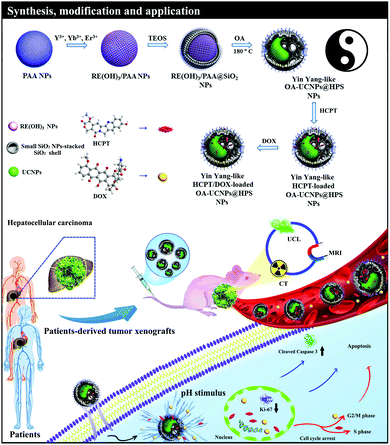
Engineering of Yin Yang-like nanocarriers for varisized guest delivery and synergistic eradication of patient-derived hepatocellular carcinoma†
Xiangjun
Chen
a,
Xiuping
Zhang
b,
Shengnan
Li
a,
Lingyu
Zhang
a,
Qi
Zhang
a,
Zhenhua
Chen
b,
Lu
Li
 *a,
Zhong-Min
Su
*a,
Zhong-Min
Su
 a,
Shuqun
Cheng
*b and
Chungang
Wang
a,
Shuqun
Cheng
*b and
Chungang
Wang
 *a
*a
aDepartment of Chemistry, Northeast Normal University, 5268 Renmin Street, Changchun, Jilin 130024, P. R. China. E-mail: lil106@nenu.edu.cn; wangcg925@nenu.edu.cn
bDepartment of Hepatic Surgery VI, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University, Shanghai, 200433, P. R. China. E-mail: chengshuqun@aliyun.com
First published on 13th April 2019
Abstract
During the different clinical manifestations and periods in the treatment of hepatocellular carcinoma (HCC), the species and doses of drugs could be optimized to realize personalized therapy for an individual patient. Here, we firstly reported Yin Yang-like ultrasmall nanoparticles-stacked oleic acid–NaYF4:Yb,Er@hollow porous SiO2 nanoparticles (Yin Yang-like OA–UCNPs@HPS NPs) with a hydrophobic eccentric hollow structure and hydrophilic exterior surface possessing a wide pore size distribution via a novel method. The obtained NPs achieved the loading of multiple varisized hydrophobic/hydrophilic guests into the discrete rooms and the release of each one from the independent channels to reduce the undesirable adverse effects of different guests, which greatly favors the selection of drugs for an individual patient and enhances synergistic theranostics. The in vitro and in vivo (cell line-derived xenograft and patient-derived xenograft models) results demonstrated that the HCPT/DOX-loaded NPs could act as excellent theranostics agents for multimodal imaging-guided synergic dual-drug cancer therapy of HCC. Meanwhile, the potential mechanisms of action of the HCPT/DOX-loaded NPs after endocytosis by HCC cells were evaluated. The nanocomposite could further motivate the progress of new architectures of nanocarriers for varisized guest delivery and personalized treatment.
Conceptual insightsYin Yang-like nanoparticles (NPs) were firstly reported via a novel method. The unique structure of the Yin Yang-like NPs possessing hierarchical pores on the hydrophilic exterior surface and an eccentric hydrophobic cavity achieved loading of multiple varisized hydrophobic/hydrophilic guests into the discrete rooms and the release of each one from the independent channels to reduce the undesirable adverse effects of different guests compared with the most widely used carriers, for instance, hollow structured NPs and core–shell NPs. This greatly favors the selection of drugs for an individual patient and enhances synergistic theranostics. Our study not only settles the problem of NP-based drug delivery systems applied to potential hepatocellular carcinoma (HCC) treatments during the different clinical manifestations and periods needed for various drugs, but the nanocomposite could also further motivate the progress of new architectures of nanocarriers for varisized guest delivery and personalized treatment. |
Hepatocellular carcinoma (HCC) is one of the most deadly malignancies worldwide, especially in the Asia-Pacific regions where hepatitis B virus (HBV) infection is endemic.1,2 Liver resection or liver transplantation is still the first-line curative therapy for early-stage HCC, but most intermediate-advanced HCC patients do not meet the criteria. Unfortunately, the 5 year recurrence rates after curative liver resection are as high as 70–80%, which seriously restricts the long-term prognosis of HCC patients.3 According to official guidelines of HCC treatments,4 traditional chemotherapy is the recommended treatment for most inoperable and HCC-recurrent patients. However, the insensitivity of traditional chemotherapy and single drug resistance to chemotherapeutic agents seriously restrict curative effects in these patients. The treatment efficiency of two different chemical drugs with diverse mechanisms of drug action delivered in an individual nanocarrier is promising due to the possibility of achieving synchronized pharmacokinetics for both drugs and surmountable drug resistance of HCC cells.5 Furthermore, the combination of the HCC treatment and diagnosis would establish personalized treatment programs to realize synergistically improved therapeutic results compared with the use of either therapy or imaging alone. Thus, highly efficient synergistic multidrug treatment and diagnosis modalities for HCC are highly needed.
Multifunctional nanoparticles (NPs) with predefined nanostructures as synergic theranostic agents are the effective means for promoting the treatment efficiency of various types of malignancies in nanomedicine.6–10 The species and doses of drugs should be optimized at different clinical manifestations and periods in the treatment to realize personalized therapy for an individual patient. Despite the progress in NP-based drug delivery, reserving multiple drugs with diverse physiochemical properties (hydrophobicity/hydrophilicity, acidity/basicity) into separate rooms within an individual unit and releasing each drug from the independent channels still remains a challenge.11–16 In particular, the loading of varisized guests such as macromolecular drugs by a single NP is difficult to realize, because the most widely used carriers, for instance hollow structured NPs and core–shell NPs normally with symmetrical concentric structures and uniform mesopores, do not contain discrete storage space and independent channels for different drugs.17–24 A unique nanostructure possessing a wide pore size distribution and interior cavities, which is ideally suited for loading and passage of varisized guests including hydrophobic macromolecules without undesirable adverse effects to effectively enhance the combined therapeutic effect, especially for personalized therapy with alternative medicines for an individual patient, is needed. However, the growth strategy enabling a hierarchical porous structure and interior cavity control in a single NP still needs to be developed.
In this paper, we firstly developed Yin Yang-like ultrasmall NPs-stacked oleic acid–NaYF4:Yb,Er@hollow porous SiO2 NPs (designated as Yin Yang-like OA–UCNPs@HPS NPs) with a discrete hydrophilic exterior surface and possessing hierarchical pores and a hydrophobic interior cavity via a novel synthesis method. The OA–UCNPs component imbibed inside the porous SiO2 forms the hydrophobic interior domain while the hydrophilic SiO2 surface ensures dispersal of the NPs in water. Due to the unique nanostructures, the eccentric hydrophobic hollow cavity and large holes could act as a storage space and passages for the water insoluble varisized guests (5-chlorouracil (5-Fu), Mw = 130; hydroxycamptothecin (HCPT), Mw = 364; paclitaxel (PTX), Mw = 830; and insulin, Mw = 5500). Meanwhile, the smaller mesopores in the SiO2 shell can provide storage rooms and channels for water soluble drug molecules, such as hydrophilic doxorubicin (DOX). In a word, the multiple varisized hydrophobic/hydrophilic guests could be loaded into the discrete rooms and each one could be released from independent channels by the single NPs.
As shown in Scheme 1, the growth of the SiO2 shell with hierarchical pores was accompanied with the transformation of rare earth hydroxide/poly(acrylic acid) (RE(OH)3/PAA) NPs into ultrasmall OA–UCNPs upon treatment under solvothermal conditions. Meanwhile, the splendid multimode upconversion luminescence (UCL), computed tomography (CT) and magnetic resonance (MR) imaging abilities were attributed to the UCNPs. As a result, the synthesized Yin Yang-like OA–UCNPs@HPS NPs were programmed to integrate hydrophilic/hydrophobic varisized guest storage, pH responsive drug release property and UCL/CT/MR imaging capabilities into one NP. We evaluated the therapeutic effect of the HCPT/DOX-loaded NPs with a fixed molar ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) on HCC in vitro (cell lines) and in vivo (cell line-derived xenograft (CDX) and novel patient-derived xenograft (PDX) models with high fidelity) and the potential mechanisms of action after endocytosis of the NPs by cancer cells. The superior therapeutic effect and higher cell apoptosis of the HCPT/DOX-loaded NPs indicated that our NPs could be employed as excellent theranostic nanoagents for multimodal imaging-guided dual-drug combined therapy of HCC.
1) on HCC in vitro (cell lines) and in vivo (cell line-derived xenograft (CDX) and novel patient-derived xenograft (PDX) models with high fidelity) and the potential mechanisms of action after endocytosis of the NPs by cancer cells. The superior therapeutic effect and higher cell apoptosis of the HCPT/DOX-loaded NPs indicated that our NPs could be employed as excellent theranostic nanoagents for multimodal imaging-guided dual-drug combined therapy of HCC.
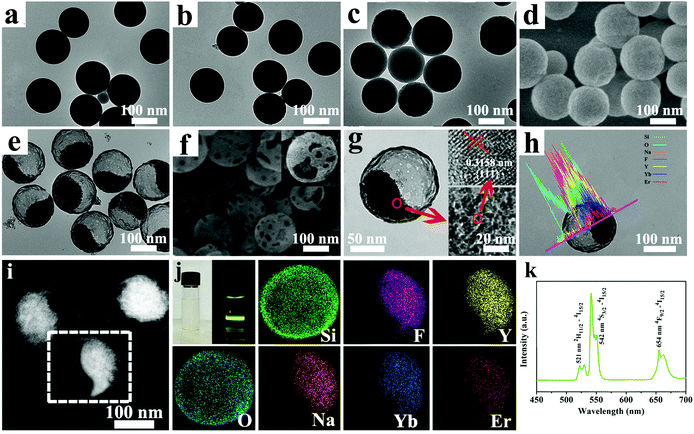
PAA NPs with a diameter of about 130 ± 15 nm were synthesized on the basis of a reported method (Fig. 1a).25 Subsequently, as PAA can absorb and reserve water molecules within its net structure, rare earth (RE) nitrate (Y![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb
Yb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Er = 78%
Er = 78%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20%
20%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2%) was hydrolyzed into small RE(OH)3 to generate monodisperse RE(OH)3/PAA NPs at an unchanged size upon the addition of RE nitrate (Fig. 1b), which were further used as templates to grow a small SiO2 NPs-stacked SiO2 shell. Subsequently, the RE(OH)3/PAA@SiO2 NPs with diameters of 136 ± 15 nm were synthesized via introducing tetraethyl orthosilicate (TEOS) since we adjusted the pH value of as-obtained RE(OH)3/PAA NPs solution to ≈8 with NH3·H2O solution (2 mol L−1) (Fig. 1c and d), and meanwhile the PAA chains were encapsulated in SiO2. Finally, the Yin Yang-like OA–UCNPs@HPS NPs with a diameter of about 136 ± 15 nm were prepared by a facile solvothermal reaction in a mixture of water, OA and ethanol (2
2%) was hydrolyzed into small RE(OH)3 to generate monodisperse RE(OH)3/PAA NPs at an unchanged size upon the addition of RE nitrate (Fig. 1b), which were further used as templates to grow a small SiO2 NPs-stacked SiO2 shell. Subsequently, the RE(OH)3/PAA@SiO2 NPs with diameters of 136 ± 15 nm were synthesized via introducing tetraethyl orthosilicate (TEOS) since we adjusted the pH value of as-obtained RE(OH)3/PAA NPs solution to ≈8 with NH3·H2O solution (2 mol L−1) (Fig. 1c and d), and meanwhile the PAA chains were encapsulated in SiO2. Finally, the Yin Yang-like OA–UCNPs@HPS NPs with a diameter of about 136 ± 15 nm were prepared by a facile solvothermal reaction in a mixture of water, OA and ethanol (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) (Fig. 1e and f) at 180 °C for 24 h. As shown in Fig. 1f and Fig. S1 (ESI†), we could observe that some pores with nonuniform sizes appeared on the SiO2 shell. We designedly chose a broken NP in order to clearly observe the location of the UCNPs in the HPS, as depicted in the inset of Fig. 1f. Obviously, a single NP had a well-defined structure with a HPS shell of approximately 6 nm and the interior structure consisted of a random packing of ultrasmall UCNPs with sizes ranging from 4 to 8 nm (average 6 nm), as shown in Fig. 1g. High-resolution transmission electron microscopy (HR-TEM) showed lattice fringes with the observed d-spacing of 0.3158 nm (illustration in Fig. 1g), which was in good consistency with the lattice spacing in the crystal surface of cubic NaYF4.26 The representative linear scan (Fig. 1h) and the elemental mapping (Fig. 1j) showed that the expected elements of Si (green), O (cyan), Na (orange), F (magenta), Y (yellow), Yb (mazarine) and Er (red) matched the relative location in one single NP. The structure of the Yin Yang-like OA–UCNPs@HPS NPs could be further confirmed through a high angle annular dark field scanning TEM (HAADF-STEM) image (Fig. 1i), which clearly showed the shell (black part) and the UCNPs (bright part). Fig. 1j and k depict the luminescence photograph and UCL spectrum of the Yin Yang-like OA–UCNPs@HPS NPs under 980 nm laser excitation. In addition, the Si, O, Na, F, Y, Yb and Er elements were revealed after the analysis of an individual NP component through energy dispersive X-ray (EDX) spectroscopy component analysis (Fig. S2, ESI†). All these results testify that the ultrasmall NPs-stacked UCNPs were successfully incorporated into the HPS shell.
3) (Fig. 1e and f) at 180 °C for 24 h. As shown in Fig. 1f and Fig. S1 (ESI†), we could observe that some pores with nonuniform sizes appeared on the SiO2 shell. We designedly chose a broken NP in order to clearly observe the location of the UCNPs in the HPS, as depicted in the inset of Fig. 1f. Obviously, a single NP had a well-defined structure with a HPS shell of approximately 6 nm and the interior structure consisted of a random packing of ultrasmall UCNPs with sizes ranging from 4 to 8 nm (average 6 nm), as shown in Fig. 1g. High-resolution transmission electron microscopy (HR-TEM) showed lattice fringes with the observed d-spacing of 0.3158 nm (illustration in Fig. 1g), which was in good consistency with the lattice spacing in the crystal surface of cubic NaYF4.26 The representative linear scan (Fig. 1h) and the elemental mapping (Fig. 1j) showed that the expected elements of Si (green), O (cyan), Na (orange), F (magenta), Y (yellow), Yb (mazarine) and Er (red) matched the relative location in one single NP. The structure of the Yin Yang-like OA–UCNPs@HPS NPs could be further confirmed through a high angle annular dark field scanning TEM (HAADF-STEM) image (Fig. 1i), which clearly showed the shell (black part) and the UCNPs (bright part). Fig. 1j and k depict the luminescence photograph and UCL spectrum of the Yin Yang-like OA–UCNPs@HPS NPs under 980 nm laser excitation. In addition, the Si, O, Na, F, Y, Yb and Er elements were revealed after the analysis of an individual NP component through energy dispersive X-ray (EDX) spectroscopy component analysis (Fig. S2, ESI†). All these results testify that the ultrasmall NPs-stacked UCNPs were successfully incorporated into the HPS shell.
To understand the formation of the Yin Yang-like OA–UCNPs@HPS NPs, a possible growth mechanism was proposed. Firstly, the RE(OH)3/PAA@SiO2 NPs were produced via introducing TEOS. The keys to the formation of the Yin Yang-like OA–UCNPs@HPS NPs were the cooperative roles of the treatment of etching the SiO2 shell and the growth of UCNPs as well as simultaneously the gradual dissolution of PAA templates with the extended reaction time under a solvothermal reaction at 180 °C. Meanwhile, there would be an adhesion (Fa) from the external SiO2 shell. Besides the adhesion, there is also an opposite contractility (Fc) induced by the RE(OH)3/PAA NPs, which would hinder the outward adhesion of the SiO2 shell. Under a solvothermal reaction at 180 °C, with Fc > Fa, eventually NPs with a clear hollow cavity are formed. The morphological evolution process of the Yin Yang-like OA–UCNPs@HPS NPs was investigated by employing TEM images at different reaction periods. When the reaction time was increased from 0.5 to 1 to 5 h, an increasing number of holes appeared on the surface of the SiO2 due to the alkaline environment, which could serve as fast-transport paths for F− and Na+ to generate NaYF4. In the meantime, with Fc > Fa, the PAA molecules in the RE(OH)3/PAA@SiO2 NPs gradually dissolved in the aqueous solution accompanied with the transformation of RE(OH)3 to ultrasmall OA–UCNPs in the presence of OA, leading to the obvious morphological evolution from solid spherical NPs (Fig. S3a and b, ESI†) to NPs with an eccentric cavity inside the HPS (Fig. S3c–h, ESI†). Since the reaction time was prolonged to 24 h, the PAA molecules in the RE(OH)3/PAA@SiO2 NPs were fully replaced with OA, forming OA-coated UCNPs, and the size and morphology of the Yin Yang-like OA–UCNPs@HPS NPs stayed unchanged (Fig. S3i and j, ESI†). The results of the X-ray diffraction (XRD) and UCL prove that the optimized reaction time for the fabrication of the Yin Yang-like OA–UCNPs@HPS NPs is 24 h (Fig. S3k and l, ESI†).
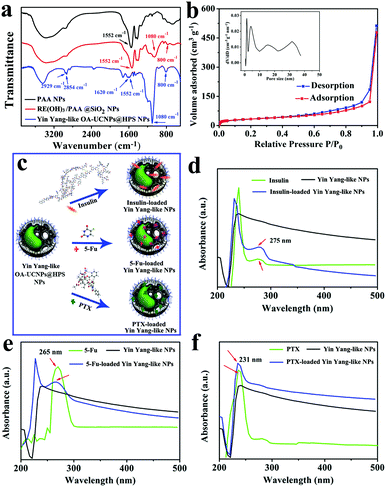
To further verify the composition and surface structure of the products in each step, Fourier transform infrared (FTIR) spectroscopy analysis was conducted on the precursors and final products. In the FTIR spectrum of the PAA NPs (Fig. 2a), the characteristic peak of 1552 cm−1 could be attributed to the carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the carboxylic acid (–COO−) group, which confirmed the occurrence of PAA. After SiO2 coating, the bands at 1080 and 800 cm−1 displayed the characteristic peaks of Si–O–Si symmetry and asymmetric vibrations. For the Yin Yang-like OA–UCNPs@HPS NPs, the peaks at 1620, 2923 and 2854 cm−1 were related to C
O) of the carboxylic acid (–COO−) group, which confirmed the occurrence of PAA. After SiO2 coating, the bands at 1080 and 800 cm−1 displayed the characteristic peaks of Si–O–Si symmetry and asymmetric vibrations. For the Yin Yang-like OA–UCNPs@HPS NPs, the peaks at 1620, 2923 and 2854 cm−1 were related to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and the asymmetric and symmetric stretching vibrations of –CH3 and –CH2 in the long alkyl chain of OA. Dynamic light scattering (DLS) data (Fig. S4, ESI†) proved the hydrodynamic sizes and size distribution of PAA NPs, RE(OH)3/PAA NPs, RE(OH)3/PAA@SiO2 NPs and OA–UCNPs@HPS NPs. These results are in accordance with the obtained products of each step. The Yin Yang-like OA–UCNPs@HPS NPs exhibited superior homogeneity and stability in water, phosphate buffer (PBS), culture medium or fetal bovine serum (FBS) for 24 h (Fig. S5, ESI†). The hydrodynamic diameters of the Yin Yang-like OA–UCNPs@HPS NPs dispersed in water, PBS (pH = 7.4), culture medium and FBS were measured, which confirmed that the Yin Yang-like OA–UCNPs@HPS NPs were monodisperse in different solvents, as shown in Fig. S6 (ESI†). The N2 adsorption–desorption isotherm and the corresponding pore size distribution curve depicted that the obtained NPs were of 133.7 m2 g−1 Brunauer–Emmett–Teller (BET) surface area and the pore size distributions centered at 2.0, 4.8, 15 and 31 nm, agreeing well with the TEM observation, which can be attributed to the hierarchical porous shell (Fig. 2b). The different pore sizes of the Yin Yang-like OA–UCNPs@HPS NPs provide a favorable environment and specialized channels for loading and releasing varisized hydrophobic/hydrophilic guests. As shown in Fig. 2c, the eccentric hydrophobic cavity and large holes of the NPs can serve as a storage space and passages for water insoluble varisized guests (insulin, 5-Fu and PTX). The UV-vis absorption curves proved the successful loading of insulin, 5-Fu and PTX into the NPs (Fig. 2d–f). Meanwhile, the loading capacity could reach 52.0 wt%, 70.0 wt%, and 28.6 wt% for 5-Fu, PTX and insulin, respectively.
C and the asymmetric and symmetric stretching vibrations of –CH3 and –CH2 in the long alkyl chain of OA. Dynamic light scattering (DLS) data (Fig. S4, ESI†) proved the hydrodynamic sizes and size distribution of PAA NPs, RE(OH)3/PAA NPs, RE(OH)3/PAA@SiO2 NPs and OA–UCNPs@HPS NPs. These results are in accordance with the obtained products of each step. The Yin Yang-like OA–UCNPs@HPS NPs exhibited superior homogeneity and stability in water, phosphate buffer (PBS), culture medium or fetal bovine serum (FBS) for 24 h (Fig. S5, ESI†). The hydrodynamic diameters of the Yin Yang-like OA–UCNPs@HPS NPs dispersed in water, PBS (pH = 7.4), culture medium and FBS were measured, which confirmed that the Yin Yang-like OA–UCNPs@HPS NPs were monodisperse in different solvents, as shown in Fig. S6 (ESI†). The N2 adsorption–desorption isotherm and the corresponding pore size distribution curve depicted that the obtained NPs were of 133.7 m2 g−1 Brunauer–Emmett–Teller (BET) surface area and the pore size distributions centered at 2.0, 4.8, 15 and 31 nm, agreeing well with the TEM observation, which can be attributed to the hierarchical porous shell (Fig. 2b). The different pore sizes of the Yin Yang-like OA–UCNPs@HPS NPs provide a favorable environment and specialized channels for loading and releasing varisized hydrophobic/hydrophilic guests. As shown in Fig. 2c, the eccentric hydrophobic cavity and large holes of the NPs can serve as a storage space and passages for water insoluble varisized guests (insulin, 5-Fu and PTX). The UV-vis absorption curves proved the successful loading of insulin, 5-Fu and PTX into the NPs (Fig. 2d–f). Meanwhile, the loading capacity could reach 52.0 wt%, 70.0 wt%, and 28.6 wt% for 5-Fu, PTX and insulin, respectively.
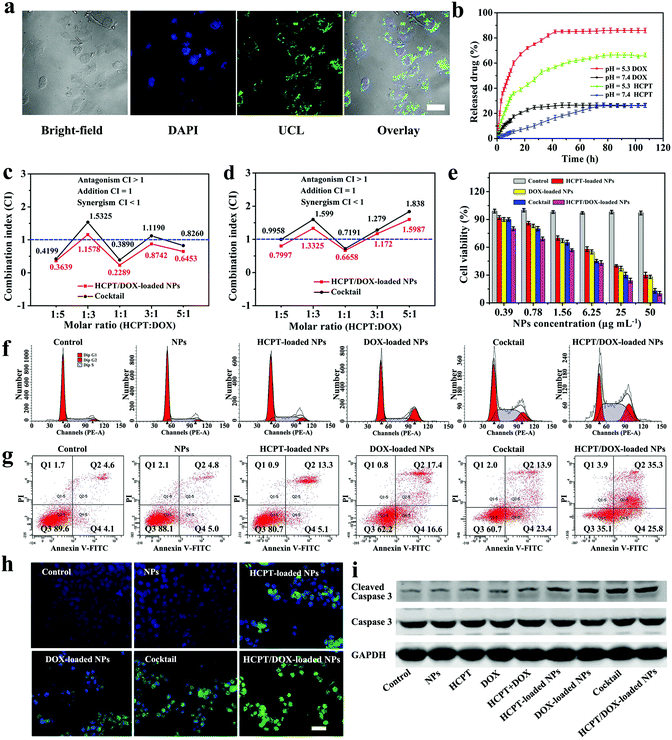
In order to test the produced sample for use as an UCL imaging contrast agent, HepG2 cells incubated with Yin Yang-like OA–UCNPs@HPS NPs for 2 h were investigated using UCL microscopy (Fig. 3a). The green UCL signal of the Yin Yang-like OA–UCNPs@HPS NPs could be observed from the UCL images via an inverted fluorescence microscope with laser excitation at 980 nm. After overlaying nuclei with the UCL image, it was further confirmed that the observed green luminescence was located in the cytoplasm of the cells. The results of UCLM confirm that the Yin Yang-like OA–UCNPs@HPS NPs are excellent candidates for UCL imaging in vitro. DOX and HCPT were chosen to evaluate the drug co-loading capacity of the Yin Yang-like OA–UCNPs@HPS NPs due to their non-overlapping toxicity profiles. Meanwhile, the combination of topoisomerase I inhibitors (hydrophobic HCPT) with topoisomerase II inhibitors (hydrophilic DOX) is an attractive strategy for efficiently combating HCC.27 In Fig. S7 (ESI†), pure HCPT and pure DOX exhibited characteristic peaks at 376 nm and 480 nm, respectively. In the meanwhile, these obvious peaks could also be easily observed from the UV-vis curve of the HCPT/DOX-loaded NPs, which suggested that the NPs have the ability to load both hydrophobic HCPT and hydrophilic DOX. The loading efficiency (LE) was measured to be as high as 98% for DOX (loading capacity = 0.21 mg DOX per mg of Yin Yang-like OA–UCNPs@HPS NPs) accounting for electrostatic attraction between the electropositive DOX and electronegative PAA in the HPS28,29 and 36% (loading capacity = 0.40 mg HCPT per mg of Yin Yang-like OA–UCNPs@HPS NPs) for HCPT via hydrophobic interaction.30–32 On the basis of the theory of similarity and intermiscibility, the OA and HCPT will maintain strong intermolecular forces in PBS (pH = 7.4), for this reason, the loaded hydrophobic HCPT cannot easily release in the physiological environment. With regard to DOX, the electrostatic interaction that can be expressed as: –COO− + DOX+ ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) –COO−DOX+ at pH 7.4. According to the above results, drug leaking could be prevented well in our work, and may be attributed to the electrostatic interaction and the stable intermolecular forces under physiological pH. Furthermore, it is essential to prove that the HCPT and DOX were loaded into different compartments of the Yin Yang-like OA–UCNPs@HPS NPs. We mixed HCPT with HPS to evaluate their loading capacity by UV-vis measurement (Fig. S8, ESI†). As a result, no HCPT was loaded into the HPS. Meanwhile, the sample was immersed into the PBS (pH = 5.3) to test the drug release. During 24 h, no HCPT was found in the PBS solution (Fig. S9, ESI†), further demonstrating that the HCPT was not stored in the HPS. On the contrary, the hydrophilic DOX can be successfully loaded into the HPS with a loading capacity of 21 wt%, which is consistent with the Yin Yang-like OA–UCNPs@HPS NPs, indicating that the DOX was loaded in the HPS part. These results manifest that the HCPT and DOX are stored separately in different compartments of the Yin Yang-like OA–UCNPs@HPS NPs. By virtue of the higher loading content of the hydrophobic/hydrophilic drugs, the loading content of HCPT and DOX in the Yin Yang-like OA–UCNPs@HPS NPs could be accurately managed via simply controlling the ratio of HCPT
–COO−DOX+ at pH 7.4. According to the above results, drug leaking could be prevented well in our work, and may be attributed to the electrostatic interaction and the stable intermolecular forces under physiological pH. Furthermore, it is essential to prove that the HCPT and DOX were loaded into different compartments of the Yin Yang-like OA–UCNPs@HPS NPs. We mixed HCPT with HPS to evaluate their loading capacity by UV-vis measurement (Fig. S8, ESI†). As a result, no HCPT was loaded into the HPS. Meanwhile, the sample was immersed into the PBS (pH = 5.3) to test the drug release. During 24 h, no HCPT was found in the PBS solution (Fig. S9, ESI†), further demonstrating that the HCPT was not stored in the HPS. On the contrary, the hydrophilic DOX can be successfully loaded into the HPS with a loading capacity of 21 wt%, which is consistent with the Yin Yang-like OA–UCNPs@HPS NPs, indicating that the DOX was loaded in the HPS part. These results manifest that the HCPT and DOX are stored separately in different compartments of the Yin Yang-like OA–UCNPs@HPS NPs. By virtue of the higher loading content of the hydrophobic/hydrophilic drugs, the loading content of HCPT and DOX in the Yin Yang-like OA–UCNPs@HPS NPs could be accurately managed via simply controlling the ratio of HCPT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOX. The accurate control of the drug to drug ratio is a great progress, which could effectively govern combination treatment, especially successfully surmounting drug resistance to a single chemotherapeutic agent for HCC. The release profiles of the drug-loaded NPs were tested under different pH conditions by UV-vis measurements. Only 26% of HCPT and 26.9% of DOX were released in phosphate buffer saline (PBS) at pH 7.4 after 108 h. Nevertheless, the release content reached 80.2% for DOX and 65% for HCPT at pH 5.3 (Fig. 3b). The protonation of NH2 groups in the DOX molecules gives DOX positive charges and thus enhanced hydrophilicity to trigger DOX release through the smaller mesopores of HPS.33–35 For HCPT, it was very likely that at pH 5.3, HCPT became more hydrophilic and water-soluble, thus leading to the release of more HCPT from the NPs into the aqueous solution.36 The hydrophilic PAA occupied the smaller sized release channels, and so the hydrophobic HCPT did not easily pass through the smaller mesopores of HPS for drug release. As a consequence, the release of HCPT was preferentially through the large holes on the shell. It follows that the two drugs were released independently with no mutual interference under acidic conditions. At pH 6.5, the tumor microenvironment medium,37 Yin Yang-like OA–UCNPs@HPS NPs exhibited a faster release profile than in PBS 7.4, but slower than in PBS 5.3 (Fig. S10, ESI†), which indicated that the release rate of the Yin Yang-like OA–UCNPs@HPS NPs increased with the enhanced acidic environment. Consequently, the pH-responsive release property of the Yin Yang-like OA–UCNPs@HPS NPs is beneficial for tumor-targeting therapy while the biotoxicity is low for the normal tissues due to their neutral condition. The above results display that the Yin Yang-like OA–UCNPs@HPS NPs can be employed as candidates for pH stimulus responsive release of hydrophilic/hydrophobic drugs.
DOX. The accurate control of the drug to drug ratio is a great progress, which could effectively govern combination treatment, especially successfully surmounting drug resistance to a single chemotherapeutic agent for HCC. The release profiles of the drug-loaded NPs were tested under different pH conditions by UV-vis measurements. Only 26% of HCPT and 26.9% of DOX were released in phosphate buffer saline (PBS) at pH 7.4 after 108 h. Nevertheless, the release content reached 80.2% for DOX and 65% for HCPT at pH 5.3 (Fig. 3b). The protonation of NH2 groups in the DOX molecules gives DOX positive charges and thus enhanced hydrophilicity to trigger DOX release through the smaller mesopores of HPS.33–35 For HCPT, it was very likely that at pH 5.3, HCPT became more hydrophilic and water-soluble, thus leading to the release of more HCPT from the NPs into the aqueous solution.36 The hydrophilic PAA occupied the smaller sized release channels, and so the hydrophobic HCPT did not easily pass through the smaller mesopores of HPS for drug release. As a consequence, the release of HCPT was preferentially through the large holes on the shell. It follows that the two drugs were released independently with no mutual interference under acidic conditions. At pH 6.5, the tumor microenvironment medium,37 Yin Yang-like OA–UCNPs@HPS NPs exhibited a faster release profile than in PBS 7.4, but slower than in PBS 5.3 (Fig. S10, ESI†), which indicated that the release rate of the Yin Yang-like OA–UCNPs@HPS NPs increased with the enhanced acidic environment. Consequently, the pH-responsive release property of the Yin Yang-like OA–UCNPs@HPS NPs is beneficial for tumor-targeting therapy while the biotoxicity is low for the normal tissues due to their neutral condition. The above results display that the Yin Yang-like OA–UCNPs@HPS NPs can be employed as candidates for pH stimulus responsive release of hydrophilic/hydrophobic drugs.
For safe bio-application of the Yin Yang-like OA–UCNPs@HPS NPs, their biocompatibility with living cells was investigated. From the MTT (3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) results in three cell lines (HeLa cells, HepG2 cells and MCF-7 cells) (Fig. S11, ESI†), the cell viabilities were over 90% even when the Yin Yang-like OA–UCNPs@HPS NP concentration was as high as 200 μg mL−1 for 48 h, suggesting that the NPs were lowly cytotoxic to cells. In addition, we also carried out the hemolysis of red blood cells to further check the biocompatibility of the Yin Yang-like OA–UCNPs@HPS NPs. According to Fig. S12 (ESI†), negligible hemolysis could be found at all indicated concentrations of the Yin Yang-like OA–UCNPs@HPS NPs, which indicated excellent blood compatibility.
Thus, the obtained Yin Yang-like OA–UCNPs@HPS NPs are promising for using as non-toxic drug delivery systems and enhancing the therapeutic efficacy. Multidrug delivery systems can promote drug synergism and prepare for precision design and tailoring in cancer chemotherapeutics. Furthermore, the efficacy of synergistic combinations could be improved by controlling drug ratios.38–40 The combination index (CI) was used to describe synergistic interactions (CI < 1, = 1 or >1 represent synergism, addition and antagonism, respectively).41,42 The relevant explanation and equation regarding how to calculate the CI is provided in the supporting information. Fig. 3c and d showed the CI values at IC50 and IC75 measurements of the HCPT/DOX-loaded NPs and a cocktail at different molar ratios. The cocktail system was obtained by means of blending HCPT-loaded NPs and DOX-loaded NPs subject to the ratio being equivalent to the HCPT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOX molar ratio in the dual-drug loaded NPs. The CI values at IC50 and IC75 for HCPT/DOX-loaded NPs with a fixed molar ratio of HCPT
DOX molar ratio in the dual-drug loaded NPs. The CI values at IC50 and IC75 for HCPT/DOX-loaded NPs with a fixed molar ratio of HCPT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOX (1
DOX (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were 0.2289 and 0.6658, which achieved the best combination therapy against HepG2 cells. Additionally, we studied the cytotoxicity of the cocktail compared with the HCPT/DOX-loaded NPs for HepG2 cells. It can be concluded that the HCPT/DOX-loaded NPs were consistently more effective than the cocktail system, as depicted in Fig. S13a–e (ESI†). The enhanced cytotoxicity in the HCPT/DOX-loaded NPs could be explained through the fact that the dual-drug loaded NPs could deliver more consistent drug combinations than the cocktail to enhance the combination effect. In the cocktail, the Yin Yang-like OA–UCNPs@HPS NP uptake and the random drug distribution in cells possibly might compromise the therapeutic effect of the drug combinations. Since the lowest CI value for the HCPT/DOX combination was obtained at a 1
1) were 0.2289 and 0.6658, which achieved the best combination therapy against HepG2 cells. Additionally, we studied the cytotoxicity of the cocktail compared with the HCPT/DOX-loaded NPs for HepG2 cells. It can be concluded that the HCPT/DOX-loaded NPs were consistently more effective than the cocktail system, as depicted in Fig. S13a–e (ESI†). The enhanced cytotoxicity in the HCPT/DOX-loaded NPs could be explained through the fact that the dual-drug loaded NPs could deliver more consistent drug combinations than the cocktail to enhance the combination effect. In the cocktail, the Yin Yang-like OA–UCNPs@HPS NP uptake and the random drug distribution in cells possibly might compromise the therapeutic effect of the drug combinations. Since the lowest CI value for the HCPT/DOX combination was obtained at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, this combination was chosen as the ideal synergistic ratio for executing the following experiments. The IC50 and IC75 values of DOX and HCPT are shown in Fig. S13f (ESI†). The viabilities of HepG2 cells were measured using a standard MTT assay to evaluate the combination drug therapy efficiency of the nanocarrier in vitro (Fig. 3e). Obviously, the groups treated with HCPT-loaded NPs, DOX-loaded NPs, the cocktail and HCPT/DOX-loaded NPs showed obvious cell death in a concentration-dependent manner. In contrast, the group with HCPT/DOX-loaded NPs showed the best cytotoxicity for all the doses tested in HepG2 cells. Fluorescence staining based on propidium iodide (PI) and calcein acetoxymethyl ester (calcein AM) was executed for visualization of the dead cells (red) and live cells (vivid green). HepG2 cells treated with Yin Yang-like OA–UCNPs@HPS NPs alone demonstrated a vivid green (Fig. S14, ESI†), suggesting that our NPs did not compromise cell viability. Nevertheless, the number of the destroyed HepG2 cells were obviously increased when the HepG2 cells were treated HCPT/DOX-loaded NPs. The enhancement in therapeutic behaviour is caused by the two different drug combinations, which suggests that the dual-drug loaded NPs have promising potential as excellent therapeutic agents in cancer therapy of HCC.
1 molar ratio, this combination was chosen as the ideal synergistic ratio for executing the following experiments. The IC50 and IC75 values of DOX and HCPT are shown in Fig. S13f (ESI†). The viabilities of HepG2 cells were measured using a standard MTT assay to evaluate the combination drug therapy efficiency of the nanocarrier in vitro (Fig. 3e). Obviously, the groups treated with HCPT-loaded NPs, DOX-loaded NPs, the cocktail and HCPT/DOX-loaded NPs showed obvious cell death in a concentration-dependent manner. In contrast, the group with HCPT/DOX-loaded NPs showed the best cytotoxicity for all the doses tested in HepG2 cells. Fluorescence staining based on propidium iodide (PI) and calcein acetoxymethyl ester (calcein AM) was executed for visualization of the dead cells (red) and live cells (vivid green). HepG2 cells treated with Yin Yang-like OA–UCNPs@HPS NPs alone demonstrated a vivid green (Fig. S14, ESI†), suggesting that our NPs did not compromise cell viability. Nevertheless, the number of the destroyed HepG2 cells were obviously increased when the HepG2 cells were treated HCPT/DOX-loaded NPs. The enhancement in therapeutic behaviour is caused by the two different drug combinations, which suggests that the dual-drug loaded NPs have promising potential as excellent therapeutic agents in cancer therapy of HCC.
To further demonstrate the co-administration of HCPT and DOX, the time dependent uptake and intracellular release behaviors of HCPT/DOX-loaded NPs were inspected using a confocal laser scanning microscope (CLSM) in HepG2 cells (Fig. S15, ESI†). It was obvious that the DOX and HCPT molecules passed through the nuclear membrane and gradually gathered in the nucleus, which confirmed that the current system has the ability to achieve the synchronized release of multiple therapeutic agents. We deeply study the inhibitory mechanism of solo drug loaded NPs, cocktail and dual-drug loaded NPs to HCC cells. Cell cycle, cell apoptotic and immunofluorescence assays on cell lines after various treatments of HepG2 cells were investigated. For cell cycle analysis, our Yin Yang-like OA–UCNPs@HPS NPs manifested no influence on cell-cycle arrest compared with the control. The percentage of cells in S phase increased when treated with HCPT-loaded NPs, indicating that HCPT successfully disturbed the S phase. Meanwhile, the DOX-loaded NP treated cells were blocked in G2/M phase. Notably, the treatment with HCPT/DOX-loaded NPs caused the most significant inhibitory effect in G0/G1 phase and increased both S phase and G2/M phase compared with the control, thus HCPT/DOX-loaded NPs could enhance the synergistic therapeutic effect (Fig. 3f). From the annexin V-fluorescein isothiocyanate (FITC) double staining assay (Fig. 3g), the percentage of apoptotic HepG2 cells was assessed to be 18.4%, 34.0%, 37.3% and 61.1% after treatment with HCPT-loaded NPs, DOX-loaded NPs, the cocktail and HCPT/DOX-loaded NPs, respectively. For caspase-3 evaluation in the immunofluorescence assays, HCPT/DOX-loaded NPs revealed the strongest fluorescence intensities compared to that of other groups (Fig. 3h). Furthermore, Western blot analyses of the protein levels of cleaved caspase-3 were carried out to study the apoptotic effect on HepG2 cells. The sole drug loaded NPs and cocktail group upregulated cleaved caspase-3 compared with the control group, but the HCPT/DOX-loaded NPs showed the highest protein level of cleaved caspase-3 among all the groups (Fig. 3i). All the results reveal the superior therapeutic effect and the highest cell apoptotic effect of the dual-drug loaded NPs, which demonstrated the efficient cellular uptake of dual-drug loaded NPs and the enhanced cytotoxicity in resistant HCC cells.
Deeply inspired by the high transverse relaxation rate and high X-ray absorption capacity of Yb3+, the UCNPs were also utilized for MR and CT imaging of tumors except optical imaging.43–45 From the slope of 1/T2 relative to the curve of Yb3+ concentration (Fig. S16a, ESI†), the ionic transverse relaxivity (r2) of the Yin Yang-like OA–UCNPs@HPS NPs (9.7512 s−1 mM−1) was higher in comparison with clinical Gd-DTPA values (5.8 s−1 mM−1). Meanwhile, the Yin Yang-like OA–UCNPs@HPS NPs in aqueous solution displayed significant signal enhancement when the Yb3+ concentration increased. The Hounsfield unit (HU) value versus ion concentration of Yb3+ was 7.047 HU mM−1 (Fig. S16b, ESI†), which is superior to the commercial X-ray contrast medium based on iodine (Iopromide, 5.33 HU mM−1). Benefiting from the excellent MR and CT imaging abilities of Yb3+, HCC tumor-bearing nude mice were analysed by injecting the Yin Yang-like OA–UCNPs@HPS NPs into the tail vein. Based on Fig. 4a–d, high contrast imaging of the tumors was detected in the T2-weighted MR and the CT investigations 24 h after injection, demonstrating that the NPs are highly promising for potential applications for multiplex theranostic nanoplatforms. As illustrated in Fig. S17 (ESI†), it was expected that the Yin Yang-like OA–UCNPs@HPS NPs mainly accumulated in the tumor, liver and kidney, similar to the in vivo behavior of other nanomaterials.46–48 The tumor uptake of the Yin Yang-like OA–UCNPs@HPS NPs could attain 14.8% ID g−1via the enhanced permeability and retention (EPR) effect49–51 24 h after injection, which greatly facilitated the synergetic cancer therapy.
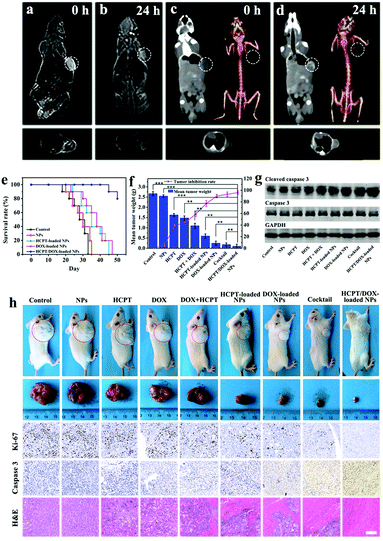
We carried out in vivo (CDX) experiments on server combined immune-deficiency (SCID) mice to testify the effect of synergic chemotherapy using HCPT/DOX-loaded NPs. The animal experiment procedures were in agreement with the guidelines of the Regional Ethics Committee for Animal Experiments and the care regulations approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital. There existed no obvious difference in average weight between the control group and the Yin Yang-like OA–UCNPs@HPS NPs group, suggesting that empty NPs served as drug delivery carriers but did not affect the growth of the mice (Fig. S18a, ESI†). What is more, the survival rate of the HCPT/DOX-loaded NPs group at day 40 remained at 100%, which was obviously for longer than other groups (Fig. 4e). The tumor volumes of the HCPT, DOX, and HCPT + DOX groups increased rapidly to ≈1950, ≈1835 and ≈1600 mm3, respectively, which were much larger than the DOX-loaded NPs and HCPT-loaded NPs groups (Fig. S18b, ESI†). Obviously, the smallest tumor volume appeared in the group treated with HCPT/DOX-loaded NPs (≈60.9 mm3). It worth mentioning that the dual-drug administrated group exhibited better tumor inhibition compared with the other groups. The tumor suppression was as high as 95.0% in the HCPT/DOX-loaded NPs group compared with the other experimental groups under the tested conditions due to the high accumulation of the Yin Yang-like OA–UCNPs@HPS NPs in the tumor via the EPR effect (Fig. 4f). Representative pictures of tumors and mice under various treatments are presented in Fig. 4h. Furthermore, to examine the mechanism of action of drug-loaded NPs in vivo, Ki-67, caspase-3 and hematoxylin and eosin (H&E) staining of tumor sections isolated from mice at day 15 were carried out (Fig. 4h). The stronger suppression on cell proliferation in the HCPT/DOX-loaded NPs group was observed by Ki-67 antibody staining to evaluate the proliferative activities, while the free drugs showed trivial inhibition on the proliferative activity of cancer cells. The most severe apoptotic and necrotic cells in the tumor region were observed in the HCPT/DOX-loaded NPs group from immunohistochemistry of cleaved caspase 3. In the H&E assay, the most severe nuclear shrinkage, fragmentation and absence appeared in the HCPT/DOX-loaded NPs group, which was possibly the reason that this group caused the most significant reduction of the tumors. Western blot analyses of the levels of cleaved caspase-3 were performed to study the apoptotic effect of HCC after various treatments (Fig. 4g). These in vivo results clearly reveal the considerably higher inhibition efficacy and synergistic therapeutic effect on HCC by the dual-drug loaded NPs. Meanwhile, no significant organ damage (Fig. S19, ESI†) was observed for the treated mice.
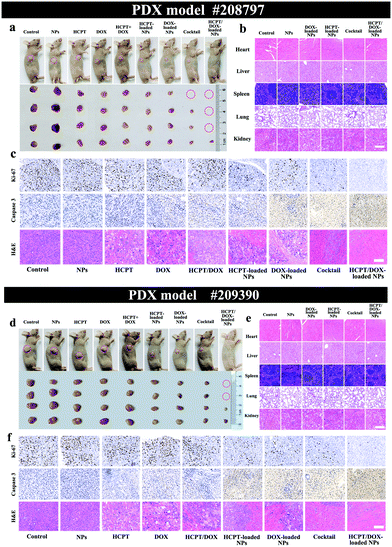
In addition, to prove the effective treatment of the HCPT/DOX-loaded NPs, we here established a novel PDX mice model with high fidelity bearing different HCC xenografts (#208797-PDX and #209390-PDX) obtained by direct implantation of two patients’ resection HCC tissue fragments in immunocompromised nude mice. Written informed consent was received from the two patients for their HCC tissue to be utilized for potential studies. As displayed in the two cases of PDX models, the HCPT/DOX-loaded NPs group possessed the most potent inhibition of tumor growth and showed little effect on mouse body weight (Fig. S20a and b, ESI†). The tumors were inhibited well in the HCPT/DOX-loaded NPs group (#208797-PDX, 28 mm3 and #209390-PDX, 57 mm3) while the average tumor volume of the control group increased to over 645 mm3 (#208797-PDX) and 1200 mm3 (#209390-PDX) after 20 days (Fig. S20c and d, ESI†). The tumor inhibition ratios of the HCPT/DOX-loaded NPs group were ∼98.0% (#208797-PDX) and ∼97% (#209390-PDX) showing the effective inhibition of tumor growth in the two PDX models (Fig. S21, ESI†). Representative photographs of PDX mice and tumors isolated from mice on day 20 are shown in Fig. 5a and d. Thereafter, the mechanism of action of drug-loaded NPs on in vivo PDX models was evaluated (Fig. 5c and f). The in vivo proliferative activities were analysed out by Ki-67 antibody staining and stronger suppression on cell proliferation was observed in the HCPT/DOX-loaded NPs group compared with all other groups. Caspase-3 and H&E analysis showed the most severe apoptosis and necrosis of HCC cells after synergistic combined therapy compared to the other groups. This may well prove the synergistic therapy effect, in which the integration of two drugs with different inhibition mechanisms could better induce the apoptosis of tumor cells thus obstructing tumor growth. No significant organ damage (Fig. 5b and e) was observed for each group in the H&E assay, which indicated the safety of the NPs. As depicted in Fig. S22 (ESI†), the safety profiles of the NPs and HCPT/DOX-loaded NPs for liver and kidney were examined using liver and kidney function indicators and other biochemical parameters. Our in vivo and in vitro results demonstrate that the NPs can play the part of superior diagnostic and therapeutic agents for efficient combating of HCC.
Conclusion
To sum up, we firstly developed a novel synthesis method to prepare water-dispersible Yin Yang-like ultrasmall NPs-stacked OA–UCNPs@HPS NPs with discrete hydrophilic exterior surface possessing hierarchical pores and a hydrophobic interior cavity. For the Yin Yang-like OA–UCNPs@HPS NPs, the OA–UCNPs component imbibed inside the HPS forms the hydrophobic interior domain while the hydrophilic exterior surface could ensure dispersal of the Yin Yang-like OA–UCNPs@HPS NPs in water. The loading of multiple varisized hydrophobic/hydrophilic guests including a hydrophobic macromolecule into the discrete rooms by a single NP was achieved, which reduced the undesirable adverse effects of different guests. The release of hydrophobic/hydrophilic drugs can be controlled by a pH stimulus from the independent channels. The nanostructure of the NPs greatly favors the selection of drugs for an individual patient and effectively enhances synergistic theranostics. The HCPT/DOX-loaded NPs with a fixed molar ratio of HCPT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOX (1
DOX (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) have great potential for the efficient and significant multimodal imaging-guided dual-drug synergic cancer therapy of HCC. This work not only offers a demonstration of the high therapeutic efficacy of combined therapy but also can lead to further development of novel synthetic methods and unique architectures of multifunctional NPs needed for cancer theranostics.
1) have great potential for the efficient and significant multimodal imaging-guided dual-drug synergic cancer therapy of HCC. This work not only offers a demonstration of the high therapeutic efficacy of combined therapy but also can lead to further development of novel synthetic methods and unique architectures of multifunctional NPs needed for cancer theranostics.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
X. J. Chen and X. P. Zhang contributed equally to this work. This work was supported by the National Natural Science Foundation of China (Grant No. 21573040, 21603029 and 21872024), the Natural Science Foundation and Science and Technology Development Planning of Jilin Province (20170520148JH), the Fundamental Research Funds for the Central Universities, the Education Department of Jilin Province “13th Five-Year” Science and Technology Research (JJKH20190272KJ), the Jilin Provincial Research Foundation for Basic Research (20160519012JH) and Jilin Provincial Key Laboratory of Micro-Nano Functional Materials (Northeast Normal University).Notes and references
- J. X. Lu, J. Wang and D. S. Ling, Small, 2018, 14, 1702037 CrossRef PubMed.
- S. Whittaker, R. Marais and A. X. Zhu, Oncogene, 2010, 29, 4989–5005 CrossRef CAS PubMed.
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 87–108 CrossRef PubMed.
- J. A. Marrero, L. M. Kulik, C. Sirlin, A. X. Zhu, R. S. Finn, M. M. Abecassis, L. R. Roberts and J. K. Heimbach, Hepatology, 2018, 68, 723–750 CrossRef PubMed.
- J. Bruix, M. Reig and M. Sherman, Gastroenterology, 2016, 150, 835–853 CrossRef PubMed.
- Q. Wang, X. Y. Zhang, H. Z. Liao, Y. Sun, L. Ding, Y. W. Teng, W. H. Zhu, Z. R. Zhang and Y. R. Duan, Adv. Funct. Mater., 2018, 28, 1706124 CrossRef.
- Z. L. Li, H. Zhang, J. Han, Y. Chen, H. Lin and T. Yang, Adv. Mater., 2018, 1706981 CrossRef PubMed.
- L. L. Feng, R. Xie, C. Q. Wang, S. L. Gai, F. He, D. Yang, P. P. Yang and J. Lin, ACS Nano, 2018, 12, 11000–11012 CrossRef CAS PubMed.
- D. Yang, G. X. Yang, S. L. Gai, F. He, C. X. Li and P. P. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 6829–6838 CrossRef CAS PubMed.
- W. S. Chen, J. Ouyang, H. Liu, M. Chen, K. Zeng, J. P. Sheng, Z. J. Liu, Y. J. Han, L. Q. Wang, J. Li, L. Deng, Y.-N. Liu and S. J. Guo, Adv. Mater., 2017, 29, 1603864 CrossRef PubMed.
- Z. Liu, Z. H. Li, J. H. Liu, S. Gu, Q. H. Yuan, J. S. Ren and X. G. Qu, Biomaterials, 2012, 33, 6748–6757 CrossRef CAS PubMed.
- L. S. Lin, X. Y. Yang, Z. J. Zhou, Z. Yang, O. Jacobson, Y. J. Liu, A. Yang, G. Niu, J. B. Song, H. H. Yang and X. Y. Chen, Adv. Mater., 2017, 29, 1606681 CrossRef PubMed.
- Y. N. Huang, Q. B. Xiao, H. S. Hu, K. C. Zhang, Y. M. Feng, F. J. Li, J. Wang, X. G. Ding, J. Jiang, Y. F. Li, L. Y. Shi and H. Z. Lin, Small, 2016, 12, 4200–4210 CrossRef CAS PubMed.
- D. L. Ni, W. B. Bu, S. J. Zhang, X. P. Zheng, M. Li, H. Y. Xing, Q. F. Xiao, Y. Y. Liu, L. P. Zhou, W. J. Peng, K. L. Zhao and J. L. Shi, Adv. Funct. Mater., 2014, 24, 6613–6620 CrossRef CAS.
- C. M. Cobley, J. Y. Chen, E. C. Cho, L. H. V. Wang and Y. N. Xia, Chem. Soc. Rev., 2011, 40, 44–56 RSC.
- X. M. Li, L. Zhou, Y. Wei, A. M. El-Toni, F. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2014, 136, 15086–15092 CrossRef CAS PubMed.
- Y. Zhao, L. N. Lin, Y. Lu, S. F. Chen, L. Dong and S. H. Yu, Adv. Mater., 2010, 22, 5255–5259 CrossRef CAS PubMed.
- Y. Chen, H. Chen, D. Zeng, Y. Tian, F. Chen, J. Feng and J. Shi, ACS Nano, 2010, 4, 6001–6013 CrossRef CAS PubMed.
- J. Fu, T. Chen, M. Wang, N. Yang, S. Li, Y. Wang and X. Liu, ACS Nano, 2013, 7, 11397–11408 CrossRef CAS PubMed.
- P. W. Gong, Q. Zhao, D. J. Dai, S. M. Zhang, Z. Z. Tian, L. Sun, J. S. Ren and Z. Liu, Chem. – Eur. J., 2017, 23, 17531–17541 CrossRef CAS PubMed.
- Y. Chen, P. F. Xu, H. R. Chen, Y. S. Li, W. B. Bu, Z. Shu, Y. P. Li, J. M. Zhang, L. X. Zhang, L. M. Pan, X. Z. Cui, Z. L. Hua, J. Wang, L. L. Zhang and J. L. Shi, Adv. Mater., 2013, 25, 3100–3105 CrossRef CAS PubMed.
- F. Zhang, G. B. Braun, A. Pallaoro, Y. Zhang, Y. Shi, D. Cui, M. Moskovits, D. Y. Zhao and G. D. Stucky, Nano Lett., 2012, 12, 61–67 CrossRef CAS PubMed.
- W. Fan, B. Shen, W. Bu, F. Chen, K. Zhao, S. Zhang, L. Zhou, W. Peng, Q. Xiao, H. Xing, J. Liu, D. Ni, Q. He and J. Shi, J. Am. Chem. Soc., 2013, 135, 6494–6503 CrossRef CAS PubMed.
- J. Liu, J. Bu, W. Bu, S. Zhang, L. Pan, W. Fan, F. Chen, L. Zhou, W. Peng, K. Zhao, J. Du and J. Shi, Angew. Chem., Int. Ed., 2014, 53, 4551–4555 CrossRef CAS PubMed.
- L. Li, L. Y. Zhang, T. T. Wang, X. T. Wu, H. Ren, C. G. Wang and Z. M. Su, Small, 2015, 11, 3162–3163 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed.
- V. M. M. Herben, W. W. Ten Bokkel Huinink, A. C. Dubbelman, I. A. M. Mandjes, Y. Groot, D. M. van Gortel-van Zomeren and J. H. Beijnen, Br. J. Cancer, 1997, 76, 1500–1508 CrossRef CAS PubMed.
- L. Y. Zhang, S. N. Li, X. J. Chen, T. T. Wang, L. Li, Z. M. Su and C. G. Wang, Adv. Funct. Mater., 2018, 28, 1803815 CrossRef.
- L. L. Chen, L. Li, L. Y. Zhang, S. X. Xing, T. T. Wang, Y. A. Wang, C. G. Wang and Z. M. Su, ACS Appl. Mater. Interfaces, 2013, 5, 7282–7290 CrossRef CAS PubMed.
- G. Zhang, L. Ding, R. Renegar, X. M. Wang, Q. Lu, S. Q. Huo and Y.-H. Chen, Cancer Sci., 2011, 102, 1216–1222 CrossRef CAS PubMed.
- T. K. Jain, M. A. Morales, S. K. Sahoo, D. L. Leslie-Pelecky and V. Labhasetwar, Mol. Pharmaceutics, 2005, 2, 194–205 CrossRef CAS PubMed.
- M. Khalkhali, S. Mohammadinejad, F. Khoeini and K. Rostamizadeh, Int. J. Pharm., 2019, 559, 173–181 CrossRef CAS PubMed.
- Z. L. Li, Y. Hu, K. A. Howard, T. T. Jiang, F. L. Fan, Z. H. Miao, Y. Sun, F. Besenbacher and M. Yu, ACS Nano, 2016, 10, 984–997 CrossRef CAS PubMed.
- X. Y. Zhong, K. Yang, Z. L. Dong, X. Yi, Y. Wang, C. C. Ge, Y. L. Zhao and Z. Liu, Adv. Funct. Mater., 2015, 25, 7327–7336 CrossRef CAS.
- C. Wang, H. Xu, C. Liang, Y. Liu, Z. Li, G. Yang, L. Cheng, Y. Li and Z. Liu, ACS Nano, 2013, 7, 6782–6795 CrossRef CAS PubMed.
- L. M. Zhang, J. G. Xia, Q. H. Zhao, L. W. Liu and Z. J. Zhang, Small, 2010, 6, 537–544 CrossRef CAS PubMed.
- J. A. Shen, H. P. Sun, P. F. Xu, Q. Yin, Z. W. Zhang, S. L. Wang, H. J. Yu and Y. P. Li, Biomaterials, 2013, 34, 1581–1590 CrossRef CAS PubMed.
- L. Ma, M. Kohli and A. Smith, ACS Nano, 2013, 7, 9518–9525 CrossRef CAS PubMed.
- A. Goldman, A. Kulkarni, M. Kohandel, P. Pandey, P. Rao, S. K. Natarajan, V. Sabbisetti and S. Sengupta, ACS Nano, 2016, 10, 5823–5834 CrossRef CAS PubMed.
- L. Y. Liao, J. Liu, E. C. Dreaden, S. W. Morton, K. E. Shopsowitz, P. T. Hammond and J. A. Johnson, J. Am. Chem. Soc., 2014, 136, 5896–5899 CrossRef CAS PubMed.
- X. L. Liang, C. Gao, L. G. Cui, S. M. Wang, J. R. Wang and Z. F. Dai, Adv. Mater., 2017, 29, 1703135 CrossRef PubMed.
- Y. B. Cai, H. S. Shen, J. Zhan, L. H. Lin, C. H. Ren, Y. Shi, J. F. Liu, J. Gao and Z. M. Yang, J. Am. Chem. Soc., 2017, 139, 2876–2879 CrossRef CAS PubMed.
- R. C. Lv, P. P. Yang, F. He, S. L. Gai, C. X. Li, Y. L. Dai, G. X. Yang and J. Lin, ACS Nano, 2015, 9, 1630–1647 CrossRef CAS PubMed.
- X. Y. Lv, X. Wang, T. Li, C. G. Wei, Y. A. Tang, T. Yang, Q. L. Wang, X. L. Yang, H. B. Chen, J. K. Shen, H. Yang and H. T. Ke, Small, 2018, 14, 1802904 CrossRef PubMed.
- Y. N. Huang, Q. B. Xiao, H. S. Hu, K. C. Zhang, Y. M. Feng, F. J. Li, J. Wang, X. G. Ding, J. Jiang, Y. F. Li, L. Y. Shi and H. Z. Lin, Small, 2016, 12, 4200–4210 CrossRef CAS PubMed.
- M. G. Li, K. T. Al-Jamal, K. Kostarelos and J. Reineke, ACS Nano, 2010, 4, 6303–6317 CrossRef CAS PubMed.
- I.-F. Li, C. H. Su, H. S. Sheu, H. C. Chiu, Y. W. Lo, W. T. Lin, J. H. Chen and C. S. Yeh, Adv. Funct. Mater., 2008, 18, 766–776 CrossRef CAS.
- D. Pan, C. O. Schirra, A. Senpan, A. H. Schmieder, A. J. Stacy, E. Roessl, A. Thran, S. A. Wickline, R. Proska and G. M. Lanza, ACS Nano, 2012, 6, 3364–3370 CrossRef CAS PubMed.
- T. M. Sun, Y. S. Zhang, B. Pang, D. C. Hyun, M. X. Yang and Y. N. Xia, Angew. Chem., Int. Ed., 2014, 53, 12320–12364 CAS.
- Y. Chen, K. l. Ai, J. H. Liu, G. Y. Sun, Q. Yin and L. H. Lu, Biomaterials, 2015, 60, 111–120 CrossRef CAS PubMed.
- A. T. Rad, C. W. Chen, W. Aresh, Y. Xia, P. S. Lai and M. P. Nieh, ACS Appl. Mater. Interfaces, 2019, 11, 10505–10519 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nh00467f |
| This journal is © The Royal Society of Chemistry 2019 |