a photoanode with a sandwich structure. The IPCE spectra show a similar photoelectric response in the wavelength range of 450 to 750 nm. The results show that the photoelectric response values of traditional Pt, NHC/Ni3S2/RGO, NHC/Ni3S2, and NHC were 61.05%, 71.54%, 50.20% and 44.71%, respectively (Fig. 4d). Therefore, NHC/Ni3S2/RGO can be used as a promising alternative electrode for platinum-free DSSCs.
Based on the photoelectric conversion properties, the electrocatalytic properties were examined by cyclic voltammetry (CV) curves in a three-electrode system with a scanning step of 25 mV s−1. Fig. S1a (ESI†) indicates that the NHC/Ni3S2/RGO nanocomposite had better catalytic activity than the other as-prepared samples and conventional Pt for I3− in the electrolyte. The two pairs of redox peaks are labeled Ox1 and Red1, and Ox2 and Red2. Eqn (1) and (2) describe the cycling of I3− catalyzed by the CE in a DSSC system.43
| I3− + 2e− = 3I−, | (1) |
| 3I2 + 2e− = 2I3−. | (2) |
The strength of the peak represents the redox ability for I3− of the material, and a strong peak indicates a high current density, which mainly reflects the adsorption and transfer of ions on the surface of the CE material and is also an important factor for the evaluation of iodide current density. The order of peak strength of the materials is NHC/Ni3S2/RGO > NHC/Ni3S2 > Pt > NHC, showing that NHC/Ni3S2/RGO has a huge specific surface area, which not only takes up more electrons and increases the current density, but also achieves super catalytic reduction of I3−. Epp is another important parameter for CE materials to assess the catalytic activity for I3− reduction.44 In the redox reaction of iodide ions, a narrower Epp indicates higher catalytic velocity. NHC/Ni3S2/RGO had the smallest Epp value of the CEs, which was 23% lower than the Epp value of conventional Pt. The values are shown in Fig. S1b (ESI†). The corrosive nature of the electrolyte destroys TMS, which hampers the continuous generation of surface active sites and affects the lifespan of the material. Thus, flexible RGO was used to encapsulate the NHC/Ni3S2 nanocomposites and provide a layer of protection. We also performed electrochemical stability analysis on the materials in the electrolyte by increasing the number of CV scans to 30 (Fig. S2, ESI†). Only the CV trace for NHC/Ni3S2/RGO remained virtually unchanged, revealing that NHC/Ni3S2/RGO had stable electrochemical properties and good corrosion resistance. Incorporating RGO has other advantages, such as creating an RGO network structure that provides more electron transmission paths and increasing the specific surface area, which provides more active sites. Thus, RGO allows excellent conductivity and remarkable catalytic activity to be achieved.45
The electrocatalytic properties were also assessed via electrochemical impedance spectroscopy (EIS) and Tafel polarization measurements. An EIS test was performed to evaluate the electron transfer process and exchange current density data at the interface between the electrolyte and the CE (Fig. S3, ESI†). In the figure, the equivalent circuit model, containing series resistance (Rs) and charge transfer resistance (Rct), was used to fit the recorded data. The results contained two semicircles. The high-frequency region is the semicircle on the left in the Nyquist curve, which is usually related to the electrocatalytic performance of the CE.46 The intercept between the left semicircle and the x-axis in the high-frequency region represents Rs for the entire cell. The left semicircle in the high-frequency region represents Rct, and is the sum of resistances generated during the redox process at the interface between the CE and the electrolyte.47 The right semicircle is the low-frequency region, indicating the Nernst diffusion impedance (ZN) of redox-coupled transport in the electrolyte.48 The values are shown in Table 1. The order of Rct and Rs values for the four samples is NHC/Ni3S2/RGO < NHC/Ni3S2 < Pt < NHC because NHC/Ni3S2/RGO has multiple channels formed by the crossing nanosheets. The results show that NHC/Ni3S2/RGO has excellent electrocatalytic properties. The addition of RGO greatly increases the contact area of ions, prevents dead volume, increases the number of electron transmission channels and greatly improves the conductivity. The smaller impedance allows the electrons to flow more smoothly between the electrode material and the electrolyte.
Tafel polarization measurements were carried out in a symmetrical battery (Fig. S4, ESI†). Theoretically, Tafel curves can be divided into the polarization region, Tafel region and diffusion region. The Tafel and diffusion zones are used to elucidate the electrocatalytic activity of the CE in the reduction of I3−.49 Exchange current density (J0) and diffusion-limited current density (Jlim) are extracted from the Tafel region and diffusion region, respectively. The values of J0 and Jlim are shown in Table 1. Both J0 and Jlim of NHC/Ni3S2/RGO are the largest values among the materials, including Pt, indicating that as a CE, this material has excellent electrocatalytic activity for I3− reduction, which is also consistent with the electrochemical test results. The excellent catalytic activity of the material was attributed to the hollow spherical shell that has a large specific surface area, the high electrical conductivity of Ni3S2 nanosheets, and creation of electronic transmission channels by RGO. Thus, the current density remained high throughout the electrochemical reaction.
2.5 Electrochemical properties of supercapacitors (pseudocapacitance)
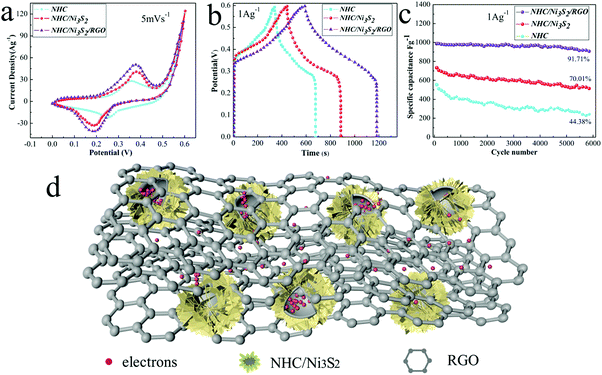
To evaluate the electrochemical pseudocapacitance of the electrode materials, we first determined their CV curves with a constant-current charge–discharge technique in a three-electrode system, with KOH solution (6 M) as the electrolyte. The CV curves for NHC, NHC/Ni3S2, and NHC/Ni3S2/RGO (Fig. 5a) were typical, with a potential window of 0–0.6 V (vs. Ag/AgCl) at a scan rate of 5 mV s−1, indicating typical pseudocapacitive behaviors.50 The capacitance is mainly affected by the Faraday redox reaction.51 All the CV curves consisted of a pair of strong redox peaks corresponding to the conversion reaction of the materials in KOH solution. At a potential of 0.37 V, the CV curves of NHC/Ni3S2 and NHC/Ni3S2/RGO both had a clear anode oxidation peak, but the peak for NHC/Ni3S2/RGO was much higher, demonstrating an excellent oxidation reaction. The cathode peak was observed near a potential of 0.185 V, and corresponded to the reduction process in the Faraday reaction. The anode and cathode peaks were symmetric, showing that the NHC/Ni3S2/RGO active material electrode had excellent reversibility. In addition, when the scan rate was increased from 5 to 100 mV s−1, the shape of the CV curves did not change substantially (Fig. S5, ESI†), which indicated that the mass transfer rates were increased, the electron conduction was increased, and the equivalent series resistance was decreased. In addition, the anode peaks moved to the positive pole and the cathode peaks moved to the negative pole because of the internal resistance of the electrode materials.51Specific capacitance is a key factor in determining whether an active material can be used as the electrode of a supercapacitor, which is represented by C (F g−1). The specific capacitance is calculated by eqn (3).
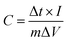 | (3) |
The mechanism of the excellent capacitance of the electrodes consisting of the active materials was studied further by electrochemical EIS (Fig. S8, ESI†). Fig. S9 (ESI†) is an enlargement of the high-frequency region. The high-frequency region of the NHC/Ni3S2/RGO electrode active material is a pseudo-semicircle, whereas those of the other two materials are not, suggesting the NHC/Ni3S2/RGO electrode active material has smaller charge transfer resistance.52 In the low-frequency region, the slope of the NHC/Ni3S2/RGO electrode active material is higher than those of the other two materials, and indicating that the NHC/Ni3S2/RGO electrode has lower diffusion resistance and faster ion velocity.
To evaluate the durability and the performance during long-term cycling of each active material at a current density of 1 A g−1, the GCD curves were measured for each active material for 6000 cycles. After 6000 cycles, the specific capacitance of the NHC/Ni3S2/RGO electrode remained at 91.71% of the original value, whereas those of NHC/Ni3S2 and NHC were only 70.01% and 44.38%, respectively (Fig. 5c). These results also demonstrate that the NHC/Ni3S2/RGO electrode material had more stable electrochemical and cyclic properties that the other electrode materials. Fig. 5d shows the electrochemical mechanism of the materials. NHC/Ni3S2/RGO had good specific capacitance because the hollow carbon spheres function as a huge electronic vault, the electrical conductivity of the Ni3S2 nanosheets is large, and RGO forms electronic transmission channels. The electrolyte can easily diffuse into NHC/Ni3S2/RGO through the open spaces between the crisscross nanosheets. The hollow sandwich structure ensures that NHC/Ni3S2/RGO is in close contact with the electrolyte, and the electrons and electrolyte ions can easily diffuse.
2.6 Electrocatalytic acidic HER
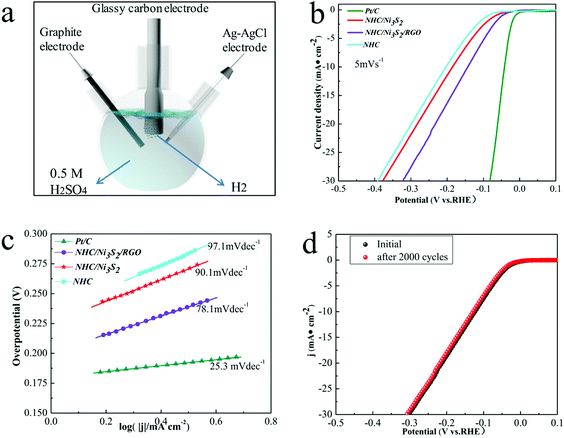
Hollow carbon materials and TMS have been widely used for the electrocatalytic HER. However, composite materials consisting of TMS grown on the surface of hollow carbon materials, particularly the hollow sandwich structure formed by combining carbon materials with different morphologies and TMS, have seldom been used in this field. For the acidic electrocatalytic HER, a NHC/Ni3S2/RGO catalyst with large pores, high specific surface area and non-noble metals was prepared. The excellent performance of the NHC/Ni3S2/RGO nanocomposite in DSSCs and supercapacitors suggested that it may have catalytic activity in the electrocatalytic HER.To test whether our assumption was correct, we measured the electrochemical HER performance of the NHC/Ni3S2/RGO catalyst by linear scanning voltammetry (LSV).53 The HER catalytic performances of NHC/Ni3S2/RGO, NHC/Ni3S2, NHC and Pt/C (20 wt%) were evaluated with a three-electrode standard test system with a scan range of 0.1 to −0.6 V (vs. RHE), and the electrolyte was H2SO4 (0.5 M) (Fig. 6a). The as-prepared catalyst materials were placed on a rotating disk electrode and the scan rate was 5 mV s−1 at 2000 rpm. Owing to the subjectivity of the determination of the onset potential, the overpotential corresponding to the current density of 0.05–5 mA cm−2 is usually determined as the onset overpotential.54 In this paper, the overpotential corresponding to the current density of 0.25 mA cm−2 was determined as the onset overpotential. The LSV polarization curves show that the onset overpotential of the NHC/Ni3S2/RGO catalyst was closer to that of the Pt/C catalyst (20 wt%) in Fig. 6b. The preliminary results showed that the NHC/Ni3S2/RGO had good electrocatalytic activity. When the current density was increased to 10 mA cm−2, the overpotentials of NHC/Ni3S2 and NHC were −186.27 and −205.46 mV, respectively, whereas it was only −142 mV for NHC/Ni3S2/RGO because of the quicker discharge reaction of H+ (2H+ → H2) in acid solution (Fig. S10, ESI†).55 For a current density of 15 mA cm−2, the overpotential of NHC/Ni3S2/RGO was −188.13 mV, which was only −45.84 mV higher than that of 10 mA cm−2, whereas the overpotential of NHC/Ni3S2 was −235.05 mV. In addition, the current density increases rapidly with the increase of overpotential and the current density is also an important standard factor for indicating the catalytic activity of catalysts.53,54 In further comparisons, when the overpotential reached −150 mV, the cathode current density of NHC/Ni3S2/RGO was 10.89 mA cm−2, which was larger than that of NHC/Ni3S2 (6.63 mA cm−2) and 2.1 times higher than that of NHC (5.11 mA cm−2). When the overpotential was further increased to 200 mV, the current density of NHC/Ni3S2/RGO reached 16.32 mA cm−2, which was 1.68 times that of NHC (9.69 mA cm−2). The current density of NHC/Ni3S2/RGO was much higher than those of NHC/Ni3S2 and NHC when the overpotential was the same.
To explore the electrocatalytic HER activity of NHC/Ni3S2/RGO further, the Tafel curves (Fig. 6c) of the four samples were measured with the potential negatively scanned from −0.5 to 0.1 V (vs. RHE) at a scan rate of 5 mV s−1. The Tafel curves were plotted as the overpotential change to the logarithm of the current density. The intrinsic characteristics of electrocatalytic hydrogen evolution materials are determined from the Tafel slopes, which are obtained by fitting the linear part of the Tafel curves.55 Generally, in accordance with the values of the Tafel slope, there are three classical reactions in the catalytic mechanism of HER: the Volmer reaction, the Heyrovsky reaction, and the Tafel reaction, which have slopes of 118.2, 39.4, and 29.6 mV dec−1, respectively.56,57 The Tafel slope of as-prepared NHC/Ni3S2/RGO was 98.1 mV dec−l; thus, the catalytic mechanism of HER matched the Volmer–Heyrovsky reaction. A smaller Tafel slope shows better scaling of the kinetics with voltage and faster kinetics of hydrogen evolution.57 The Tafel slope of NHC/Ni3S2/RGO was closest to that of 20 wt% Pt/C (35.3 mV dec−l) (Fig. 6c). The results show that the NHC/Ni3S2/RGO has excellent performance for electrocatalytic hydrogen evolution. Nitrogen atom doping changes the crystal structure of the material and rearranges the surface electron distribution, resulting in more surface defects.58 The rough surface of the hollow structure greatly increased the specific surface area to produce more hydrogen evolution active sites.59 RGO increased the electron transport rate and the current density.
To assess the durability of NHC/Ni3S2/RGO as a catalytic material, long-term CV between 0.1 and 0.50 V (vs. RHE) was performed. When the current density was 10 mA cm−2, the polarization curve period of the catalytic material after 2000 cycles almost overlapped with the initial period, indicating the excellent durability of the material for electrocatalysis (Fig. 6d).60,61
3. Conclusions
We demonstrated the use of NHC as substrate for preparing NHC/Ni3S2/RGO nanocomposites with a multifunctional sandwich structure for use in DSSCs, supercapacitors, and the acidic HER. Electrochemical tests proved that the NHC/Ni3S2/RGO nanocomposite exhibited better catalytic activity than NHC, NHC/Ni3S2 and Pt, demonstrating the essential role of the sandwich structure and heteroatom doping affecting the electronic modulation from the charge redistribution. The electrolyte easily diffused into the NHC/Ni3S2/RGO nanocomposite through the openings between the crisscross nanosheets. The hollow sandwich structure ensured that the NHC/Ni3S2/RGO nanocomposite was in close contact with the electrolyte, and that the electrons and electrolyte ions easily diffused in between. The nanocomposite is a promising candidate for improving the performance of various energy technologies and devices.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by National Key R&D Program of China (2017YFA0403503), National Natural Science Foundation of China (11674001), Open Fund for Discipline Construction, Institute of Physical Science and Information Technology (S01003103, Anhui University), Anhui Provincial Natural Science Foundation (1708085MA07, 1708085QE116), the Key Natural Science Research Program of Anhui Educational Committee (KJ2018ZD001), and the doctoral research start-up funds projects of Anhui University (J01003201). We are grateful for the experimental materials provided by Nanjing XianFeng Nanomaterials Technology Co., Ltd. We acknowledge the experimental instruments and technical guidance provided by PINE Company and Hong Kong PHYCHEMi Company.References
- X. Y. Yu, L. Yu and X. W. D. Lou, Adv. Energy Mater., 2016, 6, 1501333 CrossRef.
- Y. Hou, D. Wang, X. H. Yang, W. Q. Fang, B. Zhang, H. F. Wang, G. Z. Lu, P. Hu, H. J. Zhao and H. G. Yang, Nat. Commun., 2013, 4, 1583 CrossRef PubMed.
- Q. Tang, W. Zhu, B. He and P. Yang, ACS Nano, 2017, 11, 1540–1547 CrossRef CAS PubMed.
- H. Wang, G. Liu, X. Li, P. Xiang, Z. Ku, Y. Rong, M. Xu, L. Liu, M. Hu, Y. Yang and H. Han, Energy Environ. Sci., 2011, 4, 2025–2029 RSC.
- X. Meng, C. Yu, X. Song, J. Iocozzia, J. Hong, M. Rager, H. Jin, S. Wang, L. Huang, J. Qiu and Z. Lin, Angew. Chem., Int. Ed., 2018, 57, 4682–4686 CrossRef CAS PubMed.
- X. Meng, C. Yu, X. Zhang, L. Huang, M. Rager, J. Hong, J. Qiu and Z. Lin, Nano Energy, 2018, 54, 138–147 CrossRef CAS.
- J. M. Rhodes, C. A. Jones, L. B. Thal and J. E. Macdonald, Chem. Mater., 2017, 29, 8521–8530 CrossRef CAS.
- W. Yang, X. Xu, Y. Gao, Z. Li, C. Li, W. Wang, Y. Chen, G. Ning, L. Zhang, F. Yang, S. Chen, A. Wang, J. Kong and Y. Li, Nanoscale, 2016, 8, 13059–13066 RSC.
- T. Liu, C. Jiang, B. Cheng, W. You and J. Yu, J. Mater. Chem. A, 2017, 5, 21257–21265 RSC.
- F. Gong, H. Wang, X. Xu, G. Zhou and Z. S. Wang, J. Am. Chem. Soc., 2012, 134, 10953–10958 CrossRef CAS PubMed.
- S. Liu, Z. Wang, S. Zhou, F. Yu, M. Yu, C. Y. Chiang, W. Zhou, J. Zhao and J. Qiu, Adv. Mater., 2017, 29, 1700874 CrossRef PubMed.
- X. Liu, K. Yan, D. Tan, X. Liang, H. Zhang and W. Huang, ACS Energy Lett., 2018, 3, 2701–2707 CrossRef CAS.
- F. Yu, Y. Shi, X. Shen, W. Yao, S. Han and J. Ma, ACS Sustainable Chem. Eng., 2018, 6, 17427–17434 CrossRef CAS.
- C. Yu, X. Meng, X. Song, S. Liang, Q. Dong, G. Wang, C. Hao, X. Yang, T. Ma, P. M. Ajayan and J. Qiu, Carbon, 2016, 100, 474–483 CrossRef CAS.
- L. Chen, W. L. Chen, X. L. Wang, Y. G. Li, Z. M. Su and E. B. Wang, Chem. Soc. Rev., 2019, 48, 260–284 RSC.
- M. Ye, X. Wen, M. Wang, J. Iocozzia, N. Zhang, C. Lin and Z. Lin, Mater. Today, 2015, 18, 155–162 CrossRef CAS.
- J. Yao, K. Zhang, W. Wang, X. Zuo, Q. Yang, M. Wu and G. Li, Electrochim. Acta, 2018, 279, 168–176 CrossRef CAS.
- H. Jiang, Y. Lin, B. Chen, Y. Zhang, H. Liu, X. Duan, D. Chen and L. Song, Mater. Today, 2018, 21, 602–610 CrossRef CAS.
- Y. Huang, X. Song, J. Deng, C. Zha, W. Huang, Y. Wu and Y. Li, Appl. Catal., B, 2019, 245, 656–661 CrossRef CAS.
- F. Yu, H. Zhou, Y. Huang, J. Sun, F. Qin, J. Bao, W. A. Goddard, 3rd, S. Chen and Z. Ren, Nat. Commun., 2018, 9, 2551 CrossRef PubMed.
- S. Tang, B. Zhu, X. Shi, J. Wu and X. Meng, Adv. Energy Mater., 2017, 7, 1601985 CrossRef.
- R. Yan, M. Antonietti and M. Oschatz, Adv. Energy Mater., 2018, 8, 1800026 CrossRef.
- J. Zhu, S. Tang, J. Wu, X. Shi, B. Zhu and X. Meng, Adv. Energy Mater., 2017, 7, 1601234 CrossRef.
- S. S. Shinde, J. Y. Yu, J. W. Song, Y. H. Nam, D. H. Kim and J. H. Lee, Nanoscale Horiz., 2017, 2, 333–341 RSC.
- J. Balamurugan, T. D. Thanh, N. H. Kim and J. H. Lee, J. Mater. Chem. A, 2016, 4, 9555–9565 RSC.
- F. X. Ma, L. Yu, C. Y. Xu and X. W. Lou, Energy Environ. Sci., 2016, 9, 862–866 RSC.
- N. Gao and X. Fang, Chem. Rev., 2015, 115, 8294–8343 CrossRef CAS PubMed.
- C. T. Li, F. L. Wu, B. H. Lee, M. P. Yeh and J. T. Lin, ACS Appl. Mater. Interfaces, 2017, 9, 43739–43746 CrossRef CAS PubMed.
- M. Guo, J. Balamurugan, T. D. Thanh, N. H. Kim and J. H. Lee, J. Mater. Chem. A, 2016, 4, 17560–17571 RSC.
- Z. Zhang, J. Sun, F. Wang and L. Dai, Angew. Chem., Int. Ed., 2018, 57, 9038–9043 CrossRef CAS PubMed.
- G. L. Chai, K. Qiu, M. Qiao, M. M. Titirici, C. Shang and Z. Guo, Energy Environ. Sci., 2017, 10, 1186–1195 RSC.
- K. Qu, Y. Zheng, X. Zhang, K. Davey, S. Dai and S. Z. Qiao, ACS Nano, 2017, 11, 7293–7300 CrossRef CAS PubMed.
- J. Yao, K. Zhang, W. Wang, X. Zuo, Q. Yang, H. Tang, M. Wu and G. Li, Nanoscale, 2018, 10, 7946–7956 RSC.
- C. Wang, F. Wang, Z. Liu, Y. Zhao, Y. Liu, Q. Yue, H. Zhu, Y. Deng, Y. Wu and D. Zhao, Nano Energy, 2017, 41, 674–680 CrossRef CAS.
- C. Yang, M. Y. Gao, Q. B. Zhang, J. R. Zeng, X. T. Li and A. P. Abbott, Nano Energy, 2017, 36, 85–94 CrossRef CAS.
- Z. Cao, M. Wu, H. Hu, G. Liang and C.-y. Zhi, NPG Asia Mater., 2018, 10, 670–684 CrossRef CAS.
- J. Yao, K. Zhang, W. Wang, X. Zuo, Q. Yang, H. Tang, M. Wu and G. Li, ACS Appl. Mater. Interfaces, 2018, 10, 19564–19572 CrossRef CAS PubMed.
- W. Ouyang, F. Teng, J. H. He and X. Fang, Adv. Funct. Mater., 2019, 29, 1807672 CrossRef.
- S. K. Swami, N. Chaturvedi, A. Kumar, R. Kapoor, V. Dutta, J. Frey, T. Moehl, M. Grätzel, S. Mathew and M. K. Nazeeruddin, J. Power Sources, 2015, 275, 80–89 CrossRef CAS.
- B. Yang, X. Zuo, P. Chen, L. Zhou, X. Yang, H. Zhang, G. Li, M. Wu, Y. Ma, S. Jin and X. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 137–143 CrossRef CAS PubMed.
- F. Liu, J. Zhu, L. Hu, B. Zhang, J. Yao, M. K. Nazeeruddin, M. Grätzel and S. Dai, J. Mater. Chem. A, 2015, 3, 6315–6323 RSC.
- S. Liu, L. Zheng, P. Yu, S. Han and X. Fang, Adv. Funct. Mater., 2016, 26, 3331–3339 CrossRef CAS.
- X. Meng, C. Yu, X. Song, Y. Liu, S. Liang, Z. Liu, C. Hao and J. Qiu, Adv. Energy Mater., 2015, 5, 1500180 CrossRef.
- X. Meng, C. Yu, X. Song, Z. Liu, B. Lu, C. Hao and J. Qiu, J. Mater. Chem. A, 2017, 5, 2280–2287 RSC.
- F. Du, X. Zuo, Q. Yang, B. Yang, G. Li, H. Tang, H. Zhang, M. Wu and Y. Ma, Sol. Energy Mater. Sol. Cells, 2016, 149, 9–14 CrossRef CAS.
- W. Wei, K. Sun and Y. H. Hu, J. Mater. Chem. A, 2016, 4, 12398–12401 RSC.
- X. Cui, J. Xiao, Y. Wu, P. Du, R. Si, H. Yang, H. Tian, J. Li, W. H. Zhang, D. Deng and X. Bao, Angew. Chem., Int. Ed., 2016, 55, 6708–6712 CrossRef CAS PubMed.
- X. Cui, W. Xu, Z. Xie and Y. Wang, J. Mater. Chem. A, 2016, 4, 1908–1914 RSC.
- W. Hou, Y. Xiao and G. Han, Angew. Chem., Int. Ed., 2017, 56, 9146–9150 CrossRef CAS PubMed.
- P. Yu, Z. Zhang, L. Zheng, F. Teng, L. Hu and X. Fang, Adv. Energy Mater., 2016, 6, 1601111 CrossRef.
- Y. Yang, K. Shen, Y. Liu, Y. Tan, X. Zhao, J. Wu, X. Niu and F. Ran, Nano-Micro Lett., 2017, 9, 6 CrossRef PubMed.
- J. Yang, X. Duan, Q. Qin and W. Zheng, J. Mater. Chem. A, 2013, 1, 7880–7884 RSC.
- L. Yu, B. Yang, Q. Liu, J. Liu, X. Wang, D. Song, J. Wang and X. Jing, J. Electroanal. Chem., 2015, 739, 156–163 CrossRef CAS.
- B. Y. Guan, L. Yu, X. Wang, S. Song and X. W. Lou, Adv. Mater., 2017, 29, 1605051 CrossRef PubMed.
- J. Wang, D. Chao, J. Liu, L. Li, L. Lai, J. Lin and Z. Shen, Nano Energy, 2014, 7, 151–160 CrossRef CAS.
- X. Zou, X. Huang, A. Goswami, R. Silva, B. R. Sathe, E. Mikmekova and T. Asefa, Angew. Chem., Int. Ed., 2014, 53, 4372–4376 CrossRef CAS PubMed.
- R. Wu, D. P. Wang, X. Rui, B. Liu, K. Zhou, A. W. Law, Q. Yan, J. Wei and Z. Chen, Adv. Mater., 2015, 27, 3038–3044 CrossRef CAS PubMed.
- Y. Zang, S. Niu, Y. Wu, X. Zheng, J. Cai, J. Ye, Y. Xie, Y. Liu, J. Zhou, J. Zhu, X. Liu, G. Wang and Y. Qian, Nat. Commun., 2019, 10, 1217 CrossRef PubMed.
- J. Mao, C. He, J. Pei, W. Chen, D. He, Y. He, Z. Zhuang, C. Chen, Q. Peng, D. Wang and Y. Li, Nat. Commun., 2018, 9, 4958 CrossRef PubMed.
- T. Zhang, L. Hang, Y. Sun, D. Men, X. Li, L. Wen, X. Lyu and Y. Li, Nanoscale Horiz., 2019 10.1039/c9nh00177h.
- Y. Lin, L. Yang, Y. Zhang, H. Jiang, Z. Xiao, C. Wu, G. Zhang, J. Jiang and L. Song, Adv. Energy Mater., 2018, 8, 1703623 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9nh00133f |
| This journal is © The Royal Society of Chemistry 2019 |