Etching of transition metal dichalcogenide monolayers into nanoribbon arrays†
Zixing
Wang
 a,
Xiang
Zhang
b,
Jordan A.
Hachtel
a,
Xiang
Zhang
b,
Jordan A.
Hachtel
 c,
Amey
Apte
c,
Amey
Apte
 b,
Chandra S.
Tiwary
bd,
Robert
Vajtai
b,
Chandra S.
Tiwary
bd,
Robert
Vajtai
 be,
Juan Carlos
Idrobo
c,
Ramazan
Ozturk
be,
Juan Carlos
Idrobo
c,
Ramazan
Ozturk
 *b and
Pulickel
Ajayan
*b and
Pulickel
Ajayan
 *b
*b
aDepartment of Chemistry, Rice University, 6100 Main Street, Houston, TX 77005, USA
bDepartment of Materials Science and Nano Engineering, Rice University, Houston, TX 77005, USA. E-mail: rozturk@rice.edu; ajayan@rice.edu
cCenter for Nanophase Materials Sciences, Oak Ridge National Laboratory, Oak Ridge, TN 37830, USA
dMetallurgical and materials engineering, Indian Institute of Technology Kharagpur, West Bengal-721302, India
eUniversity of Szeged, Interdisciplinary Excellence Centre, Department of Applied and Environmental Chemistry, University of Szeged, Rerrich Bélatér 1, Szeged, Hungary
First published on 18th January 2019
Abstract
Two-dimensional transition metal dichalcogenide (TMDC) nanoribbons are reported to exhibit interesting properties distinctly different from their 2D analogues, including a change of transport properties depending on the edge structure, enhanced exciton correlation effect and thermoelectric property. Here we report a successful preparation method for large arrays of 2D TMDC nanoribbons without a template, using a reducing agent aqueous etchant. This method is a simple and tuneable way for generating TMDC (e.g. MoS2 and MoSe2) nanoribbons from CVD grown 2D TMDCs on Si/SiO2 through liquid phase mechano-chemical reaction. The reducing agent converts Mo(IV) from the defect sites to a lower oxidation state, thus expanding the defects. Water acts as a detaching and tearing medium that pulls the TMDC flake into nanoribbons. The area of conversion, density, and aspect ratio of the nanoribbons can be tuned by concentration and potency of the etchant. The 2D nanoribbons possess high structural integrity, zigzag edges with chalcogen termination, and an increase of bandgap when ribbon width decreases. This method allows a scalable approach for 2D nanoribbons to be prepared for various applications.
Conceptual insightsTransition metal dichalcogenide (TMDC) nanoribbons has been widely studied by computational scientists for their novel physical and electronic characters distinct from the bulk. Existing synthesis methods for TMDC nanoribbons either require a growth template, which sometimes is undesirable for electronic applications, or ion-beam etching with low efficiency and edge controllability. Different from the reported bottom-up and top-down methods, we turn to the liquid phase etching method for the production of TMDC nanoribbons. This method uses a low-cost reducing agent solution and tears the TMDC monolayers on a Si wafer within minutes through a mechano-chemical process. This method overcomes the limitation of sample size and time of ion-beam etching, while retaining the high crystal quality of CVD grown TMDC on Si wafer and gives a consistent zigzag edge. Through different reducing agents and solvents, this work explores the chemistry of reducing agents with TMDC, helping researchers understand the decomposition mechanism of ultra-thin nanomaterials and providing them a new pathway in manipulating the geometry of 2D nanomaterials. |
Two-dimensional materials have attracted vast attention in research in the past few years for their unusual properties.1–4 Monolayer TMDCs composed of molybdenum and tungsten have gained particular interest due to their highly tunable direct band gap that can range from 1.1 eV to 1.9 eV,5 which makes them ideal candidates for transistors, photodetectors, and electroluminescent devices.5–8 These versatile applications have made routes to synthesize 2D materials with controllable physical properties, such as mechanical and liquid exfoliation, atomic layer deposition (ALD), and chemical vapor deposition (CVD), well-developed but ever expanding fields.9–14
One method to control the properties of 2D materials is to reduce the dimensionality and increase the aspect ratio. One dimensional structures formed from 2D materials are expected to exhibit interesting properties and applications that differ from the parent 2D materials.15–18 Atomically thin nanoribbons, with a higher edge-to-area ratio, have shown promise for applications in electro-catalytic reactions, gas detection, sensing, and energy storage.19–23 Additionally, theoretical calculations show TMDC nanoribbons have properties that differ from the bulk phase.16,24,25 Unlike their 2D counterparts, nanoribbons have optical and electronic properties that are dependent on the geometry and edge structure of the ribbon (i.e. zigzag nanoribbons are ferromagnetic and metallic, while armchair nanoribbons are nonmagnetic and semiconducting).24 Methods to prepare nanoribbons mostly fall under two categories: bottom-up (templated growth such as pre-patterned amorphous silicon, carbon nanotubes, or Mo nanowires)26–29 and top-down (ion beam etching: direct helium ion beam milling).21 The preparation of templates for the bottom-up process is usually tedious, while top-down methods struggle with scalability, which makes generating 1D TMDC structures with controllable geometry and properties without a template an ongoing challenge for fabrication researchers.
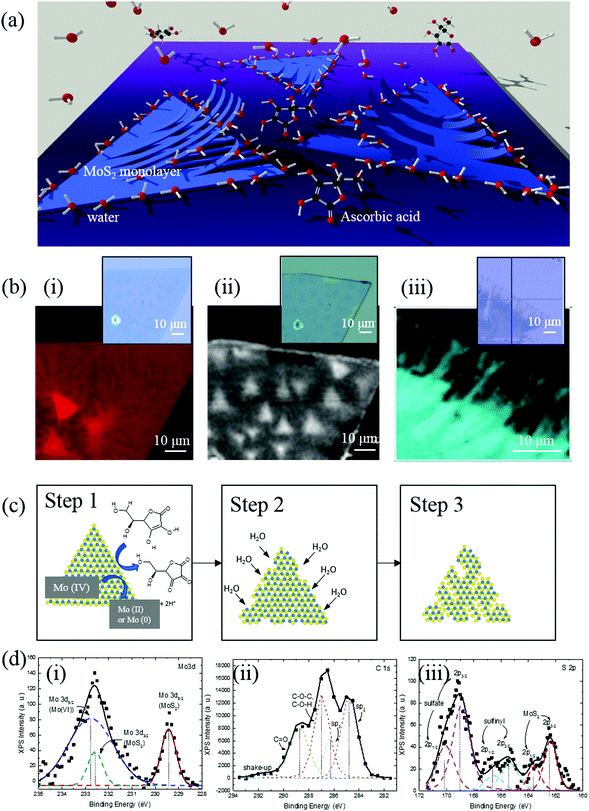
In this paper, we demonstrate a scalable, clean, and facile method to convert monolayer 2D MoS2 sheets into anisotropic nanoribbon arrays using a low-cost aqueous solution of ascorbic acid. A schematic of the process is shown in Fig. 1(a). Large (several micron) atomically-thin sheets of MoS2 are prepared using CVD and are selectively etched using an aqueous solution of reducing agents. By varying the concentration and type of the solvents and reducing agents, the width, density, and edge structure of the nanoribbons can be deliberately controlled. The ribbons are characterized via spectroscopy, electron microscopy, and photoluminescence (PL) to investigate the mechanism of the 2D to 1D conversion and demonstrate the tunability of the structure. Finally, the process is also expanded to other TMDC materials, such as MoSe2.
CVD grown MoS2 flakes are etched in 1.5 mM ascorbic acid aqueous solution, which is mildly reducing, for three minutes to form nanoribbons (details in Experimental section). The effect of solvent and reducing agent was studied respectively to explain the formation mechanism of MoS2 nanoribbons. Water as a strong polar protic solvent detaches CVD grown MoS2 off the SiO2/Si substrate. When in water, H2O molecules accumulate to the hydrophilic edges and defects of MoS2 flakes and replace the Si–S dipole–dipole bonds with Si–H2O and S–H2O hydrogen bonds and attaches to the clean S and Si atoms at the interface. The intercalation and accumulation of H2O molecules at the interface detaches MoS2 flakes from SiO2/Si substrate. Various solvents including hexane, chloroform, acetone, N,N-dimethylformamide, isopropyl alcohol, ethanol and de-ionized water have been tested for their detaching ability. After soaking MoS2/SiO2/Si pieces respectively, only polar protic solvents (isopropyl alcohol, ethanol and de-ionized water) lift the edges of MoS2 flakes off SiO2 noticeably (Fig. S1, ESI†). Isopropyl alcohol and ethanol have slower and subtler lift-off compared to water. Water causes a large amount of MoS2 lifting from both the edges and some vacancies within the flakes. MoS2 is unaffected by non-polar and slightly polar solvents (hexane and chloroform) and polar aprotic solvents (acetone and DMF). Polar protic solvents (ethanol and water) dropped on MoS2/SiO2/Si surfaces accumulate at the edges of the MoS2 flakes, proving the edges of MoS2 are hydrophilic due to dangling bonds while the SiO2 and MoS2 surfaces are not (Fig. S2, ESI†). Despite clean uncontaminated surface of MoS2 is hydrophilic due to lone pair of electrons at the surface,30 MoS2/SiO2/Si exposed in air for many days is hydrophobic. The contact angle measured on the aged MoS2/SiO2/Si shows a water contact angle of 89.6° (Fig. S2(b), ESI†). This wettability transition with aging was explained by water and hydrocarbon contamination by Chow et al.31
Ascorbic acid as a weak reducing agent reduces exposed Mo(IV) atoms at the edges, decomposing MoS2 structure, creating unevenness and promoting the formation of nanoribbons. The reaction between a reducing agent and MoS2 has been observed previously with AgNO3.32 The interaction between MoS2 and ascorbic acid was confirmed via Raman spectroscopy and X-ray photoemission spectroscopy (XPS).
Raman mapping (E2g peak at 380 cm−1) was used to compare MoS2 as grown, soaked in 0.5 M ascorbic acid DMF solution, and in 1.5 mM ascorbic acid in H2O to observe the functions of different components of the etching solution (Fig. 1(b)). Before soaking, pristine MoS2 has very little defects, as confirmed by both Raman mapping and optical microscopy images. However, after soaking in ascorbic acid in DMF for 20 minutes, defects start to develop at the edges of each MoS2 flake, indicating the reduction of exposed Mo(IV) at the edges by the ascorbic acid and disintegration of the structure. The preferred binding and reaction at the edges has also been observed in other literature, with oxygen and for water-splitting reactions.33 The reducing agents expand the defect sites on the MoS2 flake and increase the density of MoS2 nanoribbons (shown schematically in Fig. 1(c)).
XPS was performed on the product of exfoliated MoS2 powder soaked in ascorbic acid water solution for 12 hours. Exfoliated MoS2 powder was used instead of CVD grown monolayers to ensure high enough signal intensity. The results show that 71% of the Mo(IV) has been turned into Mo(VI) as shown in Fig. 1(d). This is caused by reduction of Mo to a lower oxidation state and later oxidizing it to MoO3 hydrate in water under atmosphere. Sulfur from the product shows peaks from sulfate (64%), sulfinyl (15%) together with MoS2 (21%), indicating that the sulfur in MoS2 got oxidized into sulfate and sulfinyl. C 1s spectra of as-bought ascorbic acid was compared with the C 1s spectra of MoS2/ascorbic acid reaction product (Fig. S4, ESI†). C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O
O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C–O–C, C–O–H ratio increased from 0.25 to 0.76 after the reaction. Despite the carbon spectra cannot be quantified due to contamination, it can qualitatively prove conversion of C–OH bond to C
C–O–C, C–O–H ratio increased from 0.25 to 0.76 after the reaction. Despite the carbon spectra cannot be quantified due to contamination, it can qualitatively prove conversion of C–OH bond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O during ascorbic acid oxidation.
O during ascorbic acid oxidation.
Reducing agents with different reducing strength have been tested out, as shown in Fig. S5 (ESI†). DIW without any reducing agent gives the least amount of lifting. The nanoribbons formed are stubby and mostly at the edges. However, with increasing reducing strengths, the lifting become much more significant and nanoribbons start to appear within the MoS2 flakes. The strongest reducing agent, NaBH4, produces the narrowest MoS2 nanoribbons. Formaldehyde leaves low density of very narrow ribbons and weaker reducing agents like sodium thiosulfate and ascorbic acid gives wider, higher density ribbons.
The competing kinetics of ascorbic acid etching and MoS2/SiO2 detaching allows MoS2 monolayers to tear instead of being lifted off the surface entirely. A theoretical calculation done by Li et al. shows the weakest ultimate strength and strain within both MoS2 and MoSe2 are along the zigzag direction at (16.9 GPa, 0.19 and 12.86 GPa, 0.16).34 A study on MoS2 monolayers transferred onto Si/SiO2 surface measured adhesion energy between the two materials to be 0.17 J m−2,35 which is much lower than the weakest strength within MoS2 flakes. However, since the MoS2 flakes used in this experiment were directly grown with CVD under Ar/H2 atmosphere, the contaminant between the MoS2 flakes and Si wafer was less, thus the bonding is stronger with dipole–dipole bonding between S and Si, in addition to van der Waals force. Consequently, with appropriate MoS2 detaching speed and etching kinetic, MoS2 can be torn into ribbon like form in an aqueous solution of reducing agents. The quality of CVD grown MoS2 flakes also have an influence on the dimensions of MoS2 nanoribbons. MoS2 flakes grown with more defects tend to etch faster, leaving lower density, narrower nanoribbons. If the flakes have large grain size, long ribbons will form.
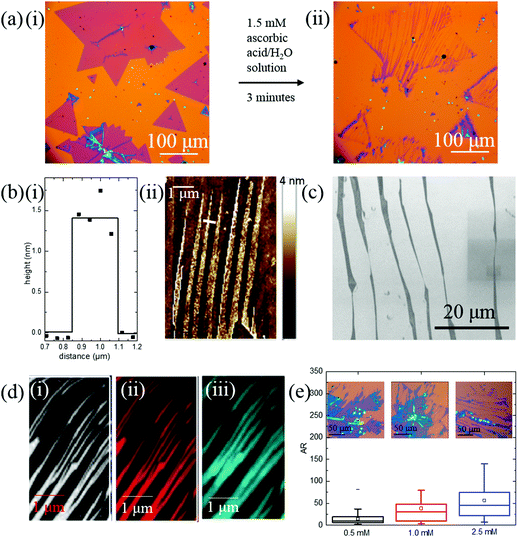
Fig. 2(a) shows optical microscopy images of the nanoribbons before and after conversion. The ribbons are anisotropic and have width down to the sub-micron regime. The ribbons mostly start from the edge of the 2D MoS2 flakes and can extend across the entire diameter of the 2D parent. The dimensions of the nanoribbons were measured with an atomic force microscope (AFM) and are shown in Fig. 2(b). The depth profile of a single ribbon is shown in Fig. 2(b-i), where the nanoribbon has a width of 300 ± 50 nm, and a height of 1.5 nm, indicating it is composed of monolayer MoS2.36 An AFM map of several nanoribbons is shown in Fig. 2(b-ii), demonstrating that the ribbons are monolayer or bilayer across their entire length and grow parallel to one another. Scanning electron microscope (SEM) images in Fig. 2(c) shows the width of the nanoribbons is not uniform and can change in ∼30° steps. The tearing of MoS2 is independent of the flow direction of water and only parallel to one of the edges of 2D parent MoS2 (Fig. S8, ESI†).
The structural integrity of the ribbons was investigated with Raman spectroscopy and photoluminescence (PL). The Raman spectra has peaks at 380 cm−1 and 402 cm−1, and Fig. 2(d) shows maps of the Raman intensity at those wavenumbers (380 cm−1 (i) and 402 cm−1 (ii)). The Raman maps show uniform intensity over the entire ribbon, indicating MoS2 ribbons are clean and defect free. Fig. 1(d-iii) shows the PL map of the peak emission at 1.82 eV. The PL mapping also shows uniform intensity, confirming that the MoS2 structure is not disturbed.
The dimensions of the nanoribbons can be controlled with the concentration of ascorbic acid, using similar quality CVD grown MoS2 sheets. Higher concentration of ascorbic acid etches away larger portions of MoS2 flakes (Fig. S9, ESI†), producing narrower and longer nanoribbons. Fig. S9(f) (ESI†) shows the width and length distribution of nanoribbons when treated with different concentrations of ascorbic acid (0.5 mM, 1.0 mM, and 2.5 mM) for 3 minutes. When the concentration of the ascorbic acid increases, the width decreases. The median of the nanoribbon width decreases from 1316 nm to 950 nm, and 684 nm. An increase of ascorbic acid concentration increases nanoribbons length. The aspect ratio box graph from Fig. 2(e) shows that lower concentration of ascorbic acid produces stubbier nanoribbons while higher concentration of ascorbic acid etches MoS2 flakes into lankier nanoribbons.
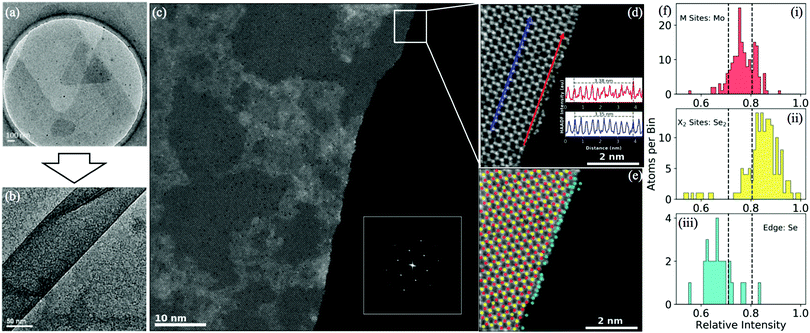
Transmission electron microscopy (TEM) images of the MoS2 before and after turning into nanoribbons are presented in Fig. 3(a and b). Fig. 3(b) shows a single post-treatment ribbon, less than 100 nm wide. The edges of the nanoribbon are parallel over the ribbon.
In Fig. 3(c) a high angle annular dark field (HAADF) scanning transmission electron microscopy (STEM) image shows an atomic-resolution picture of a MoSe2 nanoribbon. In the inset is the fast Fourier transform (FFT) of the ribbon. The Fourier points of a single hexagonal structure are clearly observed indicating that the lattice is single crystal with few defects. Fig. 3(d) shows a higher-resolution picture of the edge of the nanoribbon in Fig. 3(c), illustrating that the growth direction mostly coincides with the zigzag-direction. The angle between the edge of the ribbons and the edge of MoS2 parental flake has been measured via optical microscopy as well (Fig. S11, ESI†). Among 120 measurements, the direction of MoS2 nanoribbons growth was highly selective and occurred at 62.5°–67.5° to the edge of the flake, which corresponds to the angle of a zigzag edge, corroborating the STEM analysis. This type of anisotropic degradation has also been observed in WS2 under laser irradiation.37
Line profiles of the HAADF image measure a lattice strain of ∼1% at the edge. The two line profiles shown in the inset of Fig. 3(d) are along the growth direction on the Se2 columns. The average distance over 10 atomic sites is 3.35 Å (bulk) to 3.38 Å (edge).
The edge-termination is determined by measuring the HAADF intensity of the different atomic sites. HAADF imaging uses Z-contrast, meaning heavier atomic columns are brighter than the lighter atomic columns. In order to identify the species at the edge, the atomic columns in Fig. 3(d) are sorted into three categories: metal sites (red), chalcogenide sites (yellow), and edge sites (blue). Then a histogram of the HAADF intensities of each type of site is shown in Fig. 3(f). The metal sites and chalcogenide sites are shown in Fig. 3(f-i and ii) respectively, and match well with the predicted intensities from Z-values of each element (Mo: Z = 42, Se2: Zeff = 51). The intensity of the edge sites is shown in Fig. 3(f-iii) and are distributed of lower intensities than either Mo or Se2 indicating they are single Se atoms (Z = 36), indicating that the MoSe2 nanoribbons are chalcogenide terminated.
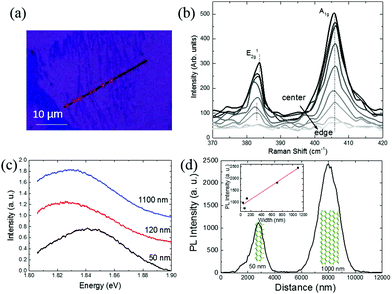
The effect of the reduced dimensionality of MoS2 nanoribbons was further studied through Raman spectroscopy and PL (Fig. 4). To measure the Raman spectra of the nanoribbons accurately, a laser has been swept across the ribbons as shown in Fig. 4(a). The Raman spectrum across a single 160 nm wide nanoribbon is shown in Fig. 4(b), where a change of position and intensity of Raman peaks (from the center to the edge: dark to light) is observed. Due to much larger size of laser spot size with respect to the nanoribbon width, moving the laser further away from the ribbon reduces the intensity, while allowing the signal from the nanoribbon edge to have a more significant effect. The E2g peak shifts to a lower wavenumber as the laser spot moves further away, while the A1g peak is unchanged. A shift of ∼1 eV for the E2g peak indicates the nanoribbon has around 1% strain at the edge according to the calculation by Rice et al., which agrees with the result obtained from STEM.38
Most nanoribbons show a bandgap of 1.82 eV while for ribbons below 100 nm a blue-shift to 1.84 eV is observed, likely due to higher density of defects in the ribbon (Fig. 4(c)). Fig. 4(d) shows PL intensity profile at 1.82 eV across two nanoribbons, 50 and 1000 nm wide. The wider ribbon predictably shows a higher intensity, and by measuring the intensities of a series of ribbons, a linear relationship between the PL and nanoribbon width can be found. The inset in Fig. 4(d) shows a series of such measurements and demonstrates that PL intensity increases with the width of nanoribbons with a rough linear relationship. This linear relationship can help identify nanoribbon width from PL.
The same method can be applied to CVD grown MoSe2 (Fig. S12, ESI†). The MoSe2 ribbons are partially monolayer (1 nm) and partially bilayer (1.7 nm) (Fig. S6, ESI†).8
Experimental section
MoS2 and MoSe2 thin films were grown on SiO2/Si substrates by CVD method. MoO3 powder was placed in the bottom of a ceramic boat, above which a SiO2/Si substrate lied face down. The entire boat was then inserted into the center of a quartz tube in a furnace. Another ceramic boat containing S or Se powder was placed upstream of the SiO2/Si substrate. The furnace was heated up to 750 °C in 15 min and remained at 750 °C for another 15 min before cooled down naturally. Ar/H2 (15% H2) was used as the carrier gas during the growth process. The CVD grown MoS2 monolayers were stored in air-tight glass vials before treatment.To etch monolayer MoS2, 300 μL of freshly prepared 0.05 M, L-ascorbic acid (Sigma Aldrich, reagent grade) aqueous solution was dropped in 10 mL de-ionized water. MoS2 wafer was then immersed in the solution for 3 minutes. Then, the wafer was picked out of the solution and submerged in 20 mL de-ionized water for 5 seconds and air dried. To change the concentration of ascorbic acid in the end solution, different volume of 0.05 M ascorbic acid was added (100 μL, 200 μL, and 500 μL). The same MoS2 wafer was cut into multiple pieces for batch comparison to keep the results accountable. For other reducing agents (iron(II) sulfate, sodium thiosulfate, formaldehyde, sodium borohydride, all from Sigma Aldrich), the same procedure was executed as with ascorbic acid. To study the effect of solvents, the above condition was kept the same (300 μL of freshly prepared 0.05 M L-ascorbic acid in 10 mL of solvents, soak for 3 minutes) except the water was replaced with hexane, chloroform, acetone, N,N-dimethylformamide, isopropyl alcohol, or ethanol (all from Sigma Aldrich).
The synthesized MoS2 were first observed under optical microscope. AFM (Bruker Multimode 8) was conducted under ScanAsyst™ self-optimizing mode. FEI Quanta 400 ESEM was used to take higher magnification images of the nanoribbons on Si wafer under 10 V beam voltage. PHI Quantera XPS was used to study the elemental composition of the end-product between MoS2 and ascorbic acid. Pass energy used was 26 eV. Raman and PL spectrum were obtained on Renishaw InVia Raman Microscope. Laser wavelength was at 532 nm. To reduce damage to the nanoribbons, laser power was reduced to 10% for Raman and 5% for PL.
For STEM and TEM characterization, the nanoribbons were transferred onto holey carbon coated TEM grids using a PMMA-assisted transferring technique. The sample was spin-coated with PMMA 950 K at 2000 rpm for 60 s and then SiO2 was etched with KOH. PMMA film after being transferred onto TEM grid was washed with acetone and IPA. TEM (JEOL 2010 Transmission Electron Microscope), STEM characterization was performed on a Nion aberration-corrected UltraSTEM 100 operated at 60 kV accelerating voltage.
The angle, width and length of the nanoribbons were measured with ImageJ using 50× optical images. The angles were measured using angle measurement tool. The width was measured by measuring the FWHM of the peak from intensity measurement across and perpendicular to the nanoribbons. The length of the nanoribbons was measured using line measurement tool.
To study the effect of water flow direction to tearing, a MoS2/SiO2/Si was placed in a narrow tube and had water flow across in one direction for one minute.
Conclusions
In summary, we have successfully produced a simple but effective way to produce TMDC (MoS2 and MoSe2) nanoribbons from CVD grown thin layers on Si/SiO2 wafer. Aqueous solution of reducing agents was used to etch the TMDC flakes. The process works through reducing agents expanding the defects in the TMDC and water detaching TMDC flakes from the SiO2 surface and tears the layer along its weakest direction, the zigzag direction. The density of the nanoribbons produced can be controlled by adjusting the concentration and the reducing strength of reducing agents. Higher concentration of reducing agent can increase the percentage of TMDC flakes turned into nanoribbons. Stronger reducing agent not only increase the percentage of TMDC film turning into ribbons, but also reduce the width and density of the ribbons synthesized. Raman and HAADF STEM show an increased strain at the edge of each ribbon. An increase of bandgap has been observed for MoS2 nanoribbons down to 50 nm and PL intensity can be used to estimate nanoribbon width. This method provides a solution towards producing a large area of nanoribbons economically and efficiently.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Army Research Office MURI grant (W911NF-11-1-0362) and the Air Force Office of Scientific Research (AAFOSR-Grant No. Fa9550-14-1-0268). Microscopy research performed as part of a user proposal at Oak Ridge National Laboratory's Center for Nanophase Materials Sciences (CNMS), which is a U.S. Department of Energy, Office of Science User Facility (J. A. H. and J. C. I.). We acknowledge Dr Yongji Gong for providing TEM picture of triangular MoS2 flakes used in Fig. 3(a).Notes and references
- S. Z. Butler, S. M. Hollen, L. Cao, Y. Cui, J. A. Gupta, H. R. Gutiérrez, T. F. Heinz, S. S. Hong, J. Huang, A. F. Ismach, E. Johnston-Halperin, M. Kuno, V. V. Plashnitsa, R. D. Robinson, R. S. Ruoff, S. Salahuddin, J. Shan, L. Shi, M. G. Spencer, M. Terrones, W. Windl and J. E. Goldberger, ACS Nano, 2013, 7, 2898–2926 CrossRef CAS PubMed.
- M. Chhowalla, D. Jena and H. Zhang, Nat. Rev. Mater., 2016, 1, 16052 CrossRef CAS.
- J. Huang, Z. Wei, J. Liao, W. Ni, C. Wang and J. Ma, J. Energy Chem., 2018 DOI:10.1016/j.jechem.2018.09.001.
- Q. H. Wang, K. Kalantar-Zadeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- G. He, K. Ghosh, U. Singisetti, H. Ramamoorthy, R. Somphonsane, G. Bohra, M. Matsunaga, A. Higuchi, N. Aoki, S. Najmaei, Y. Gong, X. Zhang, R. Vajtai, P. M. Ajayan and J. P. Bird, Nano Lett., 2015, 15, 5052–5058 CrossRef CAS PubMed.
- S. Najmaei, S. Lei, R. A. Burke, B. M. Nichols, A. George, P. M. Ajayan, A. D. Franklin, J. Lou and M. Dubey, Sci. Rep., 2016, 6, 39465 CrossRef CAS PubMed.
- F. Liu, S. Zheng, X. He, A. Chaturvedi, J. He, W. L. Chow, T. R. Mion, X. Wang, J. Zhou, Q. Fu, H. J. Fan, B. K. Tay, L. Song, R.-H. He, C. Kloc, P. M. Ajayan and Z. Liu, Adv. Funct. Mater., 2016, 26, 1169–1177 CrossRef CAS.
- K. Keyshar, Y. Gong, G. Ye, G. Brunetto, W. Zhou, D. P. Cole, K. Hackenberg, Y. He, L. Machado, M. Kabbani, A. H. C. Hart, B. Li, D. S. Galvao, A. George, R. Vajtai, C. S. Tiwary and P. M. Ajayan, Adv. Mater., 2015, 27, 4640–4648 CrossRef CAS PubMed.
- X. Wang, Y. Gong, G. Shi, W. L. Chow, K. Keyshar, G. Ye, R. Vajtai, J. Lou, Z. Liu, E. Ringe, B. K. Tay and P. M. Ajayan, ACS Nano, 2014, 8, 5125–5131 CrossRef CAS PubMed.
- Z. Wang, V. Kochat, P. Pandey, S. Kashyap, S. Chattopadhyay, A. Samanta, S. Sarkar, P. Manimunda, X. Zhang, S. Asif, A. K. Singh, K. Chattopadhyay, C. S. Tiwary and P. M. Ajayan, Adv. Mater., 2017, 29, 1700364 CrossRef PubMed.
- J. Shen, Y. He, J. Wu, C. Gao, K. Keyshar, X. Zhang, Y. Yang, M. Ye, R. Vajtai, J. Lou and P. M. Ajayan, Nano Lett., 2015, 15, 5449–5454 CrossRef CAS PubMed.
- Y. Kim, J.-G. Song, Y. J. Park, G. H. Ryu, S. J. Lee, J. S. Kim, P. J. Jeon, C. W. Lee, W. J. Woo, T. Choi, H. Jung, H.-B.-R. Lee, J.-M. Myoung, S. Im, Z. Lee, J.-H. Ahn, J. Park and H. Kim, Sci. Rep., 2016, 6, 18754 CrossRef CAS PubMed.
- Z. Liu, L. Ma, G. Shi, W. Zhou, Y. Gong, S. Lei, X. Yang, J. Zhang, J. Yu, K. P. Hackenberg, A. Babakhani, J.-C. Idrobo, R. Vajtai, J. Lou and P. M. Ajayan, Nat. Nanotechnol., 2013, 8, 119–124 CrossRef CAS PubMed.
- J. Liang, Z. Wei, C. Wang and J. Ma, Electrochim. Acta, 2018, 285, 301–308 CrossRef CAS.
- Z. Liu and K. Aydin, Nano Lett., 2016, 16, 3457–3462 CrossRef CAS PubMed.
- J. Kim, W. S. Yun and J. D. Lee, J. Phys. Chem. C, 2015, 119, 13901–13906 CrossRef CAS.
- C.-H. Lee, J. Lin and C.-K. Yang, Sci. Rep., 2018, 8, 13307 CrossRef PubMed.
- Y.-N. Wen, M.-G. Xia and S.-L. Zhang, Phys. Lett. A, 2018, 382, 2354–2360 CrossRef CAS.
- L. Wang, H. Dong, Z. Guo, L. Zhang, T. Hou and Y. Li, J. Phys. Chem. C, 2016, 120, 17427–17434 CrossRef CAS.
- Z. Zhang, Y. Xie, Q. Peng and Y. Chen, Sci. Rep., 2016, 6, 21639 CrossRef CAS PubMed.
- A. N. Abbas, G. Liu, B. Liu, L. Zhang, H. Liu, D. Ohlberg, W. Wu and C. Zhou, ACS Nano, 2014, 8, 1538–1546 CrossRef CAS PubMed.
- S. Yang, D. Li, T. Zhang, Z. Tao and J. Chen, J. Phys. Chem. C, 2012, 116, 1307–1312 CrossRef CAS.
- Y. Pak, N. Lim, Y. Kumaresan, R. Lee, K. Kim, T. H. Kim, S.-M. Kim, J. T. Kim, H. Lee, M.-H. Ham and G.-Y. Jung, Adv. Mater., 2015, 27, 6945–6952 CrossRef CAS PubMed.
- Y. Li, Z. Zhou, S. Zhang and Z. Chen, J. Am. Chem. Soc., 2008, 130, 16739–16744 CrossRef CAS PubMed.
- F. López-Urías, A. L. Elías, N. Perea-López, H. R. Gutiérrez, M. Terrones and H. Terrones, 2D Mater., 2015, 2, 015002 CrossRef.
- M. H. Heyne, J. F. De Marneffe, A. Delabie, M. Caymax, E. C. Neyts, I. Radu, C. Huyghebaert and S. De Gendt, Nanotechnology, 2017, 28, 04LT01 CrossRef PubMed.
- Z. Wang, H. Li, Z. Liu, Z. Shi, J. Lu, K. Suenaga, S. K. Joung, T. Okazaki, Z. Gu, J. Zhou, Z. Gao, G. Li, S. Sanvito, E. Wang and S. Iijima, J. Am. Chem. Soc., 2010, 132, 13840–13847 CrossRef CAS PubMed.
- Y. Pak, Y. Kim, N. Lim, J.-W. Min, W. Park, W. Kim, Y. Jeong, H. Kim, K. Kim, S. Mitra, B. Xin, T.-W. Kim, I. S. Roqan, B. Cho and G.-Y. Jung, APL Mater., 2018, 6, 076102 CrossRef.
- L. Vieira, J. d. R. Martins Neto, O. P. Ferreira, R. M. Torresi, S. I. Cordoba de Torresi and O. L. Alves, RSC Adv., 2018, 8, 30346–30353 RSC.
- S. Lei, X. Wang, B. Li, J. Kang, Y. He, A. George, L. Ge, Y. Gong, P. Dong, Z. Jin, G. Brunetto, W. Chen, Z.-T. Lin, R. Baines, D. S. Galvão, J. Lou, E. Barrera, K. Banerjee, R. Vajtai and P. Ajayan, Nat. Nanotechnol., 2016, 11, 465 CrossRef CAS PubMed.
- P. K. Chow, E. Singh, B. C. Viana, J. Gao, J. Luo, J. Li, Z. Lin, A. L. Elias, Y. Shi, Z. Wang, M. Terrones and N. Koratkar, ACS Nano, 2015, 9(3), 3023–3031 CrossRef CAS PubMed.
- B. Mondal, A. Som, I. Chakraborty, A. Baksi, D. Sarkar and T. Pradeep, Nanoscale, 2016, 8, 10282–10290 RSC.
- H. Nan, Z. Wang, W. Wang, Z. Liang, Y. Lu, Q. Chen, D. He, P. Tan, F. Miao, X. Wang, J. Wang and Z. Ni, ACS Nano, 2014, 8, 5738–5745 CrossRef CAS PubMed.
- J. Li, N. V. Medhekar and V. B. Shenoy, J. Phys. Chem. C, 2013, 117, 15842–15848 CrossRef CAS.
- S. Deng, E. Gao, Z. Xu and V. Berry, ACS Appl. Mater. Interfaces, 2017, 9, 7812–7818 CrossRef CAS PubMed.
- I. Bilgin, F. Liu, A. Vargas, A. Winchester, M. K. L. Man, M. Upmanyu, K. M. Dani, G. Gupta, S. Talapatra, A. D. Mohite and S. Kar, ACS Nano, 2015, 9, 8822–8832 CrossRef CAS PubMed.
- Z. Mutlu, M. Ozkan and C. S. Ozkan, Mater. Chem. Phys., 2016, 176, 52–57 CrossRef CAS.
- C. Rice, R. J. Young, R. Zan, U. Bangert, D. Wolverson, T. Georgiou, R. Jalil and K. S. Novoselov, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 081307 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8nh00364e |
| This journal is © The Royal Society of Chemistry 2019 |