Keggin-type polyoxometalate cluster as an active component for redox-based nonvolatile memory†
Xiaoli
Chen
a,
Pu
Huang
a,
Xin
Zhu
b,
Suixing
Zhuang
a,
Hengcheng
Zhu
a,
Jingjing
Fu
b,
Arun S.
Nissimagoudar
 b,
Wu
Li
b,
Xiuwen
Zhang
a,
Li
Zhou
a,
Yan
Wang
a,
Ziyu
Lv
a,
Ye
Zhou
b,
Wu
Li
b,
Xiuwen
Zhang
a,
Li
Zhou
a,
Yan
Wang
a,
Ziyu
Lv
a,
Ye
Zhou
 *b and
Su-Ting
Han
*b and
Su-Ting
Han
 *a
*a
aShenzhen Key Laboratory of Flexible Memory Materials and Devices, College of Electronic Science and Technology, Shenzhen University, Shenzhen, 518060, P. R. China. E-mail: sutinghan@szu.edu.cn
bInstitute for Advanced Study, Shenzhen University, Shenzhen, 518060, P. R. China. E-mail: yezhou@szu.edu.cn
First published on 16th January 2019
Abstract
Redox-based nonvolatile memory, where the nanoscale redox process is generally conceived to account for resistance change in external electrical stimuli, is attracting much scientific interest because of its high potential for application in next-generation memory and neuromorphic computing systems. However, materials used in current redox-based resistive switching memory are usually restricted by bulk transition metal oxides. Polyoxometalates (POMs)—a class of discrete early transition metal oxide molecular clusters—are gaining popularity in emerging applications of molecular electronic devices owing to their nanoscale size, high stability, and rich reversible redox potential. This paper reports nonvolatile memory associated with the redox behavior of POMs. Resistive switching mechanisms of POM-based resistive switching memory were systematically investigated for the first time. The intrinsic redox property of POMs was confirmed to initiate the formation of conductive filaments, which are developed by the migration of oxygen ions, as verified through electron energy loss spectroscopy. The results of this study could provide valuable insight into application of redox molecular-based memory for high-density data storage.
Conceptual insightsThe continuous scaling down of memory devices is one of the most effective approaches to cope with challenges resulting from the unpredictability of data types and sizes. Two terminal memories preserve the most promising perspective as next-generation memory due to its excellent scalability beyond 10 nm feature size. Meanwhile, molecular memories can work with few electrons at the molecular level, thereby having the potential for high-density data storage. This offers us an opportunity by combining molecular materials into RRAM devices for boosting the capability of the data storage. Here, we utilized a type of nanoscale metal oxide molecular cluster, polyoxometalates (POMs), as the active component of the memory device and the resistive switching mechanism was systematically investigated for the first time. We demonstrate that the valence change associated with a new reduced state resulting from the localized redox reaction of POM molecules initializes the resistive switching behavior. The formation/rupture of the conductive filaments by the migration of oxygen vacancies in POM molecules is confirmed to account for the resistive switching mechanism. We anticipate that the featured characteristics observed in this work will benefit the comprehensive understanding of the practical incorporation of POM molecules in non-volatile memory with minimized size towards advanced molecule-based high-density memory technologies. |
Since the seminal work of Williams and Strukov (2008), the field of resistive random access memory (RRAM) has expanded rapidly because of the excellent scalability of RRAM beyond 10 nm in feature size, high switching speed, low power consumption, and wide material variety.1 RRAM devices are considered next-generation memory devices that could address the limitations of commercial flash memory, including complicated fabrication processes, high fabrication costs, and high power consumption.2–4 In particular, driven by the technological demands for novel materials and innovative device concepts, redox-based RRAM with transition metal oxides sandwiched between two metal electrodes holds great promise for future data storage and logic circuits, and its commercial potential—including neuromorphic configurations5–7 and low-power microcontrollers—is under evaluation.8–10 In RRAM systems, the low-resistance state (LRS) and high-resistance state (HRS) of the oxide layer involving the formation and rupture the conducting filaments (CFs) are driven by the migration of ions or donor-type defects induced by local electrochemical redox reactions, which may modulate the resistance of the oxide layer or the barrier of the electrode–oxide interface.11–13 Typically, valence change (VC) and electrochemical metallization (ECM) are the two major switching mechanisms of redox-based RRAM, depending on the species of mobile ions.14,15 ECM is related to the motion of metal cations generated from metal electrodes, whereas VC is related to oxygen ion migration in the oxides layer. Various transition metal oxides including SrTiO3,10,16,17 HfO2,18,19 TiO2,20,21 and TaOx22,23 have been demonstrated to exhibit the redox-based resistive switching phenomenon under external electrical stimuli. The formation and rupture of CFs in these oxides have been explained at length in relation to the migration of oxygen ions accompanied by the formation of oxygen vacancies, which lead to VC in transition metals.
Inspired by the aforementioned innovative redox-based RRAM device concept, polyoxometalates (POMs)—a type of evolutionary molecular transition metal oxide cluster that exhibits properties superior to those of bulk transition metal oxides—show promise for construction of nonvolatile memory devices for future device scaling owing to their molecular nature.24–26 As soluble materials, POMs are easy to handle and compatible with low-pressure and high-temperature processes in microelectronics;27 in addition, they are highly suited to integration into stacked materials in complementary metal–oxide-semiconductor technology.28,29 Two notable properties of POMs are their reliance on fine-tuning redox and high ability to accept electrons with negligible structural modifications; such accepted electrons can be delocalized throughout their metal framework, a property that renders them suitable for application in electronic devices.30–35 Recently, Cronin et al. utilized “Dawson-like” archetype core–shell POM molecules ([W18O54(SeO3)2]4−) as the functional component in flash memory, where the oxidation of selenite in the cluster core and the reduction of the metal oxide in the cluster cage dictated the write/erase process.29 Keggin-type POM molecules were integrated into a Ta2O5 matrix for low-power switching and fast-access memory applications.36 Moreover, Anderson-type heteropolymolybdate POMs-based ternary resistive switching memory was demonstrated by Zhang et al., who claimed that observed ternary resistive switching phenomena originated from the two oxidation states of Mn(II) to Mn(III) and Mn(III) to Mn(IV) and one reduction state of Mn(III)/Mn(IV) to Mn(II).37 Nevertheless, to the best of our knowledge, no study has systematically investigated the underlying resistive switching mechanism of redox-based RRAM associated with the redox property of POMs; thus, this topic remains of significant importance for future designation of POMs-based molecular electronics.
In the present study, a core–shell “Keggin-like” POM archetype with the general formula of H3PW12O40 (denoted as “PW” hereafter) was employed as the functional aspect of nonvolatile memory, and fabricated memory exhibited high cell-to-cell uniformity and outstanding stability under ambient conditions. Through a series of experiments, characterization techniques, and density functional theory (DFT) calculations, VC associated with a new W5+ state resulting from the localized redox reaction of PW molecules was confirmed to initiate resistive switching behavior. Furthermore, the formation or rupturing of CFs via the migration of oxygen ions in PW molecules accompanied by the formation of oxygen vacancies accounted for the resistive switching mechanism. The featured characteristics observed in this study suggested a route to practical incorporation of POM molecules in nonvolatile memory of minimal size with a view to developing advanced molecule-based high-density memory technologies.
Results and discussion
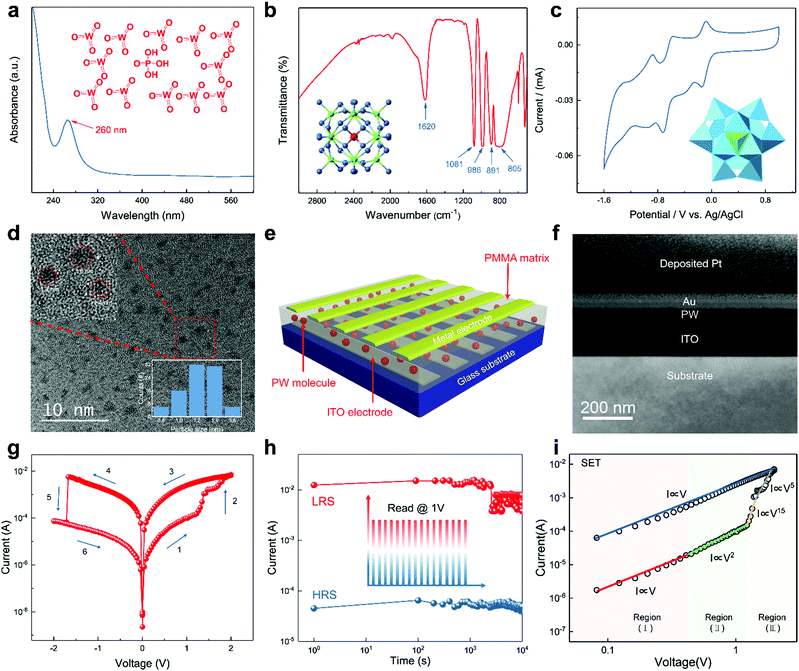
For the purpose of verifying the integrity of the material we utilized, conventional characterization approaches of POMs including ultraviolet-visible (UV-vis) absorption and Fourier transform infrared spectrum (FT-IR) measurements were carried out. As shown in Fig. 1a, an intense peak centered at 260 nm representing the typical W (3d)→O (2p) charge transfer of POMs was observed in UV-vis absorption spectrum of PW. The four representative peaks observed in the FT-IR spectrum at 1081 cm−1 for the P–Oa bond, 986 cm−1 for the W–Od bond, 891 cm−1 for the W–Ob–W bond, and 805 cm−1 for the W–Oc–W bond suggest the typical Keggin structure of PW, where Oa is the central oxygen, Ob is the bridging oxygen linking two corner-sharing octahedra, Oc is the bridging oxygen linking two edge-sharing octahedra, and Od is the terminal oxygen of the POM Keggin structure (Fig. 1b). Both UV-vis absorption and FT-IR spectrum ensure the intact Keggin structure of PW.38 To identify the reversible redox property of PW, cyclic voltammetry (CV) was measured using a glassy carbon electrode in acetonitrile solution and a scanning range of −2.5 to 1.0 V against a Ag/AgCl reference. As shown in Fig. 1c, the CV result demonstrates the reversible redox behavior of PW. The molecular structure, ball-and-stick model, and polyhedral representation of the PW molecule are shown in the inset images of Fig. 1a, b, and c, respectively. Fig. 1d shows a transmission electron microscopy (TEM) image of PW molecular clusters with diameters ranging from 0.8 to 1.6 nm; the result of particle size distribution is illustrated in the inset of Fig. 1d. Energy-dispersive X-ray spectroscopy provided in Fig. S1 (ESI†) further verified that the nanoparticle we utilized is PW clusters.To demonstrate the utilization of PW molecules in RRAM, a vertical geometry was employed on a glass substrate with 185 nm of indium tin oxide (ITO) as the bottom electrode; a three-dimensional schematic of the memory device is shown in Fig. 1e. This study applied poly(methyl methacrylate) (PMMA)—a proven suitable host material—as the polymer matrix to encapsulate PW molecules. Notably, pristine PMMA does not possess the resistive switching effect, as demonstrated in Fig. S2 (ESI†). A blended solution of PW and PMMA in propylene glycol monomethyl ether acetate at a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was spin coated onto a glass substrate (2 cm × 2 cm) with a patterned ITO bottom electrode (width: 500 μm). Subsequently, Au was deposited as the top electrode through thermal evaporation with a shadow mask (width: 500 μm) to form 5 × 5 crossbar patterns in order to render a single device cell 0.25 mm2 in size and rule out variations arising from the device fabrication process. The surface morphology of the as-fabricated PW-PMMA film was characterized through atomic force microscopy (AFM; Fig. S3, ESI†). Fig. 1f displays a cross-sectional image of the device structure measured using high-resolution TEM; the cross-section sample was obtained through focused ion beam technology, and the thickness of the PW layer was 35 nm. An optical image of the as-fabricated memory device is provided in Fig. S4 (ESI†).
1 was spin coated onto a glass substrate (2 cm × 2 cm) with a patterned ITO bottom electrode (width: 500 μm). Subsequently, Au was deposited as the top electrode through thermal evaporation with a shadow mask (width: 500 μm) to form 5 × 5 crossbar patterns in order to render a single device cell 0.25 mm2 in size and rule out variations arising from the device fabrication process. The surface morphology of the as-fabricated PW-PMMA film was characterized through atomic force microscopy (AFM; Fig. S3, ESI†). Fig. 1f displays a cross-sectional image of the device structure measured using high-resolution TEM; the cross-section sample was obtained through focused ion beam technology, and the thickness of the PW layer was 35 nm. An optical image of the as-fabricated memory device is provided in Fig. S4 (ESI†).
Fig. 1g displays the typical current–voltage (I–V) curve of the as-fabricated RRAM device measured using the sweeping mode (Vstep = 0.05 V) at a compliance current (ICC) of 0.1 A. The device initially exhibited high resistance of 1.93 × 104 Ω in the OFF state and reading bias of 0.5 V (stage 1). When subjected to positive bias sweeping, the resistance progressively decreased to 324.15 Ω at 1.2 V (stage 2), and the RRAM switched to the LRS, which was considered the SET process with 1.2 V as the set voltage (Vset). The device remained in the LRS during the subsequent 2 to 0 V (stage 3) and 0 to −2 V (stage 4) bias sweeping processes. Subsequently, the memory device switched abruptly from the LRS to HRS at a reset voltage (Vreset) of −1.71 V (stage 5); this was defined as the RESET process. Notably, the SET and RESET processes demonstrated different characteristics with respect to resistive switching performance; this is discussed in the following section. Overall, the ITO/PW/Au memory device exhibited a bipolar resistive switching characteristic and an ON/OFF ratio of ∼6 × 102.
Randomly selected 5 × 5 device cells were tested to analyze the distribution of ON and OFF current to investigate the reproducibility of the devices. As shown in Fig. S5 (ESI†), the current levels with low fluctuation suggested high uniformity of the memory array, which was an indication of repeatability and accuracy of the electrical characteristics. To assess the retention ability, the devices were set in the HRS and LRS at time zero, as depicted in Fig. 1h. No significant degradation of the ON/OFF ratio was observed after 104 s with a reading voltage of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V. Moreover, 100 switching cycles were obtained with respect to the endurance characteristic (Fig. S6, ESI†).
V. Moreover, 100 switching cycles were obtained with respect to the endurance characteristic (Fig. S6, ESI†).
To elucidate the charge transport mechanisms involved in the PW-based device during the resistive switching process, electrical characterization and appropriate theoretical analysis were performed. First, the tunneling behavior with respect to voltage versus current density (J–V)—illustrated in Fig. S7 (ESI†)—indicated a saturation effect of barrier-limited conduction arising from Au and PW interface barriers and bulk-limited conduction arising from carrier transportation among the PW molecules. Tunneling between POM molecules reportedly follows a “random-walk” path passing through energetically favorable sites toward other electrodes; the POMs act as electron traps during this process.32,35,39,40 A double logarithmic plot of the I–V curve in the positive region was analyzed to acquire in-depth understanding of the charge transport characteristics. The fitting results shown in Fig. 1i suggest that the charge transport property followed the trap-controlled space charge limited conduction (SCLC) law, and thus, the model could be divided into three regions: (I) a low-voltage region following an ohmic law (I ∝ V) featuring formation of a Schottky barrier resulting from contact resistance between the metal electrode and PW; (II) a region around the conductivity peak following Child's law (I ∝ V2), where the trapped electrons overcome and pass through the thin Schottky-like barrier via Fowler–Nordheim tunneling; and (III) an SCLC region exhibiting an evident current increase (I ∝ V5 to I ∝ V15), indicating a transition from trap-unfilled SCLC to trap-filled SCLC. After the device had been switched abruptly, ohmic behavior began to dominate the charge transport in the LRS as the voltage swept back to zero. The linearly relationship between the resistance of the LRS and temperature, as shown in Fig. S8 (ESI†), further prove the typical metallic behaviour. SCLC phenomenon is commonly observed in CF-based memory devices associated with charge carrier tunneling between adjacent filled traps involving oxygen vacancy, clusters, and metal atoms.41,42
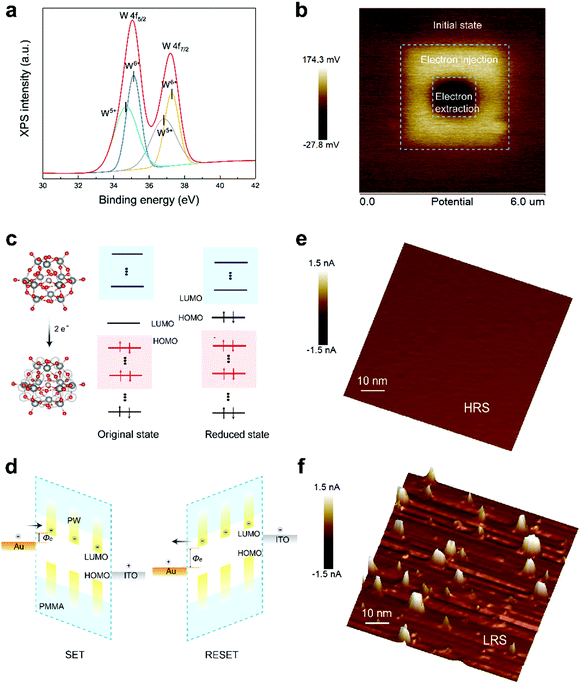
The aforementioned discussion of the conduction mechanism is unanimous in indicating modulation of transport behavior between molecules to ensure easier flow of charge carriers, which are conceivably derived from the electrochemical reduction process from PW12O403− to PW12O405− following trapping of electrons. According to Vasilopoulou,30 a solid-state reduction process of a series of representative Keggin and Dawson POM films is prone to occur upon contact with a metallic electrode; in particular, Keggin-type POM clusters prefer to obtain two electrons at the interface. The reduction of POMs at the interface was of vital importance with respect to effective electron transport through the electronic devices. To support the reduction process of PW molecules in the device structure, X-ray photoelectron spectroscopy equipped with an etching technique for removal of the top metal electrode was applied to monitor the VC of tungstate in the cage of the PW cluster. W 4f photo signals after bias application are shown in Fig. 2a. The photoemission peaks and deconvoluted doublets in W 4f are respectively attributed to the presence of W6+ and W5+, whereas no W5+ signals arising from the W 4f signals of the as-fabricated PW film were observed (Fig. S9, ESI†), indicating that the quantity of PW molecules was lower at the interface. In addition, a broad peak attributed to intervalence charge transfer appeared in the visible region of the UV-vis spectra (Fig. S10, ESI†), indicating a reduction of POM species.30,43 The electron-trapping process of PW was further demonstrated by Kelvin probe force microscopy; evident changes in surface potential were detected when charges were trapped in the PW film by scanning of the surface of the material by using a conductive AFM tip. As shown in Fig. 2b, the bright region within an area measuring 3 μm × 3 μm indicated increased surface potential owing to electron incorporation within the PW molecules, whereas the well-defined contrast (1 μm × 1 μm) after negative bias application suggested the electron extraction and recombination process. Based on these findings, it is reasonable to believe that resistive switching behavior is directly related to the reversible reduction process of PW molecules, and molecular reconfiguration is thought to underpin this behavior.
To gain further insight into the molecular reconfiguration process of POM for comprehensive understanding of the resistive switching process, DFT was utilized to analyze the state transformation of PW before and after two electrons were accepted. The frontier orbitals depicting the charge density states of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) in the PW cluster before and after two electrons were accepted are shown in Fig. 2c. The energy level alignments of PW in the original state and reduced state estimated using DFT calculation are shown in Fig. S11 (ESI†). The injection of two electrons leading to evident variations in the HOMO and LUMO levels corresponded to the increase in the electron injection barrier from 0.08 to 2.17 eV, which was in strong agreement with the ability of trapping at the interface to affect the adjacent Schottky barrier at the metal–oxide interface.44 This phenomenon can adequately explain the difference in resistive switching performance between the SET and RESET processes. During the SET process, the reduction of PW molecules (e.g., the electron-trapping process at the interface) and the transportation of generated charge carriers between PW molecules consecutively dominated the conduction mechanism, resulting in progressively resistance switching behavior. By contrast, the distinct and clear resistance switching from the LRS to HRS during the RESET process was ascribed to enhanced electron extraction and recombination efficiency, which improved the charge transport, as confirmed in multiple studies. The corresponding operation principle of the memory device and the molecular reconfiguration of PW during the SET and RESET processes are illustrated in Fig. 2d. Consequently, the reduction process of PW was believed to initiate the resistive switching process. One possibility is that electrons are injected into PW molecules via Fowler–Nordheim tunneling through the Au/PW interface, followed by charge transportation between PW molecules at energetically favored sites, which ultimately affects the resistance of the active layer as well as the interfacial barrier. As demonstrated in multiple studies regarding transition metal oxides, CFs comprising either electrochemically active metal electrode materials or mobile oxygen vacancies are supposed to be formed or ruptured during the SET and RESET processes.45–47 To support the participation of CFs during the memory switching process, conductive AFM (C-AFM) was characterized to analyze the HRS and LRS. As shown in Fig. 2e, when the memory device stayed in the HRS, no localized current paths were observed. By contrast, when the memory device switched to the LRS, a certain number of localized current paths were formed (Fig. 2f). Therefore, the formation and rupture of conductive filamentary path in the LRS and HRS accounted for the final resistive switching process after the initial reduction process of PW. In addition, nanoscale C-AFM characterization certified the scaling potential of PW-based RRAM, as have been demonstrated in Fig. S12 (ESI†).
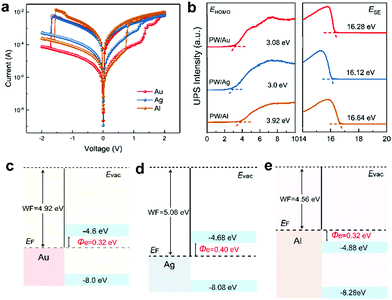
As mentioned, VC and ECM are the two major switching mechanisms of redox-based RRAM depending on the species of mobile ions. ECM is related to the motion of metal cations generated from metal electrodes, whereas VC is related to oxygen vacancy migration in the oxide layer.48,49 To identify the composition of CFs, PW-based RRAMs with electrochemically active Ag and air-stable Al anodes were fabricated to investigate the influence of electrode materials on memory performance. I–V characteristics displayed in Fig. 3a—statistical data provided in Fig. S13 (ESI†)—suggest almost identical resistive switching characteristics of the Au- and Ag-based devices, excepting for the lower ON/OFF ratio of the Ag-based device, which implies that CF formation is independent of electrode materials. However, although comparable ON/OFF ratios were observed in the Au- and Al-based devices, a clear and abrupt resistance switching during the SET and RESET processes was observed in Al-based devices.
To investigate the discrepancy between Au-, Ag-, and Al-anode-based memory devices, ultraviolet photoelectron spectroscopy (UPS) was conducted to determine the energy level alignment of original-state PW molecules in Au, Ag, and Al electrodes (PW/Au, PW/Ag, and PW/Al, respectively); the UPS spectra are displayed in Fig. 3b and the corresponding energy level alignments are illustrated in Fig. 3c–e. The work function of metal and the HOMO position of PW were determined from secondary electron cutoffs (ESE) and evolution of HOMO onset (EHOMO) in the UPS spectra, respectively. The band gap (Eg) of PW was estimated on the basis of the absorption band position and corresponding Tauc plot.50 The LUMO level of PW was determined by the equation ELUMO = EHOMO − Eg; the electron injection barriers defined as the energy difference between the LUMO level of original PW and the Fermi level of metal electrodes were thus calculated. The analogous energy alignment and similar electron injection barriers accounted for the almost identical resistive switching performance in the Au- and Ag-based devices. In addition, the lower ON/OFF ratio of the Ag-based device was believed to arise from Ag+ migration at the interface owing to high electrochemical activity, which led to a lower level of the HRS during the initial bias sweeping stage. In contrast to the energy alignment of the Au- and Ag-based devices, the LUMO level of the PW molecule was lower than the Fermi level of the Al electrode after deposition; this made the molecule energetically favorable for reduction without external bias stimulation. Consequently, in contrast to the Au- and Ag-based devices where PW reduction (electron trapping) and carrier transportation (CF formation) contributed to gradual resistance switching, the PW reduction process was exempted from the resistive switching period during the SET process, and the CF formation process led to the abrupt and well-defined resistance transition from the HRS to LRS. The above discussion suggest an electrode independent resistive switching characteristic.
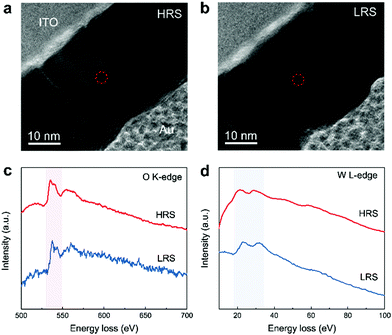
Oxygen vacancies have been demonstrated to be considerably more mobile than transition metal cations in many transition metal–oxide based RRAM studies.16,20 Furthermore, bridging oxygen species in POM structures could be depleted alongside the reduction process, which is equivalent to the formation of oxygen vacancies in the closest vicinity of the molecular lacuna.24,36 The generation or depletion of oxygen vacancies influences the valence state of the metal cations, thereby leading to marked change in electronic conductivity.51 To clarify the composition of CFs and the VCM characteristics of PW-based RRAM, electron energy loss spectroscopy with spectrum energy covering the O–K (O 1s→2p) and W–L (W 2p→3d) edges was conducted in the circled regions of the HRS and LRS, as indicated in Fig. 4a and b. Comparison of the oxygen count in both resistance states clearly showed that oxygen concentration in the LRS was significantly lower than that in the HRS (Fig. 4c); this finding strongly suggests that CFs are formed through the migration of oxygen vacancies. Meanwhile, the reduction of intensity and shifting of representative peaks at the W–L edge revealed the reduction of W6+ and corresponding increase in the W5+ state (Fig. 4d). Furthermore, the VC of W6+ was believed to have been generated by the donating effect of oxygen vacancy after confirmation of two electrons to the 3d state of W. The observed results were highly consistent with those of related reports regarding transition metal oxide-based RRAM and oxygen vacancy-induced resistive switching.52 Hence, we can confirm that the CFs formed inside the active layer of PW-based RRAM comprised oxygen vacancies.
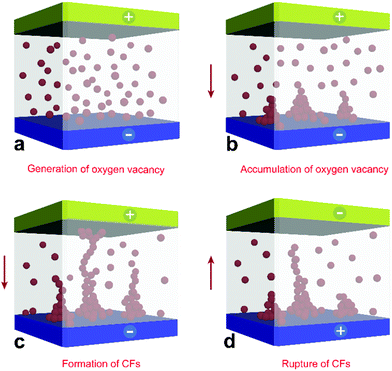
In light of the aforementioned results, a possible scenario of the resistive switching process is illustrated in Fig. 5. First, the positive bias applied to the top metal electrode triggered an electrochemical redox reaction in the vicinity of the interface, accompanied by the generation of mobile oxygen vacancies, mostly near the sites of original bridging oxygen in the POM structure. Because of the intrinsic property of POM, these generated oxygen vacancies were initially randomly distributed throughout the devices (I). Despite there are no oxygen vacancies in PMMA matrix and PMMA gaps are supposed to be exist, the movement of oxygen vacancies will not be fully hindered under the executed electric field. On the contrary, the discontinuous oxygen vacancies can migrate via a favourable alternative path through tunnelling.38 Driven by constant forward biasing, the mobile oxygen vacancies will migrate toward the bottom electrode and accumulate in its vicinity, thereby creating nuclei of oxygen vacancy CFs (II). Subsequently, the nuclei extended and developed into a conductive path. Once a complete CF had formed, the memory device switched to the LRS and ohmic behavior began to dominate the charge transport, leading to a metallic characteristic (III). After the application of opposite bias, the oxygen vacancies surrounding the top electrode began to deplete, thereby rupturing the CFs and enabling resistance switching from the LRS to HRS (IV).
Conclusions
In summary, resistance change in POM-based memory is initiated by a spatially confined redox reaction, which in turn leads to a measurable VC between the HRS and LRS, thereby verifying the so-called VC mechanism for resistive switching in POM-based RRAM. In light of the findings of this study, POMs are confirmed to feature a new class of robust electronic materials owing to their downscaled size to nanometers and accessible multilevel redox potentials realized through elaborate designation. In addition to the guidance aspect of this research, namely direct in-depth investigation of sophisticated POM-based devices, the application of POM molecules as a storage medium brings us one step further toward realizing electronic devices on a nanometer scale.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the grants from China Postdoctoral Science Foundation (Grant No. 2017M622775), Natural Science Foundation of China (Grant No. 61604097 and 61601305), the Science and Technology Innovation Commission of Shenzhen (Grant No. JCYJ20170818143618288, JCYJ20170302145229928 and JCYJ20170302151653768), Shenzhen Peacock Technological Innovation Project (Grant No. KQJSCX20170727100433270 and KQJSCX20170327150812967), Guangdong Provincial Department of Science and Technology (Grant No. 2017TQ04X082, 2018B030306028 and 2017A010103026), the Department of Education of Guangdong Province (Grant No. 2016KTSCX120) and the Natural Science Foundation of SZU.Notes and references
- C. Baeumer, C. Schmitz, A. Marchewka, D. N. Mueller, R. Valenta, J. Hackl, N. Raab, S. P. Rogers, M. I. Khan, S. Nemsak, M. Shim, S. Menzel, C. M. Schneider, R. Waser and R. Dittmann, Nat. Commun., 2016, 7, 12398 CrossRef CAS PubMed.
- M. Lanza, H.-S. P. Wong, E. Pop, D. Ielmini, D. Strukov, B. C. Regan, L. Larcher, M. A. Villena, J. J. Yang, L. Goux, A. Belmonte, Y. Yang, F. M. Puglisi, J. Kang, B. Magyari-Köpe, E. Yalon, A. Kenyon, M. Buckwell, A. Mehonic, A. Shluger, H. Li, T.-H. Hou, B. Hudec, D. Akinwande, R. Ge, S. Ambrogio, J. B. Roldan, E. Miranda, J. Suñe, K. L. Pey, X. Wu, N. Raghavan, E. Wu, W. D. Lu, G. Navarro, W. Zhang, H. Wu, R. Li, A. Holleitner, U. Wurstbauer, M. C. Lemme, M. Liu, S. Long, Q. Liu, H. Lv, A. Padovani, P. Pavan, I. Valov, X. Jing, T. Han, K. Zhu, S. Chen, F. Hui and Y. Shi, Adv. Electron. Mater., 2019, 5, 1800143 CrossRef.
- H. Wu, X. H. Wang, B. Gao, N. Deng, Z. Lu, B. Haukness, G. Bronner and H. Qian, Proc. IEEE, 2017, 105, 1770 CAS.
- S. Liu, N. Lu, X. Zhao, H. Xu, W. Banerjee, H. Lv, S. Long, Q. Li, Q. Liu and M. Liu, Adv. Mater., 2016, 28, 10623 CrossRef CAS PubMed.
- Z. Wang, S. Joshi, S. E. Savel'ev, H. Jiang, R. Midya, P. Lin, M. Hu, N. Ge, J. P. Strachan, Z. Li, Q. Wu, M. Barnell, G. L. Li, H. L. Xin, R. S. Williams, Q. Xia and J. J. Yang, Nat. Mater., 2017, 16, 101 CrossRef CAS PubMed.
- X. Yang, Y. Fang, Z. Yu, Z. Wang, T. Zhang, M. Yin, M. Lin, Y. Yang, Y. Cai and R. Huang, Nanoscale, 2016, 8, 18897 RSC.
- X. Wang, B. Gao, H. Wu, X. Li, D. Hong, Y. Chen and H. Qian, Nanoscale, 2017, 9, 13449 RSC.
- C. Liu, X. Yan, X. Song, S. Ding, D. W. Zhang and P. Zhou, Nat. Nanotechnol., 2018, 13, 404–410 CrossRef CAS PubMed.
- L. Q. Zhu, C. J. Wan, L. Q. Guo, Y. Shi and Q. Wan, Nat. Commun., 2014, 5, 3158 CrossRef PubMed.
- C. Baeumer, C. Schmitz, A. H. Ramadan, H. Du, K. Skaja, V. Feyer, P. Muller, B. Arndt, C. L. Jia, J. Mayer, R. A. De Souza, C. Michael Schneider, R. Waser and R. Dittmann, Nat. Commun., 2015, 6, 8610 CrossRef CAS PubMed.
- S. Cho, C. Yun, S. Tappertzhofen, A. Kursumovic, S. Lee, P. Lu, Q. Jia, M. Fan, J. Jian, H. Wang, S. Hofmann and J. L. MacManus-Driscoll, Nat. Commun., 2016, 7, 12373 CrossRef CAS PubMed.
- C. Baeumer, R. Valenta, C. Schmitz, A. Locatelli, T. O. Mentes, S. P. Rogers, A. Sala, N. Raab, S. Nemsak, M. Shim, C. M. Schneider, S. Menzel, R. Waser and R. Dittmann, ACS Nano, 2017, 11, 6921 CrossRef CAS PubMed.
- Y. Yang, P. Gao, L. Li, X. Pan, S. Tappertzhofen, S. Choi, R. Waser, I. Valov and W. D. Lu, Nat. Commun., 2014, 5, 4232 CrossRef CAS PubMed.
- R. Waser and M. Aono, Nat. Mater., 2007, 6, 833 CrossRef CAS PubMed.
- J. Zhu, Y. Yang, R. Jia, Z. Liang, W. Zhu, Z. U. Rehman, L. Bao, X. Zhang, Y. Cai, L. Song and R. Huang, Adv. Mater., 2018, e1800195 CrossRef PubMed.
- J. Zheng, J. Zhang, Z. Wang, L. Zhong, Y. Sun, Z. Liang, Y. Li, L. Jiang, X. Chen and L. Chi, Adv. Mater., 2018, e1802731 CrossRef PubMed.
- A. Wedig, M. Luebben, D. Y. Cho, M. Moors, K. Skaja, V. Rana, T. Hasegawa, K. K. Adepalli, B. Yildiz, R. Waser and I. Valov, Nat. Nanotechnol., 2016, 11, 67 CrossRef CAS PubMed.
- R. Waser, R. Dittmann, G. Staikov and K. Szot, Adv. Mater., 2009, 21, 2632 CrossRef CAS.
- D. Cooper, C. Baeumer, N. Bernier, A. Marchewka, C. La Torre, R. E. Dunin-Borkowski, S. Menzel, R. Waser and R. Dittmann, Adv. Mater., 2017, 29, 1700212 CrossRef PubMed.
- K. T. Kang, H. Kang, J. Park, D. Suh and W. S. Choi, Adv. Mater., 2017, 29, 1700071 CrossRef PubMed.
- F. Yuan, Z. Zhang, C. Liu, F. Zhou, H. M. Yau, W. Lu, X. Qiu, H. S. P. Wong, J. Dai and Y. Chai, ACS Nano, 2017, 11, 4097 CrossRef CAS PubMed.
- C. Li, B. Gao, Y. Yao, X. Guan, X. Shen, Y. Wang, P. Huang, L. Liu, X. Liu, J. Li, C. Gu, J. Kang and R. Yu, Adv. Mater., 2017, 29, 1602976 CrossRef PubMed.
- H. Tian, H. Y. Chen, B. Gao, S. Yu, J. Liang, Y. Yang, D. Xie, J. Kang, T. L. Ren, Y. Zhang and H. S. Wong, Nano Lett., 2013, 13, 651 CrossRef CAS PubMed.
- Y.-X. Zhou, Y. Li, Y.-T. Su, Z.-R. Wang, L.-Y. Shih, T.-C. Chang, K.-C. Chang, S.-B. Long, S. M. Sze and X.-S. Miao, Nanoscale, 2017, 9, 6649 RSC.
- K. Tang, A. C. Meng, F. Hui, Y. Shi, T. Petach, C. Hitzman, A. L. Koh, D. Goldhaber-Gordon, M. Lanza and P. C. McIntyre, Nano Lett., 2017, 17, 4390 CrossRef CAS PubMed.
- W. Banerjee, Q. Liu, H. Lv, S. Long and M. Liu, Nanoscale, 2017, 9, 14442 RSC.
- U. Celano, J. Op de Beeck, S. Clima, M. Luebben, P. M. Koenraad, L. Goux, I. Valov and W. Vandervorst, ACS Appl. Mater. Interfaces, 2017, 9, 10820 CrossRef CAS PubMed.
- G. S. Park, Y. B. Kim, S. Y. Park, X. S. Li, S. Heo, M. J. Lee, M. Chang, J. H. Kwon, M. Kim, U. I. Chung, R. Dittmann, R. Waser and K. Kim, Nat. Commun., 2013, 4, 2382 CrossRef PubMed.
- M. Qi, L. Bai, H. Xu, Z. Wang, Z. Kang, X. Zhao, W. Liu, J. Ma and Y. Liu, J. Mater. Chem. C, 2018, 6, 2026 RSC.
- M. Vasilopoulou, E. Polydorou, A. M. Douvas, L. C. Palilis, S. Kennou and P. Argitis, Energy Environ. Sci., 2015, 8, 2448 RSC.
- H. N. Miras, J. Yan, D. L. Long and L. Cronin, Chem. Soc. Rev., 2012, 41, 7403 RSC.
- N. Kawasaki, H. Wang, R. Nakanishi, S. Hamanaka, R. Kitaura, H. Shinohara, T. Yokoyama, H. Yoshikawa and K. Awaga, Angew. Chem., Int. Ed. Engl., 2011, 50, 3471 CrossRef CAS PubMed.
- L. Vila-Nadal, S. G. Mitchell, S. Markov, C. Busche, V. Georgiev, A. Asenov and L. Cronin, Chem. – Eur. J., 2013, 19, 16502 CrossRef CAS PubMed.
- X. Chen, Y. Zhou, V. A. L. Roy and S.-T. Han, Adv. Mater., 2018, 30, 1703950 CrossRef PubMed.
- C. Busche, L. Vila-Nadal, J. Yan, H. N. Miras, D. L. Long, V. P. Georgiev, A. Asenov, R. H. Pedersen, N. Gadegaard, M. M. Mirza, D. J. Paul, J. M. Poblet and L. Cronin, Nature, 2014, 515, 545 CrossRef CAS PubMed.
- M. Vasilopoulou, A. M. Douvas, L. C. Palilis, S. Kennou and P. Argitis, J. Am. Chem. Soc., 2015, 137, 6844 CrossRef CAS PubMed.
- Y. Zhu, Z. Yuan, W. Cui, Z. Wu, Q. Sun, S. Wang, Z. Kang and B. Sun, J. Mater. Chem. A, 2014, 2, 1436 RSC.
- A. M. Douvas, E. Makarona, N. Glezos, P. Argitis, J. A. Mielczarski and E. Mielczarski, ACS Nano, 2008, 2, 733 CrossRef CAS PubMed.
- D. J. Wales, Q. Cao, K. Kastner, E. Karjalainen, G. N. Newton and V. Sans, Adv. Mater., 2018, e1800159 CrossRef PubMed.
- H. Tanaka, M. Akai-Kasaya, A. TermehYousefi, L. Hong, L. Fu, H. Tamukoh, D. Tanaka, T. Asai and T. Ogawa, Nat. Commun., 2018, 9, 2693 CrossRef PubMed.
- F. Volatron, J.-M. Noël, C. Rinfray, P. Decorse, C. Combellas, F. Kanoufi and A. Proust, J. Mater. Chem. C, 2015, 3, 6266 RSC.
- A. Balliou, G. Papadimitropoulos, G. Skoulatakis, S. Kennou, D. Davazoglou, S. Gardelis and N. Glezos, ACS Appl. Mater. Interfaces, 2016, 8, 7212–7220 CrossRef CAS PubMed.
- B. Hu, C. Wang, J. Wang, J. Gao, K. Wang, J. Wu, G. Zhang, W. Cheng, B. Venkateswarlu, M. Wang, P. S. Lee and Q. Zhang, Chem. Sci., 2014, 5, 3404 RSC.
- H. Li, S. Pang, S. Wu, X. Feng, K. Mullen and C. Bubeck, J. Am. Chem. Soc., 2011, 133, 9423 CrossRef CAS PubMed.
- G. Chaidogiannos, Microelectron. Eng., 2004, 73-74, 746 CrossRef CAS.
- H. Ling, M. Yi, M. Nagai, L. Xie, L. Wang, B. Hu and W. Huang, Adv. Mater., 2017, 29, 1701333 CrossRef PubMed.
- B. Hwang and J. S. Lee, Adv. Mater., 2017, 29, 1701048 CrossRef PubMed.
- X. Lopez, J. J. Carbo, C. Bo and J. M. Poblet, Chem. Soc. Rev., 2012, 41, 7537 RSC.
- H. Du, C.-L. Jia, A. Koehl, J. Barthel, R. Dittmann, R. Waser and J. Mayer, Chem. Mater., 2017, 29, 3164 CrossRef CAS.
- J. Li, T. Zhang, Q. Duan, L. Li and R. Huang, in Engineering Resistive Switching Behavior in TaOx Based Memristive Devices for Non-Von Neuman Computing Applications, China Semiconductor Technology International Conference, 2018, pp. 1–3 Search PubMed.
- S. Gam Derouich, C. Rinfray, G. Izzet, J. Pinson, J. J. Gallet, F. Kanoufi, A. Proust and C. Combellas, Langmuir, 2014, 30, 2287 CrossRef CAS PubMed.
- R. Yang, H.-M. Huang, Q.-H. Hong, X.-B. Yin, Z.-H. Tan, T. Shi, Y.-X. Zhou, X.-S. Miao, X.-P. Wang, S.-B. Mi, C.-L. Jia and X. Guo, Adv. Funct. Mater., 2018, 28, 1704455 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nh00366a |
| This journal is © The Royal Society of Chemistry 2019 |