Realizing the extraction of carbon from WC for in situ formation of W/WC heterostructures with efficient photoelectrochemical hydrogen evolution†
Zongkui
Kou
 ,
Tingting
Wang
,
Zonghua
Pu
,
Lin
Wu
,
Kai
Xi
and
Shichun
Mu
,
Tingting
Wang
,
Zonghua
Pu
,
Lin
Wu
,
Kai
Xi
and
Shichun
Mu
 *
*
State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, Wuhan University of Technology, Wuhan 430070, China. E-mail: msc@whut.edu.cn
First published on 2nd October 2018
Abstract
Although extracting carbon atoms from carbides, as the reverse route to carbide derived carbon (CDC), may have more potentials for constructing advanced nanostructures, it has not been realized yet. As a proof of concept, in this work we realize the extraction of carbon atoms from carbide lattices by rationally controlling the reaction between carbides and Cl2. Thus, a homologous metallic W layer adhered on a WC (W/WC) heterostructure is created. Based on experimental results, such a W/WC heterostructure can be used as an efficient catalyst for the photoelectrocatalytic hydrogen evolution reaction (HER), where the photocurrent density at 0 V can reach up to 16 mA cm−2. Our theoretical calculations disclose that the Mott–Schottky effect accelerates electron flow across the interfaces and significantly decreases the work function of the W facet, which leads to excellent photoelectrocatalytic HER activity on the W facets. The presented results have broad implications since they demonstrate the generic capability to build homologous M/TMC heterostructures.
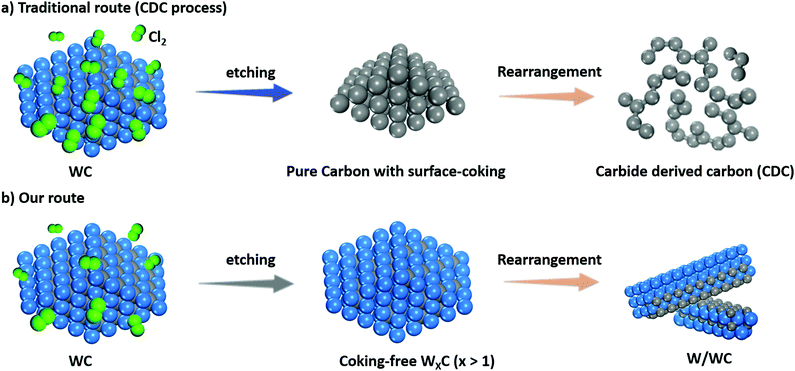
Conceptual insightsContrary to the traditional route to produce carbide-derived carbon (CDC) by extracting metal atoms from carbides via Cl2 etching, in this study, a method for partially removing the carbon atoms of WC was designed and facilely realized to produce W/WC heterostructures by regulating the chlorine-to-carbide ratio (χ). Surprisingly, the extraction of carbon atoms in the WC is thermodynamically favourable when χ decreases to an ultralow value of 1.3, which has received far less attention because the chlorine must be in excess in the synthesis of CDC (generally χ > 5). Such a W/WC heterostructure is also applied for the first time as a photoelectrocatalyst for hydrogen generation. Benefiting from the Mott–Schottky effect at the interfaces and the decreasing work function of the W facets, the W/WC heterostructure demonstrates an excellent activity and stability towards photoelectrocatalytic hydrogen evolution. |
Introduction
The heterointerface plays a predominant role in cross-cutting applications, from heterogeneous catalysis to microelectronic components and medical implants.1,2 The distinct nature of the phases in contact facilitates the transport of electrons across the interface, which can largely reduce the work function of the surface phases.3,4 The most extensively investigated heterointerfaces are those with Pt as the host metal and 3d transition metals as admetals.5–7 Such a heterointerface has a weak adsorbate binding energy and is active for hydrogenation.8 The scarcity of Pt-group metals and the poor stability of the heterointerfaces under various reaction conditions are two key challenges.Metal covered transition metal carbides (M/TMCs) as a substitute for Pt-group metals represent a new class of heterointerfacial materials that are both conductive and stable in a harsh environment.9 In the past few decades, density functional theory (DFT) calculations in model systems have been used to make a great number of achievements to disclose the atomic and electronic interface structures of the M/TMC heterostructures.10,11 Because layered phases are unrestricted by lattice matching, the M/TMC heterostructures can be obtained by artificially stacking/depositing individual layered phases on top of each other in a given sequence via van der Waals interactions.12–15 Unfortunately, harsh synthetic conditions (e.g. ultra-high vacuum and temperature as well as long synthesis periods) are necessary to overcome the thermodynamic and kinetic barriers when metal phases are deposited on the surfaces of TMCs.
The extraction of non-carbon atoms by using the chemical reaction of carbides with Cl2 can obtain an advanced class of carbon nanomaterials, which are defined as carbide derived carbons (CDCs).16 For example, partial extraction of metal atoms from TMCs with Cl2 on account of insufficient chlorination can produce homologous metal-doped graphene17 wherein the chlorine-to-carbide molar ratio (χ) could play a key role on the microstructure. Conversely, due to a large reactive barrier, the extraction of carbon atoms from carbides represents a formidable challenge. Herein, for the first time we find that a very low χ enables the partial chemical extraction of carbon atoms from WC lattices via Cl2 etching. As a result, we avoid the complex interfacial interactions between the contacting materials and realize the first facile fabrication of W/WC heterostructures via rationally controlling χ. DFT calculations, along with electrochemical tests, are performed to disclose the huge advantage of W/WC heterostructures for photoelectrocatalytic hydrogen production compared to WC alone.
Results and discussion
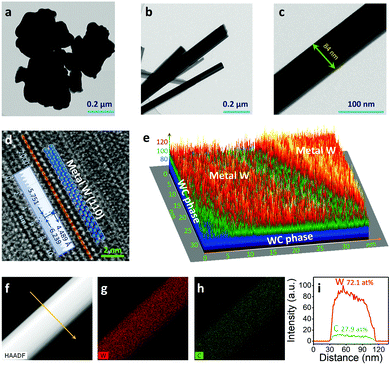
Previous theoretical predictions18 have suggested that there is an optimum value for the chlorine-to-carbide molar ratio (χ), corresponding to a large temperature range over which the extraction of metal atoms from carbides with chlorine gas can occur. Hitherto, extracting non-carbon atoms (e.g. W) with Cl2 to yield traditional carbide derived carbon (CDC, Scheme 1a) must be performed at a relatively high chlorine-to-carbide molar ratio (χ, see collected data the Table S1, ESI†).16,19,20 However, in the case of an extremely low χ (e.g. χ = 1.3), Cl2 might extract C atoms from the WC to produce a homologous W/WC heterostructure (Scheme 1b) due to a favorable thermodynamic tendency. Such a markedly different reaction process can be directly identified by the color of two volatile byproducts (WCl4 or CCl4, Fig. S2, ESI†). Clearly differently from the pristine WC with an aggregated nanoparticle morphology and average size >100 nm (Fig. 1a and Fig. S2, ESI†), the produced W/WC heterostructures, with plenty of dark contrast nanoribbons, can be readily distinguished by TEM (Fig. 1b). The width of the representative nanoribbons is about 84 nm (Fig. 1c).For the surface of such nanoribbons, an interatomic distance of ∼2.245 Å is assigned to the (110) facets of W metal,21 indicating the presence of a pure W layer on the WC (Fig. 1d). The simulated intensity profile along the 3D thickness direction exposes the enriched surface metallic W phase and the WC phase inside the nanoribbons (Fig. 1e and Fig. S3, ESI†). High-angle annular dark field scanning TEM (HAADF-STEM) imaging further reveals the full spatial dispersion of the W species along a single nanoribbon (Fig. 1f and g). Its high spatial correlation with the weak C signal (Fig. 1h) also supports the uniform distribution of W coverage on the WC. The atomic percentages of W and C in the composite are 72.1 at% and 27.9 at%, respectively (Fig. 1i), suggesting a dominant W phase on the nanoribbons.
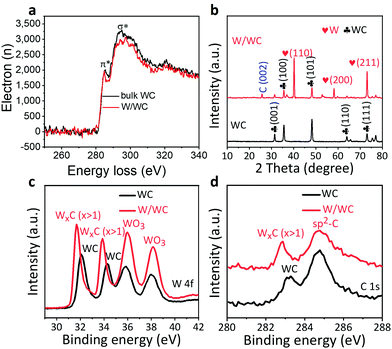
Electron energy loss spectroscopy (EELS) of the W/WC heterostructures (Fig. 2a) shows that the surface sp2-carbons have an energy downshift of ∼0.74 eV compared to the WC alone, indicating a changing orbital hybridization of the carbon bonds. This could originate from chemical bonding at the interfaces, which can promote the electron transfer across the interfaces.9 From X-ray diffraction (XRD) patterns, except for a weaker signal at 25.7° from thin carbon layers due to surface segregation of C atoms at high temperatures (which has been demonstrated by blank experiment as shown in Fig. S4, ESI†), two sets of the diffraction peaks (Fig. 2b) are separately assignable to the hexagonal WC and the pure metallic W, with no sign of other species.22 High resolution X-ray photoelectron spectroscopy (XPS) analyses of the WC precursors and produced W/WC heterostructures were performed to identify and compare their chemical states on the surface. For the W/WC heterostructure, the positions of the W 4f XPS signals shift to 31.7 eV and 33.9 eV (Fig. 2c), which are assigned to the W phase.23 This supplies another proof for the W covering on the WC. The C 1s spectrum is shown in Fig. 2d. The peak at 284.7 eV implies the possible presence of sp2 carbon, in agreement with the result from the PXRD pattern.
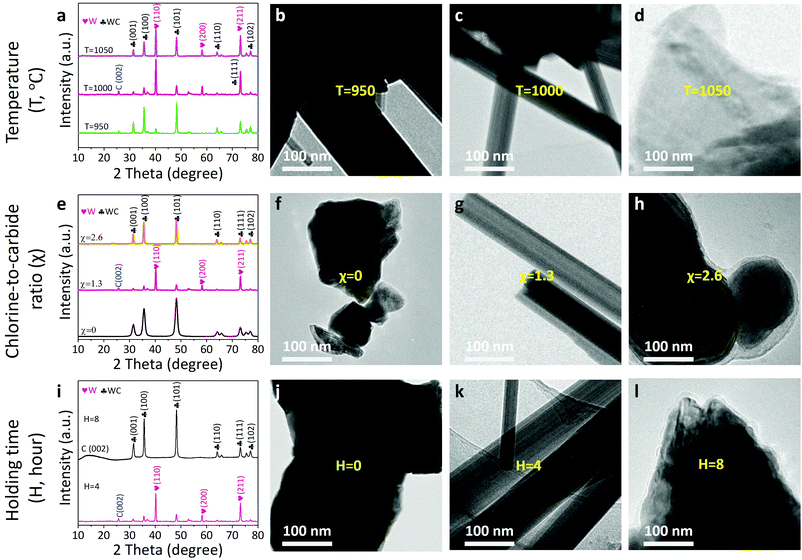
It is worth noticing that the reaction thermodynamics are crucial to control the microstructure of the products.16 First, we investigated the effect of reaction temperature on the microstructure of the resultants (Fig. 3a–d). When the temperature increases from 950 to 1000 to 1050 °C, the W phase appears and propagates, but subsequently decreases in percentage based on X-ray diffraction results (Fig. 3a and Fig. S5, ESI†). As a result, a composite consisting of the pristine WC and W/WC (WC–W/WC) forms at a relatively low temperature (950 °C; Fig. 3b and Fig. S6, ESI†); at a relatively high temperature (1050 °C), it can lead to the destruction of the W/WC heterostructures (Fig. 3d and Fig. S7, ESI†). Second, the role of the chlorine-to-carbide molar ratio (χ) on the formation of the W/WC heterostructure is also clarified (Fig. 3e–h). When the temperature is maintained at 1000 °C and χ = 0, it results in sintered WC (Fig. 3f and Fig. S8, ESI†) terminated by seven graphene layers (Fig. S4, ESI†) owing to the surface segregation of the C atoms. Significantly, increasing χ to 1.3 can produce the predominant W phase on the surface of the WC (Fig. 3g). On the other hand, in the case of excessive χ (2.6), the extraction of W atoms preferentially occurs and thus produces WC coated by in situ amorphous carbon layers (WC@CDC) as presented in Fig. 3h and Fig. S9, ESI.† This is consistent with the results reported by Jänes et al.20 Moreover, when holding time is extended from 0 to 4, and then to 8 h (Fig. 3i–l), WC coated with nine layers of graphene (WC@NLG) can be obtained (Fig. 3l and Fig. S10, ESI†). Therefore, the optimal conditions to afford W/WC heterostructures are found to be χ = 1.3 and a 1000 °C heat-treatment for 4 h (the relationship between the experimental configurations and the product phases is summarized in Table S2, ESI†).
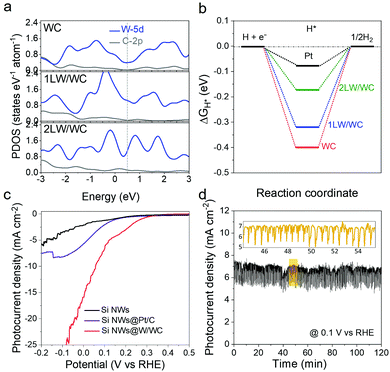
We first conducted the density functional theory (DFT) calculations to investigate the possibility of the more competitive W/WC heterostructure over popular WC toward the photoelectrocatalytic hydrogen evolution reaction (HER). The DFT lattice parameters and models are seen in Table S3 and Fig. S11, ESI.† Compared to WC, the WC (0001) surface covered by monolayered or bilayered metallic W (named as 1LW/WC and 2LW/WC, respectively) shows a gradually increasing electronic projected density of states (PDOS) near the Fermi level (Fig. 4a), indicating an enhanced carrier density and d-band center (Fig. S12, ESI†) with increasing W cover layers. These results suggest that the adsorbate could be weakly bonded to the catalyst surface.24 In addition, ΔGH has been widely adopted as a brief descriptor in the choice of appropriate HER catalysts.25 A good catalyst should follow the Sabatier principle with ΔGH ≈ 0 eV, exactly as it is for platinum.26 As shown in Fig. 4b, the WC presents a negative ΔGH of −0.4 eV above the reported result27 due to sufficient hydrogen coverage. Nevertheless, the 1LW/WC and 2LW/WC have less negative ΔGH values of −0.32 and −0.17 eV than that of the WC, suggesting a remarkable enhancement beyond WC. As a benchmark, ΔGH of a Pt reference (−0.07 eV) is still closest to 0.
We integrated the W/WC heterostructures with p-type Si nanowires and explored their performance for photoelectrochemical (PEC) hydrogen evolution. P-type Si nanowires with an average length of ∼3 μm were prepared from the Ag-assisted etching of a Si wafer.21 The W/WC heterostructures were directly spin-coated onto these nanowires. Fig. S13a, ESI† shows a vertically-viewed SEM image of the photocathode. These Si nanowires have adequate open space among them, and can be better coupled with the W/WC heterostructures. The side view reveals that the W/WC heterostructures blend into the vertically aligned Si nanowires (Fig. S13b, ESI†), indicating direct physical contact between the W/WC heterostructures and the p-Si NWs.
The p-Si NWs@W/WC photocathodes were tested for PEC hydrogen evolution in strongly acidic 0.5 M H2SO4 solutions under simulated 1 sun AM 1.5G illumination. Fig. 4c displays the current–potential curves with or without catalysts under illumination. After Si NWs were integrated with the W/WC heterostructures, the onset of the photocurrent is significantly enhanced to about 0.3 V vs. RHE due to the Mott–Schottky effect.9 Importantly, the photocurrent density at 0 V vs. RHE can reach up to 16 mA cm−2, which exceeds that of commercial 20 wt% Pt/C catalysts and most non-noble metal-based PEC HER catalysts (Table S4, ESI†). Moreover, the excellent operation durability of the Si NWs@W/WC is presented by a current–time response experiment at 0.1 V vs. RHE for 120 min (Fig. 4d). TEM, X-ray diffraction and XPS analyses of the W/WC heterostructures before and after HER durability tests reveal that the microstructure and composition are retained (Fig. S14, ESI†). All these results corroborate the great HER activity and durability of our W/WC heterostructures. The physical integration of the Si nanowires with the W/WC heterostructures also represents a high level for the first application as a HER catalyst in PEC water splitting.
Electrocatalytic HER polarization curves of different electrocatalysts were also collected (Fig. S15, ESI†). As expected, the W/WC heterostructures obtained at 1000 °C, χ = 1.3 and 4 h exhibit an impressive HER activity with a small onset overpotential of ∼31 mV, and a geometric current density of 10 mA cm−2 at η = 159 mV vs. RHE, which exceeds that of the control samples, especially the WC@CDC obtained at 1000 °C, χ = 2.6 and 4 h, suggesting that the W covered surface of the WC is relatively more active than that of the CDC. For the W/WC heterostructures, the two polarization curves before and after i–t chronoamperometric response for 10 hours nearly overlap in a wide current region (Fig. S16, ESI†), indicating excellent durability in an acidic environment.
In view of the aforementioned considerations, the amazing HER activities of the W/WC heterostructures are speculated to originate from the following: (1) the nanoribbon microstructure and the active metal covers of the W/WC heterostructures favor the exposure of an abundance of available active sites (Fig. S17, ESI†), enhancing the catalytic activity for the HER;28,29 (2) the robust in situ conjugation between the homogeneous WC and W provides a resistance-less path (Fig. S18, ESI†), favorable for fast electron transfer24 and increasing reaction rate; (3) in the W/WC heterostructure, electrons flow through the metal/TMC interface due to the Mott–Schottky effect so that the work function equilibrium is reached.30 The work function of the W facet is thus lowered with obviously improved PEC HER activity, which largely depends on the electron donating ability of an electrocatalyst to electrolyte molecules (Fig. S12, ESI†).
Conclusions
In summary, built on our improved understanding of the thermodynamic configurations associated with the chlorination technique and the great potential of W/WC heterostructures predicted by theoretical calculations, we first take this strategy to fabricate W/WC heterostructures by chemical removal of carbon atoms from common WC with chlorine. The regulation of experimental configurations provides the opportunity to preferentially extract carbon atoms and thus leads to the favorable formation of W/WC heterostructures over the traditional carbide derived carbon (CDC). As a result, the W/WC heterostructures could be physically integrated with p-type Si nanowires to enable solar-driven hydrogen production and achieve highly competitive performance wherein the photocurrent density at 0 V can reach up to 16 mA cm−2 with a long-term cycling stability. In particular, it also exhibits amazing electrocatalytic HER performance, endowing a small onset overpotential of ∼31 mV, and a cathodic current density of 10 mA cm−2 at η = 159 mV. This novel design concept could pave a way to fabricate a class of advanced homologous metal/metal carbide heterostructures towards a range of other electrocatalytic or photoelectrocatalytic reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge support from the National Natural Science Foundation of China (NSFC) through awarding No. 51672204 and No. 51372186 on catalysis studies. We express heartfelt thanks to Prof. Gaoke Zhang for the supply of computational resources in the School of Resources and Environmental Engineering, Wuhan University of Technology. We wish to thank Associate Prof. Xiaoqing Liu, Prof. Xiaoxing Ke and Dr Zhao Deng for TEM analytical measurements performed at JEM-2100F in the Materials Analysis Center of Wuhan University of Technology.Notes and references
- Z. Zhang, B. Xu and X. Wang, Chem. Soc. Rev., 2014, 43, 7870 RSC.
- M. Christensen, S. Dudiy and G. Wahnström, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 045408 CrossRef.
- M. W. Finnis, J. Phys.: Condens. Matter, 1996, 8, 5811 CrossRef CAS.
- H. Fashandi, M. Dahlqvist, J. Lu, J. Palisaitis, S. I. Simak, I. A. Abrikosov, J. Rosen, L. Hultman, M. Andersson, A. L. Spetz and P. Eklund, Nat. Mater., 2017, 16, 814 CrossRef CAS PubMed.
- L. Bu, N. Zhang, S. Guo, X. Zhang, J. Li, J. Yao, T. Wu, G. Lu, J. Ma, D. Su and X. Huang, Science, 2016, 354, 1410 CrossRef CAS PubMed.
- K. D. Gilroy, A. Ruditskiy, H. C. Peng, D. Qin and Y. Xia, Chem. Rev., 2016, 116, 10414 CrossRef CAS PubMed.
- L. Zhang, Z. Xie and J. Gong, Chem. Soc. Rev., 2016, 45, 3916 RSC.
- J. G. Chen, C. A. Menning and M. B. Zellner, Surf. Sci. Rep., 2008, 63, 201 CrossRef CAS.
- S. Yao, X. Zhang, W. Zhou, R. Gao, W. Xu, Y. Ye, L. Lin, X. Wen, P. Liu, B. Chen, E. Crumlin, J. Guo, Z. Zuo, W. Li, J. Xie, L. Lu, C. J. Kiely, L. Gu, C. Shi, J. A. Rodriguez and D. Ma, Science, 2017, 357, 389 CrossRef CAS PubMed.
- R. Benedek, A. Alavi, D. N. Seidman, L. H. Yang, D. A. Muller and C. Woodward, Phys. Rev. Lett., 2000, 84, 3362 CrossRef CAS PubMed.
- C. K. Poh, S. H. Lim, J. Lin and Y. P. Feng, J. Phys. Chem. C, 2014, 118, 13525 CrossRef CAS.
- T. G. Kellya and J. G. Chen, Chem. Soc. Rev., 2012, 41, 8021 RSC.
- P. Rivera, K. L. Seyler, H. Yu, J. R. Schaibley, J. Yan, D. G. Mandrus, W. Yao and X. Xu, Science, 2016, 351, 688 CrossRef CAS PubMed.
- Z. S. Wu, Y. Zheng, S. Zheng, S. Wang, C. Sun, K. Parvez, T. Ikeda, X. Bao, K. Müllen and X. Feng, Adv. Mater., 2017, 29, 1602960 CrossRef PubMed.
- C. Bacaksiz, A. Dominguez, A. Rubio, R. T. Senger and H. Sahin, Phys. Rev. B, 2017, 95, 075423 CrossRef.
- V. Presser, M. Heon and Y. Gogotsi, Adv. Funct. Mater., 2011, 21, 810 CrossRef CAS.
- Z. Kou, T. Meng, B. Guo, I. S. Amiinu, W. Li, J. Zhang and S. Mu, Adv. Funct. Mater., 2017, 27, 201604904 CrossRef.
- R. K. Dash, G. Yushin and Y. Gogotsi, Microporous Mesoporous Mater., 2005, 86, 50 CrossRef CAS.
- Z. Cambaz, G. Yushin, Y. Gogotsi, K. Vyshnyakova and L. Pereselentseva, J. Am. Ceram. Soc., 2006, 89, 509 CrossRef CAS.
- I. Tallo, T. Thomberg, K. Kontturi, A. Jänes and E. Lust, Carbon, 2011, 49, 4427 CrossRef CAS.
- S. Hunt, T. Nimmanwudipong and Y. Román-Leshkov, Angew. Chem., Int. Ed., 2014, 53, 5131 ( Angew. Chem. , 2014 , 126 , 5231 ) CAS.
- Q. Gong, Y. Wang, Q. Hu, J. Zhou, R. Feng, P. N. Duchesne, P. Zhang, F. Chen, N. Han, Y. Li, C. Jin, Y. Li, S. T. Lee and C. Jin, Nat. Commun., 2016, 7, 13216 CrossRef CAS PubMed.
- A. Katrib, F. Hemming, P. Wehrer, L. Hilaire and G. Maire, J. Electron Spectrosc. Relat. Phenom., 1995, 76, 195 CrossRef CAS.
- J. Li, Y. Wang, C. Liu, S. Li, Y. Wang, L. Dong, Z. Dai, Y. Li and Y. Lan, Nat. Commun., 2016, 7, 11204 CrossRef CAS PubMed.
- J. Nørskov, T. Bligaard, J. Rossmeisl and C. Christensen, Nat. Chem., 2009, 1, 37 CrossRef PubMed.
- J. Greeley, T. Jaramillo, J. Bonde, I. Chorkendorff and J. Nørskov, Nat. Mater., 2006, 5, 909 CrossRef CAS PubMed.
- J. Kitchin, J. Nørskov, M. Barteau and J. Chen, Catal. Today, 2005, 105, 66 CrossRef CAS.
- J. Lin, Z. Peng, G. Wang, D. Zakhidov, E. Larios, M. Yacaman and J. Tour, Adv. Energy Mater., 2014, 4, 1066 Search PubMed.
- F. Wang, J. Li, F. Wang, T. Shifa, Z. Cheng, Z. Wang, K. Xu, X. Zhan, Q. Wang, Y. Huang, C. Jiang and J. He, Adv. Funct. Mater., 2015, 25, 6077 CrossRef CAS.
- L. Guo, Y. Cai, J. Ge, Y. Zhang, L. Gong, X. Li, K. Wang, Q. Ren, J. Su and J. Chen, ACS Catal., 2014, 5, 388 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: S1: experimental methods; S2: digital photos of by-products; S3: morphology and structure of the WC; S4: morphology and surface information of the W/WC heterostructure; S5: morphology and structure of the sintering WC; S6: the influence of thermodynamic configurations on the composition and microstructure of the final products; S7: geometric structure and d-band center of the as-studied systems; S8: the morphology of the W/WC heterostructure integrated on the p-type Si nanowires; S9: the morphology, composition and surface information of the W/WC heterostructure before and after the HER experiment; S10: the influence of thermodynamic configurations on the HER activities of the final products; S11: the mechanism of enhancing the HER activity; S12: Tables S1–S4; S13: ref. S1–S7. See DOI: 10.1039/c8nh00275d |
| This journal is © The Royal Society of Chemistry 2019 |