Recent advances in functionalized MnO2 nanosheets for biosensing and biomedicine applications
Juan
Chen
a,
Hongmin
Meng
a,
Yuan
Tian
a,
Ran
Yang
a,
Dan
Du
b,
Zhaohui
Li
 *a,
Lingbo
Qu
*a and
Yuehe
Lin
*a,
Lingbo
Qu
*a and
Yuehe
Lin
 *b
*b
aHenan Joint International Research Laboratory of Green Construction of Functional Molecules and Their Bioanalytical Applications, College of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, P. R. China. E-mail: zhaohui.li@zzu.edu.cn; qulingbo@zzu.edu.cn
bSchool of Mechanical and Materials Engineering, Washington State University, Pullman, WA 99164, USA. E-mail: yuehe.lin@wsu.edu
First published on 15th November 2018
Abstract
As one kind of redox active layered transition-metal dioxide nanomaterials, single-layer manganese dioxide (MnO2) nanosheets have gained significant research attention in the fields of biosensing and biomedicine because of their large surface area, intense and broad optical absorption, strong oxidation ability, catalytic activity, and robust mechanical properties. This review provides a brief overview of the recent advances in the development of MnO2 nanosheet-based biosensors, bioimaging as well as drug delivery for cancer therapy. The methodologies for the preparation of MnO2 nanosheets are summarized, followed by an introduction of the nanostructure and properties of MnO2 nanosheets. Special attention is paid to their applications in biosensing, bioimaging and cancer therapy. Future perspectives and the challenges of high-performance MnO2 nanosheets are also discussed.
Introduction
There are increasing demands for nanobiotechnology in various applications, such as biosensing, bioanalysis, bioimaging, and biomedicine, which have substantially promoted the development of a variety of nanosystems. One of the key issues in biomaterial development at the nanoscale is to the exploration of advanced functional nanomaterials with high performance. As a newly emerging class of nanomaterials, two-dimensional (2D) nanosheets, especially a wide variety of transition-metal oxide nanosheets with planar topography such as manganese dioxide (MnO2), molybdenum disulfide (MOS2) and cobaltosic oxide (Co3O4), exhibit unique properties including high specific surface area (SSA) and robust physicochemical properties. Furthermore, they are degradable and easy to modify, which makes them very promising nanoplatforms for biomedical applications.1–4MnO2 nanosheets are redox active 2D nanomaterials with thickness on the nanometer scale or even smaller, while the lateral size ranges from sub-micrometers to micrometers.5,6 The MnO2 nanosheets have three atomic layers, i.e., one Mn layer sandwiched by two O layers.7 Each Mn coordinates to six O atoms to form edge-sharing MnO6 octahedra. The existence of Mn-vacancies makes MnO2 nanosheets negatively charged and repulsive to each other.8 Moreover, the d–d transitions of Mn ions in the MnO6 octahedra of MnO2 nanosheets result in a broad absorption spectrum (∼200–600 nm) with a large molar extinction coefficient (εmax = 9.6 × 103 M−1 cm−1) at 380 nm.6
The properties of nanomaterials are largely affected by their structures. The special features MnO2 nanomaterials and the corresponding applications are summarized as follows: (1) due to the edge-shared octahedral crystal structure and the large surface area, the layered MnO2 possesses good absorption and degradation ability toward different kinds of organic pollutants, dyes and heavy metal ions, which makes it an environmental-friendly material.9–11 (2) The broad absorption spectrum of MnO2 nanosheets overlaps with the fluorescence excitation and/or emission spectra of most kinds of organic dyes, quantum dots, fluorescence nanoparticles and metal nanoclusters, which endows MnO2 nanosheets with strong fluorescence quenching ability.12–15 Based on this property, Förster resonance energy transfer (FRET) and the inner filter effect (IFE) can occur between MnO2 nanosheets and the organic dyes/fluorescence nanomaterials.8 (3) MnO2 platelets can adsorb single-stranded DNA (ssDNA) through van der Waals forces between nucleobases and the basal plane of MnO2 platelets, while releasing double-stranded DNA (dsDNA) because of their negative charge, so it has often been used as a new class of biosensing platform for probing DNA hybridization and aptamer–target interactions in a homogeneous solution.16 (4) The valence state of Mn4+ in MnO2 is the intermediate valence, so the MnO2 nanosheets themselves have strong oxidation ability and catalytic activity, which can be rapidly degraded by some redox reactions with reducing substances. In the presence of a reducing substance (taking GSH as an example), MnO2 can be reduced to Mn2+, leading to the decomposition of the MnO2 nanosheets, as shown in eqn (1).
| 2GSH + MnO2 + 2H+ → GSSG + Mn2+ + 2H2O | (1) |
Due to the unique properties of MnO2 nanosheets, they have been widely used to construct nanosystems for biomedical applications including biosensing,21–24 drug delivery,25 bioimaging,26 and cancer therapy.27 (Fig. 1). A review article on this topic is timely, as a large number of studies are being reported on both chemical and biological sensors using MnO2 nanosheets and their growing commercial attention.28 Besides, varied methods have achieved great success in the preparation of MnO2-based materials with different nanostructures. However, a comprehensive summary of techniques developed for synthesizing MnO2 nanosheets has not yet been reported. In this feature article, we shed light on the recent developments in synthesizing MnO2 nanosheets and their applications in biosensing, bioimaging, and drug delivery. The remaining challenges and future prospects of MnO2 nanosheets will also be highlighted and discussed.
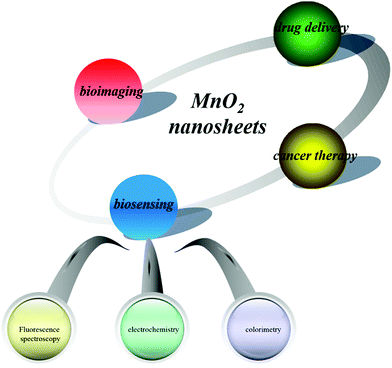 | ||
| Fig. 1 Schematic illustration of MnO2 nanosheets for applications in biosensing, bioimaging, drug delivery and cancer therapy. | ||
Synthesis of MnO2 nanosheets
There are two major approaches to preparing MnO2 nanosheets: the top-down approach and the bottom-up approach. As shown in Fig. 2A, the top-down approach for the synthesis of MnO2 nanosheets requires a multistep process: (1) a high-temperature solid-state reaction (to yield a thermodynamically stable precursor phase, e.g., K/MnO2); (2) protonation of interlayer alkali metal ions (to yield, e.g., H/MnO2), and (3) an acid–base reaction with an aqueous solution of quaternary ammonium cations (to finally yield negatively charged nanosheets, e.g., MnO2 in the form of a colloidal suspension).5,29 This approach is costly and time-consuming. Moreover, it is fairly difficult to exfoliate the protonated compounds into single-layer nanosheets, resulting in the wide thickness distribution of the final products. Herein, we mainly discuss the bottom-up approach.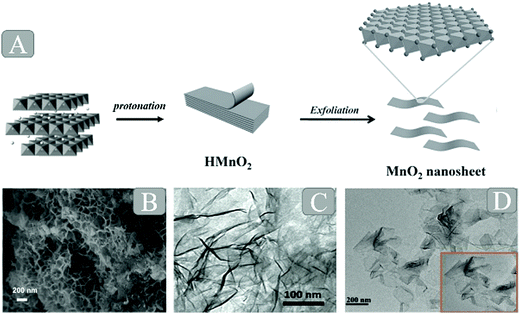 | ||
| Fig. 2 (A) Schematic illustration of the synthetic procedure for 2D MnO2 nanosheets. Reprinted with permission from ref. 99. Copyright (2014) Wiley-VCH. (B) SEM images of the MnO2 nanosheets based on the hard template method. Adapted with permission from ref. 24. Copyright (2018) Elsevier. (C) TEM image of MnO2 nanosheets based on the soft template method. Reprinted with permission from ref. 36. Copyright (2015) Wiley-VCH. (D) HRTEM images of MnO2 nanosheets based on the free template method. Adapted with permission from ref. 96. Copyright (2017) American Chemical Society. | ||
In 2007, Oaki and Imai30 reported for the first time the one-pot bottom-up synthesis of MnO2 nanosheets in aqueous solution without the requirement of high-temperature hydrothermal treatment and special equipment. The authors employed ethylenediaminetetraacetate (EDTA) as the chelating agent for Mn2+ ions so as to inhibit the rapid precipitation of Mn(OH)2. However, the obtained materials were in the multilayer structure rather than a monosheet, and the whole process required 3–5 days. On this basis, Kawamata et al.6 successfully demonstrated the first single-step approach to access the MnO2 nanosheets directly within one day. So far, this simple single-step approach has attracted tremendous attention, and a number of broad new studies on further developing MnO2 nanocomposites based on this approach have emerged. Moreover, this high-yield simple route to synthesizing the MnO2 monosheets within a day has been developed to construct a unique structure (Fig. 2B–D) by different improved methods such as the hard template method, soft template method and template-free method.
Hard template method
Hard templates are those materials that are used as scaffolds for the nanomaterial deposition or employed as the nucleus coated by the desired nanomaterials. The main hard template approach uses a simple hydrothermal method for deposition without any surfactants. For the deposition of MnO2 nanomaterials, KMnO4 was used in the hydrothermal reaction as the Mn precursor. After several hours of hydrothermal reaction, MnO2 nanosheets were formed on the surface of the hard templates, including graphene oxide (GO),31 montmorillonite K10,24 silica nanoparticles (SiO2 NPs),32 ferroferric oxide nanoparticles (Fe3O4 NPs),33 and so on.For example, Feng et al.31 proposed a hydrothermal method to prepare MnO2/graphene nanocomposites. As we know, on the surface and edge of GO, there are a lot of functional groups, e.g., ketone, quinine, carboxylic, hydroxyl, etc., which make GO negatively charged. Thus, upon the addition of MnSO4 to the GO suspension, Mn2+ can be attached to the graphene surfaces through the electrostatic effect. After the addition of the oxidant KMnO4, lots of MnO2 nuclei were immediately formed on the surface of GO due to the redox reaction between the Mn2+ and MnO4−. Then, with the process of hydrothermal reaction at 140 °C for 2 h, GO was reduced and MnO2 nanosheets were successfully prepared. The different morphologies of the final composites were controlled by the amount of Mn2+ source.
Using a similar strategy, MnO2 nanosheets on montmorillonite (MnO2 nanosheets@MMt) were synthesized by a one-pot method.24 Montmorillonite K 10 was added to a KMnO4 solution without any surfactants and then subjected to stirring and ultrasonic treatment at room temperature. The mixture was added to a Teflon-lined stainless steel autoclave and maintained at 160 °C for 24 h. After that, the honeycomb-like MnO2@MMt was obtained. Compared with the montmorillonite, the MnO2 nanosheets@MMt showed high adsorption capacity of 363.63 mg g−1 for MB dye and can improve adsorption performance and redox reactivity for organic pollutants. The morphology of MnO2 nanostructures can be adjusted by changing the reaction time, annealing process or the amount of reducing agents.
In summary, hard template methods can be used for the controlled synthesis of ordered MnO2 nanostructures through the hydrothermal reaction. Moreover, because no surface active agents are involved, the interference of other substances during the formation of MnO2 is effectively reduced. However, these hard template methods often require high temperature, high pressure, and long reaction time. Therefore, the development of low-cost and simple templating methods for fabricating unique MnO2 nanostructures it is desired.
Soft template method
The soft template method is also applied in the synthesis of MnO2 nanostructures. Soft templates include surfactants,16,34 flexible organic molecules,20 bioprotein35 or block copolymers,26 all of which can act as structure-directing agents in the formation of MnO2. During the process of synthesizing MnO2 nanostructures, micelles have different morphologies depending on the different surfactant concentrations. Taking the electrostatic attraction, hydrogen bonding and van der Waals forces between surfactant molecules and nanomaterials as driving forces, these special micellar structures are used to effectively guide the precursors of the free nanomaterials to synthesize designed nanostructures.Using sodium dodecyl sulfate (SDS) as the template (Fig. 3), colloidal MnO2 nanosheets were synthesized in one step.36 Gu et al. synthesized single-layer MnO2 nanosheets via a redox reaction between KMnO4 and SDS under acidic condition. The gradual hydrolysis of SDS was found to be the key factor for the successful formation of single-layer nanosheets. SDS serves not only as the precursor of dodecanol to reduce KMnO4 but also as a structure-inducing agent to aid the formation of single-layer MnO2 nanosheets. Zou et al.16 employed cetyltrimethylammonium bromide (CTAB) as the cationic surfactant template, 2-(N-morpholino)ethanesulfonic acid (MES) as the reducing agent and KMnO4 as the Mn precursor to prepare the water-dispersed nano-MnO2 materials at room temperature. Such a template can self-assemble into a stable supramolecular structure (oil/water emulsion) in aqueous solution; the redox reaction between KMnO4 and MES quickly occurred at the oil/water interface and produced MnO2 nuclei in situ.37 These MnO2 nuclei continually grew into nano-MnO2 materials, and thus an unstable shell of loosely packed platelets was formed. After removing CTAB and other residual reactants, the shell collapsed, yielding the purified nano-MnO2 materials.
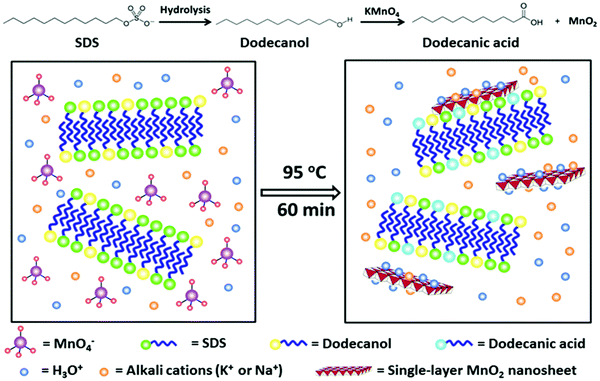 | ||
| Fig. 3 Schematic illustration of the lauric acid and SDS co-modified MnO2 nanosheets formation by in situ redox reaction between KMnO4 and dodecanol on the surface of the preformed soft-template. Reprinted with permission from ref. 36. Copyright (2015) Wiley-VCH. | ||
With the development of the bio-template synthesis method, the protein-directed in situ synthesis of nanomaterials has received a lot of attention due to the cost-effective, simple and environmentally friendly synthesis process. Most of the reported protein-templated nanomaterials show the morphologies of nanosheets (NSs),38 nanoparticles (NPs)39 and nanoclusters (NCs),40 and it is worth mentioning here that Liu et al.35 employed the capsid proteins pVIII of phage M13 as bio-templates to synthesize MnO2 nanosheets for the first time. MnO2 nanosheets were synthesized by the spontaneous oxidation of Mn2+ in an alkaline solution containing pVIII in the air at room temperature. Although the detailed mechanism is not clear and remains to be further investigated, the as-prepared protein–MnO2 nanosheets have shown intrinsic peroxidase-like activity and excellent antioxidant behaviors of natural antioxidants.
Although conceptually simple and versatile, the soft template method has its own limitations. This method often has difficulty in controlling the size, shape and uniformity of the products. Besides, the residual surfactants, organic substances and macromolecules may increase the ion resistance, proteins as bio-templates may be expensive, and the template removing process is time-consuming. Therefore, there still remains a major challenge to develop a facile, low cost, and environmentally benign method for the controllable synthesis of MnO2 materials.
Free template method
Apart from the abovementioned template-assisted methods, there are many other techniques that can be used to synthesize MnO2 nanosheets in the absence of an additional template. By employing MnCl2·4H2O as the Mn precursor and H2O2 as the oxidizing agent, Kawamata et al.6 successfully demonstrated the free template approach to directly form the MnO2 nanosheets within a day. In the presence of hydrogen peroxide (H2O2) and tetramethylammonium cations (TMA), Mn2+ ions were oxidized to MnO2 in an aqueous solution at room temperature. The cation not only served to inhibit the flocculation of the monosheets but also to exfoliate the locally aggregated monosheets in the suspension. The obtained monolayer MnO2 nanosheets possess good dispersibility and are not easily agglomerated. The reports on using this method to synthesize MnO2 nanosheets are springing up.Using this typical synthesis, Xie41 demonstrated experimentally that the chemical introduction of double-exchange in MnO2 nanosheets brought a metal–insulator transition near room-temperature. First, the bulk 2D MnO2 was prepared by adding MnCl2·4H2O into the (NH4)2S2O8 and TMA (25 wt%) solution under vigorous stirring for 60 min. The pristine MnO2 nanosheet was obtained by adding the bulk 2D MnO2 nanosheet to the water for ultrasonic treatment under constant temperature at 25 °C. The treated MnO2 nanosheet was obtained from the pristine MnO2 nanosheet by heating at 100 °C for 30 min and then at 220 °C for 2 h in a N2 atmosphere. The obtained films were characterized by X-ray diffraction (XRD), high-resolution transmission electron microscopy (HRTEM), atomic-force microscopy (AFM) and X-ray photoelectron spectra (XPS). The double-exchange Mn(III)–O–Mn(IV) structure was confirmed to be successfully produced by a low-oxygen-pressure thermoannealing of pristine MnO2 nanosheets (Fig. 4). The rapid development of nanoscience and technology has brought change to the MnO2 preparation technique and a few materials and more convenient methods are being used to prepare MnO2 nanosheets. The MnO2 nanomaterials can be fabricated by a facile one-step redox method by the addition of aqueous KMnO4 as the Mn precursor in the presence of 2-(N-morpholino)ethanesulfonic acid (MES) buffer at pH 6.0. KMnO4 was sonication-induced for 30 min directly and reduced by MES to form MnO2 nanosheets at room temperature. This method has received considerable attention for preparing MnO2 nanosheets due to the significant advantages such as less interfering substances, simple formation process and short preparation time. However, the prepared MnO2 nanosheets were thick, agglomerated easily, and unstable.
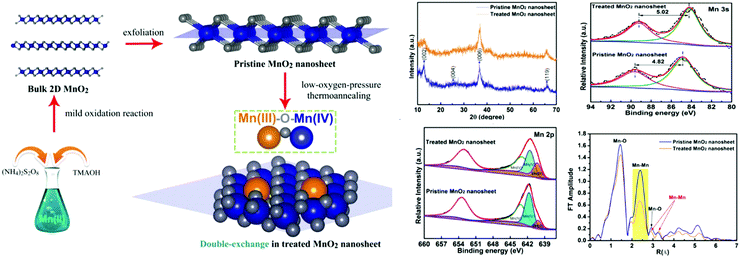 | ||
| Fig. 4 Schematic illustration of the synthesis strategy for the 2D MnO2 nanosheet with Mn(III)–O–Mn(IV) double-exchange structure, and investigation of the systematic characterization. Adapted with permission from ref. 41. Copyright (2017) American Chemical Society. | ||
An even more relevant reason is as follows: in the presence of a fluorescence reporter (e.g. CQDs), the addition of KMnO4 and MES can generate MnO2–CQDs nanocomposites in situ, and simultaneously quench the fluorescence of the labels, which can reduce the tedious steps and material interference in the formation of a fluorescence quenching system. For instance, Deng et al.21 used a template-free method to direct the growth of MnO2 nanosheets on the surface of the core–shell NaYF4:Yb/Tm@NaYF4 nanoparticles in the presence of KMnO4 and MES buffer. Subsequently, various MnO2 nanostructures have been synthesized by a template-free method with different labels.42–44 In the recent study by Chen et al.,17 the graphitic-phase C3N4 (g-C3N4) nanosheet–MnO2 sandwich nanocomposite was fabricated by a facile one-step in situ approach for the first time. The as-prepared MnO2 nanoparticles have a broad absorption band from 250 to 500 nm, which matches well with the reported optical characteristics of MnO2 nanomaterials.45 Remarkably, the absorbance spectrum of MnO2 nanoparticles overlaps well with the fluorescence emission of the g-C3N4 nanosheet, thereby leading to fluorescence resonance energy transfer (FRET) from the g-C3N4 nanosheet to the MnO2.
Biomedical applications of MnO2 nanosheets
The unique 2D planar structure and the above-mentioned series of characteristics have led to unique properties of MnO2 nanosheets for biomedical applications. To date, MnO2 nanosheets have been explored for a variety of biomedical applications such as sensing, molecular adsorption, biological imaging, drug delivery and cancer therapy. In addition, the biological effect and behavior of MnO2 nanosheets are also now under extensive exploration. It has been demonstrated that MnO2 nanosheets present low cytotoxicity and high hemo-/histo-compatibility. In this section, we mainly summarize the biomedical applications of MnO2 nanosheets in biosensing, imaging and cancer therapy.MnO2 nanosheet-based biosensor
Based on the oxidizing ability and catalytic activity of MnO2, MnO2 nanosheets have been widely used to construct biosensors. These biosensors can be mainly classified into three types: fluorescence biosensors, electrochemical biosensors and colorimetric biosensors.MnO2 nanosheet-based fluorescence biosensors
MnO2 nanosheets are generally used as fluorescence quenchers in fluorescence assays due to their strong optical absorption and fast electron transfer. MnO2 has been widely used for the detection of reducing substances due to its selective decomposition of MnO2 to Mn2+ ions. The fluorescence of the fluorescent materials is initially quenched by the MnO2 nanosheets. When in the presence of reducing target substances, the reductive reactions take place between the target and MnO2 nanosheets, and the MnO2 nanosheets are reduced to Mn2+ ions. As a result, the fluorescent labels are released and the fluorescence is recovered with the intensity directly related to the concentration of the target. By coupling with different kinds of fluorescent nanomaterials, many reducing substances can be detected based on this principle. GSH is a thiol-containing tripeptide that is generally treated as an essential endogenous antioxidant in cellular defense against toxins and free radicals.Deng et al.,21 for the first time, reported a MnO2-UCNPs based nanoprobe for GSH detection. In this nanosystem, the fluorescence of UCNPs (up-converting nanoparticles) can be effectively quenched by wrapping the MnO2 nanosheets on the surface of UCNPs. When the GSH was added to the solution, the fluorescence was restored due to the reductive reactions between the GSH and MnO2 nanosheets. The principle of a designed assay was demonstrated in Fig. 5A. By monitoring the fluorescence signal change of UCNPs, GSH in aqueous solutions and cancer cells can be detected. A limit of detection of 0.9 mM was achieved for GSH detection. Based on this principle, various fluorescence nanomaterial-MnO2 assays, such as carbon dot–MnO2 nanosheets,14,46–55 quantum dot–MnO2 nanosheets,34,56–59 organic dye–MnO2 nanosheets,8,60–63 metal nanocluster–MnO2 nanosheets,43,64,65 and polymer fluorescence nanoparticles,13,66–70 were developed for the detection of GSH and other reductive substances.
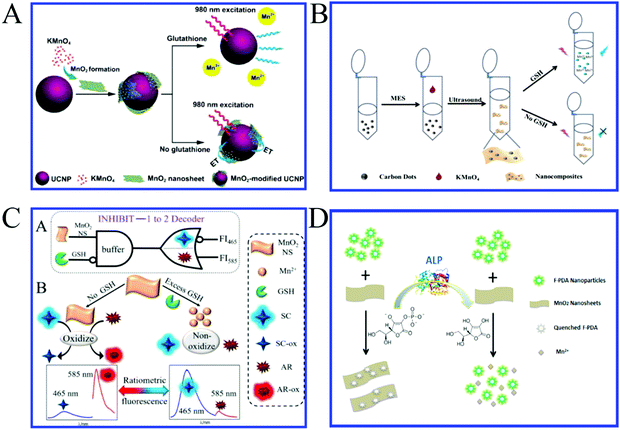 | ||
| Fig. 5 (A) Experimental design for GSH detection using MnO2-nanosheet-modified upconversion nanoparticles. Adapted with permission from ref. 21. Copyright (2011) American Chemical Society. (B) Schematic principle of C-dots–MnO2 nanocomposites for GSH detection. Adapted with permission from ref. 14. Copyright (2015) Elsevier. (C) Illustration of the cascade logic circuit (INHIBIT-1 to 2 Decoder) and schematic operations of the MnO2 NS-based ratiometric fluorescence sensor for GSH based on two fluorescent substrates. Adapted with permission from ref. 12. Copyright (2017) American Chemical Society. (D) Schematic illustration of the F-PDA–MnO2 probe for ALP detection on the basis of FRET. Adapted with permission from ref. 66. Copyright (2018) American Chemical Society. | ||
Our group14 also reported a facile one-step approach for the rapid and selective sensing of GSH based on the fluorescence resonance energy transfer (FRET) of C-dots–MnO2 nanocomposites (Fig. 5B). Soon after, a large amount of work about CQDs and MnO2 complexes for the detection of different reductive substances was reported. For example, Lin et al.54 and Zou et al.53 reported a novel FRET-based sensing platform using fluorescent C-dots and MnO2 nanosheets as the energy donor–acceptor pairs for GSH sensing in human whole blood samples and intracellular fluid, respectively. To further explore the level of GSH in living cells, our group56 developed a convenient fluorescence “turn-on” nanosensor based on graphene quantum dots (GQDs)–MnO2 nanosheets for the selective detection of GSH in living cells. The limit of detection was 150 nM for GSH.
Generally, MnO2 nanosheets are quenching agents, but Fan et al.12 found that MnO2 nanosheets can not only largely quench the fluorescence of highly fluorescent Scopoletin (SC) but also enhance that of nonfluorescent Amplex Red (AR) (Fig. 5C). Taking advantage of this property and the unique reaction between GSH and MnO2 nanosheets, they constructed the first MnO2 nanosheets-based ratiometric fluorescence sensor that is programmed for the ultrasensitive and selective detection of GSH by a cascade logic circuit. The calculated detection limit reached as low as 6.7 nM, which is the most sensitive MnO2 nanosheets-based fluorescent GSH sensor reported so far.
Due to the convenience, quickness and high accuracy, point-of-care testing (POCT) will be the main force in clinical laboratory science in the future. It can be used directly in situations that have limited resources or medical facilities. The main prospects for using these fast POCT diagnostics include portability, user-friendliness, durability, cheapness and the ability to produce fast results. Among them, the most well-known POCT technique is the lateral flow test strip assay, whose unique advantage lies in speediness; it is also low cost, simple, and convenient. Our group developed a novel fluorescence lateral flow biosensor for GSH detection based on quantum dot–MnO2 nanocomposites, which may have great potential to be used for GSH detection in point of care diagnosis.58
MnO2 nanosheets can also be degraded by hydrogen peroxide (H2O2). Based on this principle, Wang43 reported MnO2 nanosheets–CuNC nanoprobes for the rapid and sensitive detection of H2O2 and glucose in human serum. In this design, the fluorescence of CuNCs (measured at excitation/emission wavelengths of 335/410 nm) was quenched when adding MnO2 nanosheets into a colloidal solution of CuNCs. When H2O2 was added, MnO2 nanosheets were degraded to restore the fluorescence of CuNCs. Thus, the detection of H2O2 was achieved. In addition, glucose is further detected based on the characteristics of glucose producing H2O2 under the action of its oxidase. Compared with the traditional methods of glucose detection, the strategy is convenient, low-cost and without a complicated procedure. Second, it is operated under mild conditions within several minutes. Third, it provides an alternative platform for detecting other substrates (e.g., cholesterol) through oxidation by the O2-dependent oxidase (e.g., cholesterol oxidase), which can generate H2O2.
Ascorbic acid (AA), commonly known as vitamin C, is an antioxidant, an enzyme cofactor and essential nutritional factor in organisms. It can participate in hydroxylation and reduction reactions. It is also widely used in many fields, serving as an antioxidant in food, animal feed, beverages, cosmetics, and pharmaceutical formulations. A lack of AA in humans can cause a variety of diseases, such as scurvy. Mao et al.8 developed a new fluorescence method for the in vivo sensing of AA in the rat brain through suppressing the fluorescence of 7-hydroxycoumarin with the presence of MnO2 nanosheets. The mechanism for the fluorescence suppression is attributed to a combination of the inner filter effect (IFE) and static quenching effect (SQE), which is different from those reported for the traditional two-dimensional nanosheets, and the Förster resonant energy transfer (FRET) mechanism reported for MnO2 nanosheets. The limit of detection was estimated to be 8.7 ± 2.5 μM. This study not only offers a new avenue for in vivo sensing of AA but also provides new insight into MnO2 nanosheets suppressing the fluorescence of dyes. In order to avoid the poor photostability of dyes, Yang et al.66 recently reported a label-free, visual, reversible fluorescence biosensor for the convenient assaying of ALP activity based on the AA triggered reduction of MnO2 nanosheets, as well as clarified the quenching mechanism of F-PDA nanoparticles by MnO2 nanosheets (Fig. 5D).
Organophosphorus pesticides (OPs) are widely used in agricultural production because of their high efficacy and easy degradation in the environment. Because of their widespread use, organophosphorus pesticide residues in food and the environment have greatly threatened human health and caused widespread concern. Our group50 developed a facile fluorescence platform for the sensitive detection of OPs based on single-layer MnO2 nanosheets with C-dots as the signal readout. In the presence of butyrylcholinesterase (BChE) and acetylthiocholine, acetylthiocholine (ATCh) can be hydrolyzed to thiocholine by BChE; thiocholine reduced the MnO2 nanosheets to Mn2+. As a result of the decomposition of the MnO2 nanosheets, the fluorescence of C-dots was recovered. OPs as an inhibitor to suppress BChE activity can decrease the decomposition of MnO2, which again results in the fluorescence quenching. The fluorescence signal change corresponded to the degree of OPs-induced inhibition reaction. The linear detection range from 0.05 to 5 ng mL−1 was achieved for paraoxon detection with a limit of detection of 0.015 ng mL−1. This sensing system provided a new method for probing another reducing agent product-related enzyme system. Beyond this, MnO2 nanosheet-based DNA adsorption sensors are also very important. Layered transition-metal nanomaterials with one or a few atomic layers, recognized as planar covalent-network solids, have attracted growing attention in the past few years owing to their special structures with high specific surface areas. These materials possess the capability for good physisorption to aromatic and backbone phosphodiester groups, which can be taken as the platform for the adsorption of DNA chains.
The monolayer or multilayer MnO2 nanosheets have especially attractive properties due to the resemblance of their structures to graphene.71 The adsorption effect of MnO2 nanosheets on single-stranded DNA (ssDNA) is greater than that on double-stranded DNA (dsDNA). In the absence of the target, the MnO2 nanosheets adsorb the fluorophore-labeled ssDNA and quench the fluorescence of the fluorophore; in the presence of the target, the configuration of the fluorophore-labeled ssDNA changes to dsDNA, which are released from the MnO2 nanosheets and the fluorescence of the fluorophore is restored. Yuan et al.72 utilized the MnO2 nanosheet as a label-free nanoplatform for homogeneous biosensing. Two biosensors based on MnO2 nanosheets with favorable performances were constructed for ochratoxin A (OTA) and cathepsin D (Cat D) using different probes and following different sensing principles (Fig. 6A). The sensors constructed on this nanoplatform can be applied in not only aqueous solutions but also complicated sample matrixes with favorable sensing performances, high robustness and easy operations. In addition, the MnO2 nanoplatforms can easily be adapted to other luminescent materials and diverse probes, and therefore wide applications in bio-/chemo-sensing can be expected. Due to the interaction between the bases in nucleic acids and metal ions, the MnO2 nanosheet as a label-free nanoplatforms can successfully detect Ag+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 and Hg2+.74,75 For example, the Zhang et al.,73 for the first time, have developed a MnO2 nanosheet-assisted ligand–DNA interaction-based fluorescence polarization method for the sensitive detection of Ag+ (Fig. 6B). The good linearity ranges from 30–240 nM for Ag+ were observed with a detection limit (S/N = 3) of 9.1 nM. This method additionally exhibits high selectivity. This present study has opened up an avenue for determining other analytes, combining the advantages of MnO2 and the detection of strategy-fluorescence polarization.
73 and Hg2+.74,75 For example, the Zhang et al.,73 for the first time, have developed a MnO2 nanosheet-assisted ligand–DNA interaction-based fluorescence polarization method for the sensitive detection of Ag+ (Fig. 6B). The good linearity ranges from 30–240 nM for Ag+ were observed with a detection limit (S/N = 3) of 9.1 nM. This method additionally exhibits high selectivity. This present study has opened up an avenue for determining other analytes, combining the advantages of MnO2 and the detection of strategy-fluorescence polarization.
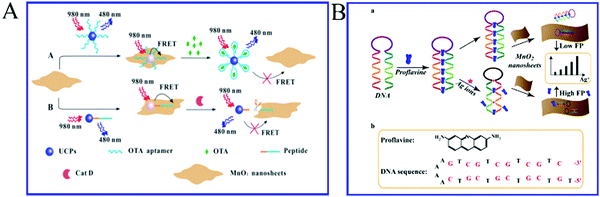 | ||
| Fig. 6 (A) Schematic illustration (not to scale) of MnO2 nanosheets-based FRET sensing: the OTA sensor with an ssDNA as the probe and the Cat D sensor with a peptide as the probe. Reproduced from ref. 72 with permission from Royal Society of Chemistry. (B) Illustration of MnO2 nanosheet-assisted ligand–DNA interaction-based fluorescence polarization sensor for the detection of Ag+ ions. Adapted with permission from ref. 73. Copyright (2017) Elsevier. | ||
In recent years, with the rapid development of various new technologies such as amplification or coupling technique, the combination of nanometer-sized MnO2 materials with these novel techniques can overcome the drawbacks of conventional sensors and further improve the sensitivity and selectivity of the sensor, which can serve as an effective sensing platform for the detection of small molecules or intracellular substances, and broaden the application of nanometer-sized MnO2. Xiang et al.76 have developed an in-cell signal amplification approach for monitoring down-regulated miRNAs in living cells based on the biodegradable MnO2 nanosheet-mediated and target-triggered assembly of hairpins. MnO2 nanosheets are graphene-like structure nanomaterials that adsorb and exhibit efficient fluorescence quenching ability toward organic dye-linked ssDNAs. The degradation of the MnO2 nanosheets by intracellular GSH and displacement reactions by proteins or nucleic acids lead to the release of the free hairpins from HCR, resulting in significantly amplified FRET signals from trace miRNA-21 in living cells. The developed method based on the MnO2 nanosheet-mediated amplification strategy can offer new opportunities for monitoring various trace intracellular miRNA targets in living cells. This MnO2 sensing platform can be developed for various targets such as DNA,16,77 RNA,78 biomolecules15 and metal ions.74 The high selectivity can be achieved by quenching the fluorescence of MnO2 nanosheets and recovering the fluorescence upon adding the targets.
MnO2 nanosheet-based colorimetric biosensors
The colorimetric biosensor has attracted considerable interest in biological science and analytical chemistry because of the potential for unaided visual readout. There are many advantages such as simplicity, cost-effectiveness and no requirement for any sophisticated instruments, so it is especially meaningful for on-site detection in real time.79 Among various colorimetric systems, because of its label-free nature and fast response, the enzyme catalytic oxidation of a chromogenic substrate such as 3,3′,5,5′-tetramethylbenzidine (TMB) has been widely applied in colorimetric biosensing. In recent years, artificial enzymes have been of great interest due to their enzyme-like reaction profiles and substrate specificities, which are more robust, stable, and tunable as compared with natural enzymes.MnO2 nanomaterials have high peroxidase-, oxidase-, and catalase-like activities due to the presence of lattice oxygen defects that were recently found to be capable of catalyzing the reaction of organic substrates [(TMB), o-phenylenediamine (OPD), and diazoaminobenzene (DAB)] in the absence of H2O2 to produce a color reaction.80 Based on this oxidase-like catalytic property, different groups have reported that MnO2 nanomaterial exhibited oxidase-like activity for the colorimetric detection of various analytes, including ions, small molecules, etc.
Liu et al.23 reported that MnO2 nanosheets possess oxidase-like activity that can catalyze the oxidization of TMB. Meanwhile, the existence of GSH can cause the reduction of oxidized TMB, which will generate a visual color change (Fig. 7A). Thus, the authors developed a novel colorimetric method for detecting GSH with a limit of detection of 300 nM in human serum. Its convenience and visibility will be very important for biomedical applications. Similarly, the MnO2 nanosheet–TMB reaction system was developed as a convenient detection platform for other antioxidants like uric acid,81 ascorbic acid,82 cysteine, etc. Nevertheless, these sensors only determine a single antioxidant, which does not allow high-throughput detection owing to their similar chemical properties.
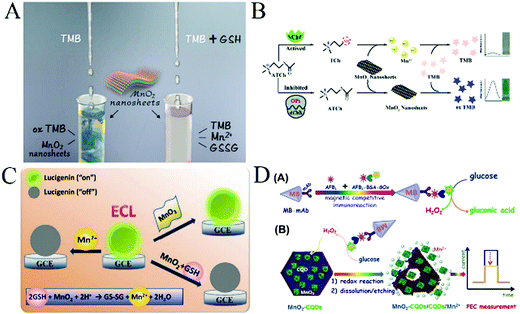 | ||
| Fig. 7 (A) Illustration of the MnO2 nanosheet-based colorimetric assay for GSH quantification. Adapted with permission from ref. 23. Copyright (2017) Elsevier. (B) Schematic of a colorimetric platform for AChE activity and its inhibitor. Reproduced ref. 84 with permission from Royal Society of Chemistry. (C) Schematic illustration for measuring GSH using lucigenin and MnO2 nanosheets. Adapted with permission from ref. 22. Copyright (2016) American Chemical Society. (D) Schematic illustration of the photoelectrochemical immunosensing platform toward aflatoxin B1 (AFB1) on carbon quantum dots-coated MnO2 nanosheets (MnO2–CQDs) by coupling an enzyme immunoassay format with a magneto-controlled microfluidic device. Adapted with permission from ref. 96. Copyright (2017) American Chemical Society. | ||
Array-based sensing approaches using nanomaterials have emerged as a powerful tool for the discrimination of a variety of analytes with similar structures and properties. Combining the advantages of the array-based sensing and MnO2 nanosheet–TMB reaction system, He et al.18 reported a unique visual colorimetric sensor array for the discrimination and identification of five antioxidants with the help of statistical analysis in serum. In this study, they employed MnO2 nanosheets to trigger a multicolor chromogenic system for discriminating five antioxidants. Different antioxidants have distinct reducing abilities, producing different inhibitions on the MnO2 nanosheet–TMB system, and therefore generating distinct colorimetric response patterns at 370, 450, and 650 nm. Using this sensor array, five antioxidants including UA, GSH, AA, Cys and Mel were successfully discriminated at a low concentration of 20 μM. Meanwhile, fetal bovine serum spiked with different antioxidants can be directly differentiated with the naked eye. Furthermore, this sensor array can also discriminate different concentrations of antioxidants and antioxidant mixtures and may hold great promise for the medical diagnosis and antioxidant biosensor.
Based on the strong affinity between thiolated compounds and metal ions, several studies have developed methods for metal ion detection in aqueous solution. Ye et al.83 prepared homogeneous MnO2 nanorods as oxidase-like mimetics to catalyze the oxidation of TMB into a blue colored cation radical in the absence of H2O2. GSH can successfully hinder the cation radical production leading to the decrease of solution absorbance, and mercury ions (Hg2+) have a strong affinity for thiolated compounds, therefore, when GSH and Hg2+ were pre-incubated, the solution was recovered to the blue colored solution in the presence of MnO2 nanorods. Based on this, the authors developed a simple and novel “off–on” colorimetric sensor for the detection of Hg2+ in aqueous solution and real water samples with a good linear relationship from 0.1 to 8.0 μM. The limit of detection was estimated to be 0.08 μM. The catalytic reaction was fast and the visible color change of the reaction system is obvious. The proposed method opens up a new possibility for monitoring trace levels of metal ions in real samples.
Although a large number of documents have reported the detection of different substances in chem/biosensing applications based on the MnO2–TMB strategy as a promising platform, there are few reports about the detection strategy that combines the specificity of the enzyme with the MnO2-catalyzed visual detection. Yan84 designed a novel colorimetric sensing platform using the specific reaction between thiocholine and the MnO2–TMB platform for the quantitative detection of acetylcholinesterase (AChE) activity and its inhibitor (Fig. 7B). First, the probe was constructed using MnO2 nanosheets as an oxidase-mimicking nanomaterial to directly oxidize TMB to oxTMB. In the presence of AChE, acetylthiocholine (ATCh) was catalytically hydrolysed to thiocholine (TCh), which easily triggered the decomposition of MnO2 nanosheets, and rendered TMB molecules colourless. When organophosphorus pesticides (OPs) were introduced, the activity of the enzyme was suppressed, preventing the generation of TCh and the decomposition of MnO2, resulting in the increase in the absorbance. By monitoring the absorbance change, they can quantitatively detect AChE activity and its inhibitor paraoxon. The integration of these two strategies not only enhanced the selectivity and sensitivity of the colorimetric sensing probe but also provided a novel strategy for enzyme detection. More importantly, the MnO2–TMB platform can also be successfully utilized in fabricating test strips for on-site monitoring of AChE activity and its inhibitor with high sensitivity and good accuracy, which also provides a new way to detect AChE activity and other organophosphorus pesticides.85
MnO2 nanosheet-based electrochemical biosensors
MnO2 has been explored as an alternative electrochemical catalyst for fabricating enzyme-free and sensitive electrochemical sensors due to its peculiar properties, including high catalytic activity, good chemical stability, low-cost, environmental friendliness and high energy density. However, the low intrinsic electrical conductivity of MnO2 (10−5–10−6 S cm−1) encourages researchers to combine MnO2 with graphene oxide,86 carbon nanotubes (CNTs),87 carbon foam,88 metal nanoparticles or metal oxides,89etc. Thus, by combining the advantages of the remarkable electrical conductivity and excellent biocompatibility of graphene with the high electrocatalytic activity of MnO2, the synergistic effect between them can make it possible to fabricate a sensor with good performance in the detection of various analytes, including ions, small molecules, immunoassays and so on. Specifically, MnO2 has a well-known electrochemical activity on H2O2 decomposition.Feng et al.90 developed the petal-shaped MnO2 nanosheet/graphene composites as an electrode material for the nonenzymatic sensing of H2O2 in a neutral environment. The fabricated sensor showed good repeatability, stability, selectivity, and high catalytic activity for the detection of H2O2. This assay has excellent anti-interference ability and displayed two wide linear ranges from 10 to 90 μM and from 0.2 to 0.9 mM with a low detection limit of 2 μM. In order to measure the trace concentration of H2O2 released by living cells/organisms, there is an urgent need to develop a much more sensitive electrochemical sensor based on a desirable nanomaterial with a unique electrocatalytic activity. Hu et al.91 successfully prepared MnO2 nanosheets with enhanced specific surface area and porous structure by a facile and efficient method, which is beneficial for their applications as electrochemical sensing materials. The H2O2 electrochemical nonenzymatic sensor was constructed based on MnO2 nanosheets to monitor H2O2 released by living cells. The proposed biosensor is highly sensitive and selective for the determination of H2O2 with a detection limit of 5 nM. The constructed electrochemical nonenzymatic sensor has been successfully applied to the real-time monitoring of the trace concentrations of H2O2 (0.1 μM) released by SP2/0 cells. The present study has shown that MnO2 nanosheets provide a new platform for developing highly sensitive electrochemical sensors with practical applications.
A recent striking find by Duan92 is that amorphous MnO2 can be used as a highly effective water oxidation catalyst to evolve molecular oxygen, which can quench the ECL for deactivating the excited state of Ru(dcbpy)32+. Based on the properties, Yuan et al.86 first described a signal-on electrochemiluminescence (ECL) aptasensor for the ultrasensitive detection of carcinoembryonic antigen (CEA) by using surface-initiated atom transfer radical polymerization (SI-ATRP) to facilitate the high density immobilization of a luminophore (Ru(dcbpy)32+-pendant PAMAM–poly-GMA) and manganese dioxide–graphene (MnO2–GO) composite, which can indirectly deactivate the excited state of Ru(dcbpy)32+. Due to the synergistic contribution of two functional components, the MnO2–GO composite can serve as an effective quencher for Ru(dcbpy)32+. The aptasensor has a good linear response for CEA ranging from 0.1 pg mL−1 to 20 ng mL−1 with a limit of detection of 25.3 fg mL−1. In addition, the ECL aptasensor exhibited high sensitivity, good stability, and satisfying selectivity, which provided a promising alternative tool for the determination of proteins in clinic analysis.
MnO2 nanosheets have little effect on the ECL of lucigenin. However, Mn2+ can obviously inhibit the ECL of lucigenin. Based on this principle, Gao et al.22 developed a new ECL method for the highly sensitive detection of GSH in human serum samples using lucigenin as the ECL luminophore and MnO2 nanosheets as the mediator. When MnO2 nanosheets were reduced to Mn2+ by GSH, an obvious inhibition on the ECL of lucigenin was observed. The ECL inhibition efficiencies gradually increased when the concentrations of GSH increased from 10 to 2000 nM. This nanosystem possessed a good linear relationship and the detection limit is 3.7 nM (Fig. 7C). This proposed method is highly sensitive, simple, fast, selective, and cost-effective. Besides, it is reported that this is the first time MnO2 nanosheets were used in the ECL detection technique, and it will stimulate the development of other ECL detection platforms.
The photoelectrochemical (PEC) sensing method is a newly grown and booming detection technique.93,94 It combines the advantages of optical methods and electrochemical sensors, and thus shows great promise in analytical applications. Owing to its band gap of about 2.1 eV, MnO2 nanosheets exhibit unique electrochemical properties and have an absorption peak centered at around 380 nm, which tails into the visible region. Sasaki et al.95 reported the observation of photocurrent generation via self-assembled MnO2 nanosheets on indium tin oxide (ITO) under visible light irradiation in nonaqueous electrolyte. Due to the strong localized d–d transition and high contact resistance, only a poor photocurrent from MnO2 nanosheets can be observed. Based on this study, Lin et al.96 designed MnO2–CQDs hybrid nanostructures for the improvement of photoelectrochemical properties (Fig. 7D). In this work, they developed a proof-of-concept high-throughput PEC immunosensing system for the simultaneous quantitative visual detection of AFB1 (as a model mycotoxin) by coupling a semiautomatic microfluidic device with the nanoparticles-based immunoassay format. In the presence of target AFB1, it initially competed with AFB1–BSA–GOx for the labeled anti-AFB1 antibody on the magnetic beads, and then the carried GOx oxidized the glucose to generate H2O2. In this case, the produced H2O2 was used as the reducing agent for the decomposition of MnO2 nanosheets. Due to the decline of MnO2–CQDs on the electrode, the photocurrent of the MnO2–CQDs-modified electrode decreased with the increasing H2O2 concentration. By monitoring the change in photocurrent, we can quantitatively determine AFB1 in real samples. MnO2–CQDs were favorably eliminated, and a visual detection could be realized by judging the color intensity change of the electrode. Due to the synergism between MnO2 and CQDs, the PEC activity of MnO2 and the efficiency of PEC sensors have been greatly improved. This present system has opened up new horizons for determining other mycotoxins or small molecules by changing the corresponding conditions.
MnO2 nanosheet-based bioimaging
Molecular imaging as a non-invasive tool is the most attractive imaging technique for the early detection and management of malignant tumors. Due to the poor tissue penetration of “turn-off’’ fluorescence imaging and the poor sensitivity of magnetic resonance imaging (MRI), two or more imaging techniques are combined to synergistically address multiple issues like sensitivity, resolution, and tissue penetration in tumor diagnosis. However, these ‘‘always on’’ imaging probes still suffer from a high non-specific background and a low signal to background ratio. To address these issues, the use of activatable imaging probes may cater to the improvement of the signal to background ratio, sensitivity, and specificity.97As two-dimensional materials, MnO2 nanosheets have a large specific surface area and a broad absorption spectrum, which has been widely used as the fluorescence quencher. Moreover, since MnO2 can achieve controlled degradation in the tumor microenvironment under the condition of low pH and high GSH concentrations, the fluorescence quenched by MnO2 can be recovered once MnO2, a high T1-weighted contrast agent with excellent MRI characteristics, is reduced to Mn2+, enabling the imaging intensity to be controllable. MnO2 nanomaterials, therefore, have shown great prospects as activatable bioimaging platforms. Recently, several MnO2 nanostructure-based activatable bioimaging platforms have been reported for intracellular or in vivo imaging.
Lee et al.98 developed urchin-shaped MnO2 nanomaterials as pH-responsive activatable T1 contrast agents for in vivo MRI imaging with a mouse tumor model. Based on that, Chen et al.99 explored the degradation of MnO2 nanosheets under conditions of low pH in tumor cells and confirmed that their degraded Mn2+ had magnetic resonance T1-weighted imaging properties. When DOX–PEG–MnO2 enters the cell through endocytosis, low pH-responsive MnO2 decomposed to Mn2+, which acts as a highly efficient T1-MRI CAs for in vivo nude mice tumor imaging. The anticancer drug, DOX, was released into the acidic microenvironment of the tumor tissue, inducing remarkable apoptosis and achieving the successful theranostics of cancer in an intelligent and on-demand manner (Fig. 8A). T1-MRI images were observed at the axial and coronal sites of 4T1 tumor-bearing nude mice before and after administration of PEG–MnO2 nanosheets within the tumor and normal subcutaneous tissue every 10 min. Quantitative T1-MRI signal intensity was recorded before and after the administration of PEG–MnO2 nanosheets on the (c) axial tumor region and (d) coronal tumor region and normal subcutaneous tissue. In order to make up for the insufficiency of single MIR imaging, Zhao et al.100 firstly developed a novel dual-activatable fluorescence/MRI bimodal platform for imaging cancer cells using a MnO2–Cy5-labeled aptamer nanoprobe that can target, recognize and enter tumor cells (Fig. 8B). The overexpressed GSH in tumor cells induced the degradation of MnO2 and released Cy5-labeled aptamers as well as Mn2+. On the one hand, the released Cy5-labeled aptamers can be used for fluorescence imaging; on the other hand, the produced Mn2+ is used as a contrast agent for MRI to increase the contrast signal, which can be used as a dual-activatable fluorescence/MRI bimodal platform in cancer cell imaging. It is demonstrated that the fluorescence activation of the nanoprobe possesses high specificity to target cells by using confocal laser scanning microscopy. The feasibility of a nanosheet–sgc8 nanoprobe for cellular MRI was also evaluated by examination of CCRF-CEM cells (human T cell acute lymphoblastic leukemia cell line cells) and Ramos cells incubated with nanoprobes at different concentrations.
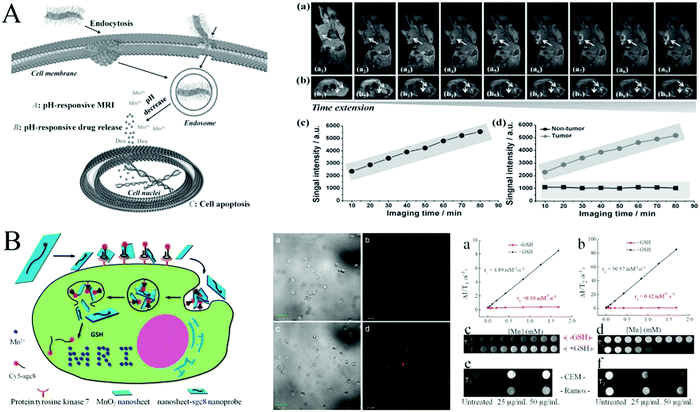 | ||
| Fig. 8 (A) Theranostic function of PEG–MnO2 nanosheets for intracellular pH-responsive drug delivery and T1-MRI, and the axial and coronal T1-MRI of 4T1 tumor-bearing nude mice before and after the administration of PEG–MnO2 nanosheets. Reprinted with permission from ref. 99. Copyright (2014) Wiley-VCH. (B) Activation mechanism of the MnO2 nanosheet–aptamer nanoprobe for fluorescence/MRI bimodal tumor cell imaging, and the confocal imaging and MRI images of CCRF-CEM cells. Adapted with permission from ref. 100. Copyright (2014) American Chemical Society. | ||
Overall, various imaging modalities and image-enhancing methods based on MnO2 nanosheets have been developed to provide help in the sensitive detection of cancer in its earliest stages and further their applications in cancer management.
MnO2 nanosheet-based drug delivery and cancer therapy
MnO2 nanosheets, as outstanding structural nanomaterials with low toxicity, strong adsorption, large surface-to-volume ratio and good biocompatibility, can load numerous guest molecules. Moreover, MnO2 nanosheets are degraded by overexpressed GSH at low pH in cancer cells and have been widely used for drug delivery and cancer therapy. Here, we discuss the progress in cancer therapy combining MnO2 nanostructure-based drug delivery with different methods, including chemotherapy, photodynamic therapy, photothermal therapy and gene therapy.In recent decades, the drug delivery system (DDS) based on nanomaterials has been widely studied, which provides hope by enhancing the selectivity of chemotherapy. Chemotherapy usually refers to the use of medicines or drugs to treat cancers. Zhao et al.101 designed a novel and facile multifunctional nanocomposite probe (MSU/MnO2) as a new DDS (Fig. 9A). They first prepared the magnetic up-conversion (MSU) composite probe through conjugated cross-linking of amino-modified Fe3O4@SiO2 particles (the core) and carboxyl functionalized NaYF4:Yb,Er nanoparticles (the shell). Layered MnO2 nanosheets were formed in situ on the surface of MSU as MSU/MnO2 for the loading and release of the model drug, congo red (CR). In this DDS, the targeted transport of Fe3O4 to tumor cells and decomposition of MnO2 nanosheets in the tumor cells can achieve the release of drugs at the lesion sites. Moreover, the quenched fluorescence was recovered and upconversion luminescence was observed. Similarly, Wang et al.20 have used the redox-responsive degradable MnO2 nanostructures as an effective nanocarrier for detecting intracellular glutathione and triggering doxorubicin (DOX) release.
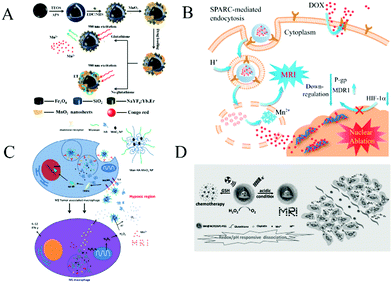 | ||
| Fig. 9 (A) Schematic illustration of the synthetic procedure for the preparation of the MSU/MnO2-CR drug delivery system. Reproduced from ref. 101 with permission from the Royal Society of Chemistry. (B) Schematic illustration of BMDN as an MDR-reversal vector and T1-weighted contrast agent for MRI and simultaneous chemotherapy on the resistant tumor. Adapted with permission from ref. 102. Copyright (2017) American Chemical Society. (C) Bioconjugated manganese dioxide nanoparticles enhanced the chemotherapy response by priming tumor-associated macrophages toward the M1-Like phenotype and attenuating tumor hypoxia. Adapted with permission from ref. 103. Copyright (2016) American Chemical Society. (D) A scheme illustrating the redox/pH-responsive behaviors of BM@NCP(DSP)–PEG composite nanoparticles in the tumor microenvironment. Reprinted with permission from ref. 104. Copyright (2017) Wiley-VCH. | ||
Chemotherapy may have serious side effects on normal cells and tissues due to the lack of selectivity, which can limit their applications in cancer treatment. Better DDS should have the characteristic of the controllable release at a target position. By conjugating the targeting ligand of the tumor-specific receptors on the surface of nanoparticles, active tumor targeting has successfully improved the tumor targeting efficiency. In recent years, some natural target molecules such as folic acid (FA) and hyaluronic acid (HA) have made great progress in the research on tumor targeting. They are widely applied in the extracellular matrix, cell surface and intracellular matrix of various body tissues, and can specifically bind to receptors that are highly expressed on the surface of tumor cells. In this way, targeted cancer therapy can be achieved without any complex nanomaterials.
Zhang et al.25 developed a multifunctional tumor targeting theranostic system for tumor-targeting therapy and magnetic resonance imaging by employing FA as an attractive targeting agent. Layered MnO2 nanosheets have been used as drug nanocarriers and potential magnetic resonance imaging (MRI) agents. The MnO2–PEG–FA/DOX nanosheets are highly efficient for delivering doxorubicin (DOX) to tumor cells in vitro and in vivo due to the presence of the folate receptors, meanwhile, MnO2 can be quickly decomposed into Mn2+, enabling magnetic resonance imaging under the slightly acidic environment with a high concentration of GSH. By taking advantage of active targeting and efficient MRI in multiple reductions, pH dual-responsive biodegradable nanosheet systems have provided a promising platform for tumor-targeting theranostics. Similarly, BSA–MnO2–DOX nanomaterials (BMDN) were fabricated by Chen et al.102 as the DOX drug loading nanoplatform for cancer magnetic resonance imaging and simultaneously reversing multidrug resistance in the resistant tumor (Fig. 9B). BMDN facilitated the delivery of DOX into MDR tumor cells through their MDR reversal effects including enhanced cellular uptake, reduced drug efflux, and a decreased hypoxic tumor microenvironment. BMDN also acted as an effective MRI contrast agent, thereby causing good in vitro and in vivo T1-weighted imaging.
Liu103 constructed HA-modified mannan-conjugated MnO2 nanoparticles (Man–HA–MnO2 NPs) as the target delivery platform to alleviate tumor hypoxia and enhance chemotherapy response by reacting with endogenous H2O2 under hypoxic conditions. Combination treatment of the tumors with Man–HA–MnO2 NPs and doxorubicin significantly increased the apparent diffusion coefficient values for breast tumors, largely inhibited tumor growth and tumor cell proliferation as compared with chemotherapy alone. In addition, the released Mn2+ ions have the ability to highly enhance T1 MRI performance for tumor imaging (Fig. 9C). Liu104,105et al. has utilized the nanoscale-coordination-polymer-shelled MnO2 composite nanomaterials as a multistage redox/pH/H2O2-responsive nanoplatform for cancer chemoradiation treatment (Fig. 9D). The MnO2-based multifunctional drug carrier nanoplatform not only broadens the application of the MnO2 nanomaterials but also provides a new approach for the simultaneous magnetic resonance imaging and cancer therapy.
Compared with chemotherapy, photodynamic therapy (PDT) is a form of phototherapy that is based primarily on the production of reactive oxygen species by photosensitizing molecules under selective illumination and is, therefore, toxic to target tissues. PDT is a minimally invasive treatment strategy to kill tumors with high specificity. It has received tremendous attention over the past decades in both preclinical studies and clinical practices.106 Tan et al. have developed a series of drug delivery and cancer treatment platforms based on MnO2 nanocomposites. For example, Meng et al.67 developed a detecting platform for GSH bioimaging in CEM cells and tissues based on activatable two-photon mesoporous silica (TP-MSNs) coated with MnO2 nanosheets fluorescence nanoprobes. Later, they improved the abovementioned work and obtained multi-functional nanoprobes for contrast-enhanced dual-mode cell imaging and targeted therapy.19 In the later work, MSNs were loaded with antitumor drug DOX and photosensitizer molecule chlorin e6 (Ce6). The Sgc8 aptamer for targeted cancer cells was adsorbed on the surface of MnO2 nanosheets. In addition, MnO2 nanosheets acted not only as a gatekeeper for TP-MSNs, but as a quencher for TP fluorescence and a contrast agent for MRI. Due to the above-mentioned effects, this design can be used not only for intracellular GSH imaging but also for targeting CEM cells. It can achieve the release of DOX and Ce6 from MSN in cancer cells, as well as the synergistic effect of chemotherapy and PDT.
Fan et al.107 found that the concentration of intracellular GSH was related to the content of singlet oxygen (1O2), which was generated through the reaction between a photosensitizer and tissue oxygen under illumination. Based on this fact, they constructed a multifunctional photosensitizer chlorin e6 (Ce6)–MnO2 nanosystem. It not only inhibits extracellular singlet oxygen generation by Ce6 with fewer side effects but can also enhance the cellular uptake of photosensitizers for highly efficient intracellular PDT as compared to free Ce6. As seen in Fig. 10A, the level of GSH is decreased in cancer cells in situ, causing the death of cancer cells. Moreover, flow cytometry and confocal laser scanning microscopy analysis of cells treated with the Ce6–MnO2 nanosystem both presented significantly higher fluorescence intensity in the cytoplasm as compared to untreated cells and cells treated with only Ce6. These results also demonstrated that the Ce6–MnO2 nanosystem was mainly distributed in the cytoplasm, and MnO2 nanosheets were mainly reduced by the GSH in the cytoplasm. The data suggest that the MnO2 nanosheets can efficiently deliver photosensitizer Ce6 into cells for photodynamic therapy. Moreover, this multifunctional MnO2 nanosheet nanosystem shows significant promise in monitoring various types of cancers and facilitating their biomedical applications, particularly cancer theranostics.
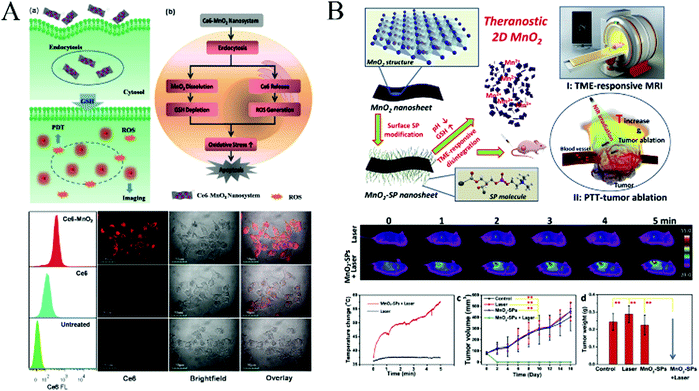 | ||
| Fig. 10 (A) Activation mechanism of the Ce6–MnO2 nanosystem for highly efficient photodynamic therapy, and flow cytometry analysis (left) and confocal fluorescence images (right) of MCF-7 cells treated with free Ce6 and the Ce6–MnO2 nanosystem. Reprinted with permission from ref. 107. Copyright (2016) Wiley-VCH. (B) Schematic illustration of the synthetic procedure for MnO2-SPs nanosheets and their specific functions for tumor theranostics with TME sensitivity, including the acidic/reducing condition-triggered T1-weighted MR imaging and efficient PTT against the tumor, and the in vivo photothermal therapy against tumor growth assisted by MnO2-SPs. Adapted with permission from ref. 108. Copyright (2018) Elsevier. | ||
Coincidentally, photodynamic therapy based on multifunctional MnO2 nanocarriers was also published by other groups. Liu27 further conjugated MnO2 nanomaterial with amino terminated polyethylene glycol (PEG–NH2) to increase its water solubility and physiological stability. They combined the advantages of the high reactivity of MnO2 nanomaterials toward endogenous H2O2 within the tumor microenvironment to generate O2 and multifunctional Ce6@MnO2–PEG to achieve enhanced tumor-specific PDT and MR imaging in the solid tumor microenvironment.
Premature leakage of photosensitizer (PS) from nanocarriers significantly reduces the accumulation of PS within a tumor, thereby increasing the nonspecific accumulation in normal tissues and inevitably leading to a limited efficacy for PDT and the more apparent systemic phototoxicity. Ma et al.,26 for the first time, designed an intelligent core–shell PDT nanoplatform utilizing SiO2–methylene blue (MB) with a high MB payload as the inner core and MnO2-based nanostructures as the intelligent “gatekeeper”. This method prevents the premature release of loaded PS in the core and elevates the O2 concentration for the in vivo imaging-guided PDT and MRI imaging by stimuli-responsive acidic H2O2 in solid tumors. This proof of concept might provide a novel route to develop the new tumor microenvironment-sensitive nanoplatform for imaging-guided cancer therapy.
Although PDT has become an excellent strategy in cancer treatment, it still faces problems such as poor tumor selectivity, hypoxia-induced drug resistance, and limitations of common photosensitizers. Photothermal therapy (PTT) can significantly inhibit the growth of cancer cells by photothermal ablation and also greatly enhance the efficacy of chemotherapy and PDT. In order to overcome the shortcomings of photodynamic therapy and achieve photothermal (PTT) therapy triggered on cancer cells under near-infrared light irradiation, Liu et al.108 reported a new finding that ultrathin 2D MnO2 nanosheets, as a new photothermal agent, intrinsically possess high photothermal-conversion capability for highly efficient PTT against tumors. Moreover, these ultrathin MnO2 nanosheets exhibited an ultrasensitive response to the endogenous tumor microenvironment (TME), which can disintegrate and release the Mn2+ in response to the low pH and high concentration of GSH for T1-weighted MR imaging of the tumor. Through the recording of IR thermal images, the tumor surface temperature of the MnO2-SPs + Laser group increased from 37 °C to 57 °C within 5 min under NIR laser irradiation, while the temperature of the laser-only group just increased by 1 °C.
Digital photos, tumor size and body weight of 4T1 tumor-bearing mice in four groups were recorded every other day for 16 days after different treatments. It was noted that the tumors were completely eliminated after the MnO2-SPs injection combined with 808 nm laser irradiation without obvious reoccurrence on the 16th-day feeding, which was further demonstrated by the tumor weight at the end of the evaluation (Fig. 10B). The high PTT efficiency of 2D MnO2 nanosheets responding to exogenous NIR irradiation has been systematically demonstrated both in vitro and in vivo to suppress the tumor growth.
Gene therapy is becoming a promising approach for the treatment of both inherited and acquired diseases. Fan et al.109 employed MnO2 nanosheets as nanocarriers loaded with DNAzymes for gene silencing therapy and cell imaging (Fig. 11). They applied the photosensitizer Ce6 as a PDT agent, and free Mn2+ ions could then be used as efficient cofactors of 10–23 DNAzyme for gene silencing. Based on these, they constructed the photosensitizer Ce6–MnO2–DNAzymes nanosystem for highly efficient photodynamic therapy (PDT) and gene silencing therapy by consuming intracellular GSH and protecting reactive oxygen species, which can be used to kill cancer cells.26 As shown in confocal laser scanning microscopy analysis (Fig. 11B), comparing the internalization of the Ce6–DNAzyme–MnO2 nanosystem and that of free DNAzyme in MCF-7 breast-cancer cells, MCF-7 cells treated with the MnO2 nanosheets can efficiently deliver Ce6-labelled DNAzymes into cells for therapeutic applications. In addition, as shown in Fig. 11C and D, an MTS assay and a soft-agar colony assay were applied to investigate the proliferation of MCF-7 cells treated with a nanosystem containing different concentrations of DNAzymes. It was found that compared with the DNAzyme only, the gene silencing therapeutic efficacy of the DNAzyme–MnO2 nanosystem gradually increased with increasing concentration of DNAzyme. These results show that the MnO2 nanosheet is both a potent delivery tool and an efficient cofactor to facilitate DNAzyme-based gene-silencing therapy.
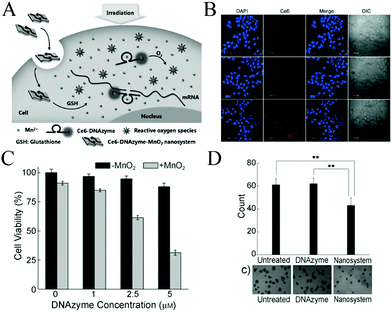 | ||
| Fig. 11 (A) Activated mechanism of the Ce6–DNAzyme–MnO2 nanosystem for gene silencing and PDT. The nanosheet can adsorb and efficiently deliver DNAzymes into cells and protect them from enzymatic digestion. (B) Confocal fluorescence images of MCF-7 cells without treatment (upper) and with treatment with Ce6–DNAzyme (middle) and the Ce6–DNAzyme–MnO2 nanosystem (lower). (C) Gene-silencing therapy with the DNAzyme–MnO2 nanosystem. (D) Statistical analysis of results from the soft-agar colony assay. (A–D) Reprinted with permission from ref. 109. Copyright (2015) Wiley-VCH. | ||
The properties of the MnO2 composite material were highly improved, and in vitro and in vivo detection/imaging indicated their successes as biodegradable probes. These studies have shown the promise of using MnO2 nanosheets for bioimaging, drug delivery, diagnosis and therapy, and should encourage in-depth studies of MnO2 composite nanomaterials for other biomedical applications.
Summary and outlook
In this review, we discussed the recent advances of MnO2 nanosheets-based biochemical application platforms, from the preparation of MnO2 nanosheets to chemical/biomedical applications including fluorescence biosensors, electrochemical biosensors, colorimetric biosensors, bioimaging, drug delivery and cancer therapy. Although MnO2 nanomaterials have been widely studied in recent years, compared with GO and other two-dimensional nanomaterials, most of the research on MnO2 nanosheets is still at the primary stage. The prospects for popularizing their related clinical products is infinite. Several unresolved issues and further research efforts for the future are listed in the following.(I) In terms of the preparation method, manganese dioxide is mostly prepared by one-pot synthesis, from which pristine MnO2 nanomaterial is obtained. Some physical or chemical exfoliating methods were adopted to obtain the lamellar MnO2, whose size, composition and dispersibility have not been determined. More controllable and functional strategies should be developed to obtain the desired structural/compositional MnO2 nanomaterials.
(II) Regarding diagnostics and therapeutics, MnO2 nanosheets can act as a nanocarrier without orientation, so it should be able to combine with other substances to achieve the targeted doses and bioimaging accuracy.
(III) Understanding the safety of MnO2 nanosheets toward living organisms for applications in bioimaging, drug delivery and cancer therapy is imperative. Although the toxicity of MnO2 is probably low as demonstrated, the potential risks, long-term toxicity, cellular-uptake mechanism and metabolic pathway are still unknown. Sufficient attention should be paid to decrease or diminish its toxicity in living organisms and accelerate the biodegradability on the premise of maintaining therapeutic effects in biological systems.
(IV) Research toward the wider application of MnO2 for biosensors and biomedicine is still in its infancy, so in-depth studies on MnO2 nanomaterials are urgently needed for other biomedical applications. MnO2 nanosheets-based biomedical applications still have room for development and there are infinite research opportunities for researchers to explore.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (21205108, 21605038), the Foundation for University Key Teacher by Henan Province (2017GGJS007), and the Key Scientific Research Project in Universities of Henan Province (19A150048).References
- T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100–102 CrossRef CAS PubMed.
- (a) W. Wen, Y. Song, X. Yuan, C. Zhu, D. Du, A. Asiri and Y. Lin, Mater. Today, 2018, 21, 164–177 CrossRef CAS; (b) C. Zhu, D. Du and Y. Lin, Biosens. Bioelectron., 2017, 89, 43–55 CrossRef CAS PubMed.
- X. Peng, L. Peng, C. Wu and Y. Xie, Chem. Soc. Rev., 2014, 43, 3303–3323 RSC.
- Y. Chen, C. Tan, H. Zhang and L. Wang, Chem. Soc. Rev., 2015, 44, 2681–2701 RSC.
- Y. Omomo, T. Sasaki, L. Wang and M. Watanabe, J. Am. Chem. Soc., 2003, 125, 3568–3575 CrossRef CAS PubMed.
- K. Kai, Y. Yoshida, H. Kageyama, G. Saito, T. Ishigaki, Y. Furukawa and J. Kawamata, J. Am. Chem. Soc., 2008, 130, 15938–15943 CrossRef CAS PubMed.
- B. A. Pinaud, Z. Chen, D. N. Abram and T. F. Jaramillo, J. Phys. Chem. C, 2011, 115, 11830–11838 CrossRef CAS.
- W. Zhai, C. Wang, P. Yu, Y. Wang and L. Mao, Anal. Chem., 2014, 86, 12206–12213 CrossRef CAS PubMed.
- Y. He, W. Huang, Y. Liang and H. Yu, Sens. Actuators, B, 2015, 220, 927–931 CrossRef CAS.
- H. Sun, K. Xu, M. Huang, Y. Shang, P. She, S. Yin and Z. Liu, Appl. Surf. Sci., 2015, 357, 69–73 CrossRef CAS.
- L. Peng, Q. Zeng, B. Tie, M. Lei, J. Yang, S. Luo and Z. Song, J. Colloid Interface Sci., 2015, 456, 108–115 CrossRef CAS PubMed.
- D. Fan, C. Shang, W. Gu, E. Wang and S. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 25870–25877 CrossRef CAS PubMed.
- Z. Lin, M. Li, S. Lv, K. Zhang, M. Lu and D. Tang, J. Mater. Chem. B, 2017, 5, 8506–8513 RSC.
- (a) Q. Y. Cai, J. Li, J. Ge, L. Zhang, Y. L. Hu, Z. H. Li and L. B. Qu, Biosens. Bioelectron., 2015, 72, 31–36 CrossRef CAS PubMed; (b) Y. Hu, L. Zhang, X. Geng, J. Ge, H. Liu and Z. Li, Anal. Methods, 2017, 9, 5653–5658 RSC.
- H.-B. Wang, Y. Li, H.-Y. Bai and Y.-M. Liu, Sens. Actuators, B, 2018, 259, 204–210 CrossRef CAS.
- D. He, X. He, K. Wang, X. Yang, X. Yang, X. Li and Z. Zou, Chem. Commun., 2014, 50, 11049–11052 RSC.
- X. L. Zhang, C. Zheng, S. S. Guo, J. Li, H. H. Yang and G. Chen, Anal. Chem., 2014, 86, 3426–3434 CrossRef CAS PubMed.
- W. Huang, Y. Deng and Y. He, Biosens. Bioelectron., 2017, 91, 89–94 CrossRef CAS PubMed.
- H. M. Meng, L. Lu, X. H. Zhao, Z. Chen, Z. Zhao, C. Yang, X. B. Zhang and W. Tan, Anal. Chem., 2015, 87, 4448–4454 CrossRef CAS PubMed.
- D. He, X. He, K. Wang, X. Yang, X. Yang, Z. Zou and X. Li, Chem. Commun., 2015, 51, 776–779 RSC.
- R. Deng, X. Xie, M. Vendrell, Y. T. Chang and X. Liu, J. Am. Chem. Soc., 2011, 133, 20168–20171 CrossRef CAS PubMed.
- W. Gao, Z. Liu, L. Qi, J. Lai, S. A. Kitte and G. Xu, Anal. Chem., 2016, 88, 7654–7659 CrossRef CAS PubMed.
- J. Liu, L. Meng, Z. Fei, P. J. Dyson, X. Jing and X. Liu, Biosens. Bioelectron., 2017, 90, 69–74 CrossRef CAS PubMed.
- Y. He, B. Jiang, J. Chen, Y. Jiang and Y. X. Zhang, J. Colloid Interface Sci., 2018, 510, 207–220 CrossRef CAS PubMed.
- Y. Hao, L. Wang, B. Zhang, H. Zhao, M. Niu, Y. Hu, C. Zheng, H. Zhang, J. Chang, Z. Zhang and Y. Zhang, Nanotechnology, 2016, 27, 025101 CrossRef PubMed.
- Z. Ma, X. Jia, J. Bai, Y. Ruan, C. Wang, J. Li, M. Zhang and X. Jiang, Adv. Funct. Mater., 2017, 27, 1604258 CrossRef.
- W. Zhu, Z. Dong, T. Fu, J. Liu, Q. Chen, Y. Li, R. Zhu, L. Xu and Z. Liu, Adv. Funct. Mater., 2016, 26, 5490–5498 CrossRef CAS.
- Y. B. Hahn, R. Ahmad and N. Tripathy, Chem. Commun., 2012, 48, 10369–10385 RSC.
- L. Wang, Y. Omomo, N. Sakai, K. Fukuda, I. Nakai, Y. Ebina, K. Takada, M. Watanabe and T. Sasaki, Chem. Mater., 2003, 15, 2873–2878 CrossRef CAS.
- Y. Oaki and H. Imai, Angew. Chem., 2007, 119, 5039–5043 CrossRef.
- X. Feng, Y. Zhang, J. Song, N. Chen, J. Zhou, Z. Huang, Y. Ma, L. Zhang and L. Wang, Electroanalysis, 2015, 27, 353–359 CrossRef CAS.
- X. Zhang, R. Kong, Q. Tan, F. Qu and F. Qu, Talanta, 2017, 169, 1–7 CrossRef CAS PubMed.
- L. Zhang, J. Lian, L. Wu, Z. Duan, J. Jiang and L. Zhao, Langmuir, 2014, 30, 7006–7013 CrossRef CAS PubMed.
- J.-L. Chen, L. Li, S. Wang, X.-Y. Sun, L. Xiao, J.-S. Ren, B. Di and N. Gu, J. Mater. Chem. B, 2017, 5, 5336–5344 RSC.
- L. Han, P. Liu, H. Zhang, F. Li and A. Liu, Chem. Commun., 2017, 53, 5216–5219 RSC.
- Z. Liu, K. Xu, H. Sun and S. Yin, Small, 2015, 11, 2182–2191 CrossRef CAS PubMed.
- H. Chen, J. He, C. Zhang and H. He, J. Phys. Chem. C, 2007, 111, 18033–18038 CrossRef CAS.
- P. Liu, L. Han, F. Wang, X. Li, V. A. Petrenko and A. Liu, Nanoscale, 2018, 10, 2825–2833 RSC.
- X. Liu, Q. Wang, H. Zhao, L. Zhang, Y. Su and Y. Lv, Analyst, 2012, 137, 4552–4558 RSC.
- J. Xie, Y. Zheng and J. Y. Ying, J. Am. Chem. Soc., 2009, 131, 888–889 CrossRef CAS PubMed.
- X. Peng, Y. Guo, Q. Yin, J. Wu, J. Zhao, C. Wang, S. Tao, W. Chu, C. Wu and Y. Xie, J. Am. Chem. Soc., 2017, 139, 5242–5248 CrossRef CAS PubMed.
- X. J. Kong, S. Wu, T. T. Chen, R. Q. Yu and X. Chu, Nanoscale, 2016, 8, 15604–15610 RSC.
- (a) H.-B. Wang, Y. Chen, N. Li and Y.-M. Liu, Microchim. Acta, 2016, 184, 515–523 CrossRef; (b) Z. M. Huang, J. Yang, L. Zhang, X. Geng, J. Ge, Y. L. Hu and Z. H. Li, Anal. Methods, 2017, 9, 4275–4281 RSC.
- F. Zhou, T. Zheng, E. S. Abdel-Halim, L. Jiang and J.-J. Zhu, J. Mater. Chem. B, 2016, 4, 2887–2894 RSC.
- G. Zhao, J. Li, L. Jiang, H. Dong, X. Wang and W. Hu, Chem. Sci., 2012, 3, 433–437 RSC.
- J. Liu, Y. Chen, W. Wang, J. Feng, M. Liang, S. Ma and X. Chen, J. Agric. Food Chem., 2015, 64, 371–380 CrossRef PubMed.
- J. Gallo, N. Vasimalai, M. T. Fernandez-Arguelles and M. Banobre-Lopez, Dalton Trans., 2016, 45, 17672–17680 RSC.
- Y. Xu, X. Chen, R. Chai, C. Xing, H. Li and X. B. Yin, Nanoscale, 2016, 8, 13414–13421 RSC.
- F. Qu, H. Pei, R. Kong, S. Zhu and L. Xia, Talanta, 2017, 165, 136–142 CrossRef CAS PubMed.
- X. Yan, Y. Song, C. Zhu, H. Li, D. Du, X. Su and Y. Lin, Anal. Chem., 2018, 90, 2618–2624 CrossRef CAS PubMed.
- L. Han, S. G. Liu, X. F. Zhang, B. X. Tao, N. B. Li and H. Q. Luo, Sens. Actuators, B, 2018, 258, 25–31 CrossRef CAS.
- D. Garg, A. Mehta, A. Mishra and S. Basu, Spectrochim. Acta, Part A, 2018, 192, 411–419 CrossRef CAS PubMed.
- D. He, X. Yang, X. He, K. Wang, X. Yang, X. He and Z. Zou, Chem. Commun., 2015, 51, 14764–14767 RSC.
- Y. Wang, K. Jiang, J. Zhu, L. Zhang and H. Lin, Chem. Commun., 2015, 51, 12748–12751 RSC.
- G. Li, N. Lv, J. Zhang and J. Ni, RSC Adv., 2017, 7, 16423–16427 RSC.
- X. Yan, Y. Song, C. Zhu, J. Song, D. Du, X. Su and Y. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 21990–21996 CrossRef CAS PubMed.
- J. Deng, D. Lu, X. Zhang, G. Shi and T. Zhou, Environ. Pollut., 2017, 224, 436–444 CrossRef CAS PubMed.
- J. Chen, Z. Huang, H. Meng, L. Zhang, D. Ji, J. Liu, F. Yu, L. Qu and Z. Li, Sens. Actuators, B, 2018, 260, 770–777 CrossRef CAS.
- Z. Liu, X. Cai, X. Lin, Y. Zheng, Y. Wu, P. Chen, S. Weng, L. Lin and X. Lin, Anal. Methods, 2016, 8, 2366–2374 RSC.
- D. Fan, C. Shang, W. Gu, E. Wang and S. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 25870–25877 CrossRef CAS PubMed.
- Z. Z. Dong, L. Lu, C. N. Ko, C. Yang, S. Li, M. Y. Lee, C. H. Leung and D. L. Ma, Nanoscale, 2017, 9, 4677–4682 RSC.
- X. He, X. Yang, L. Hai, D. He, X. He, K. Wang and X. Yang, RSC Adv., 2016, 6, 79204–79208 RSC.
- Y. Zhang, C. Zhang, J. Chen, Y. Li, M. Yang, H. Zhou, S. A. Shahzad, H. Qi, C. Yu and S. Jiang, J. Mater. Chem. B, 2017, 5, 4691–4694 CAS.
- J. Sheng, X. Jiang, L. Wang, M. Yang and Y. N. Liu, Anal. Chem., 2018, 90, 2926–2932 CrossRef CAS PubMed.
- S. Lin, H. Cheng, Q. Ouyang and H. Wei, Anal. Methods, 2016, 8, 3935–3940 RSC.
- T. Xiao, J. Sun, J. Zhao, S. Wang, G. Liu and X. Yang, ACS Appl. Mater. Interfaces, 2018, 10, 6560–6569 CrossRef CAS PubMed.
- H. M. Meng, Z. Jin, Y. Lv, C. Yang, X. B. Zhang, W. Tan and R. Q. Yu, Anal. Chem., 2014, 86, 12321–12326 CrossRef CAS PubMed.
- N. Li, W. Diao, Y. Han, W. Pan, T. Zhang and B. Tang, Chem. – Eur. J., 2014, 20, 16488–16491 CrossRef CAS PubMed.
- X. Wang, D. Wang, Y. Guo, C. Yang, X. Liu, A. Iqbal, W. Liu, W. Qin, D. Yan and H. Guo, Biosens. Bioelectron., 2016, 77, 299–305 CrossRef CAS PubMed.
- K. Radhakrishnan, P. Panneerselvam and A. Ravikumar, RSC Adv., 2017, 7, 45824–45833 RSC.
- Y. Yuan, R. Li and Z. Liu, Anal. Chem., 2014, 86, 3610–3615 CrossRef CAS PubMed.
- Y. Yuan, S. Wu, F. Shu and Z. Liu, Chem. Commun., 2014, 50, 1095–1097 RSC.
- L. Qi, Z. Yan, Y. Huo, X. M. Hai and Z. Q. Zhang, Biosens. Bioelectron., 2017, 87, 566–571 CrossRef CAS PubMed.
- K. Yang, M. Zeng, X. Hu, B. Guo and J. Zhou, Analyst, 2014, 139, 4445–4448 RSC.
- J. Li, B. Du, Y. Li, Y. Wang, D. Wu and Q. Wei, New J. Chem., 2018, 42, 1228–1234 RSC.
- J. Li, D. Li, R. Yuan and Y. Xiang, ACS Appl. Mater. Interfaces, 2017, 9, 5717–5724 CrossRef CAS PubMed.
- Q. Chen, L. Zhang, F. Jiang, B. Wang, T. Lv, Z. Zeng, W. Wu and S. Sun, Sens. Actuators, B, 2017, 244, 1138–1144 CrossRef CAS.
- M. Ou, J. Huang, X. Yang, K. Quan, Y. Yang, N. Xie and K. Wang, Chem. Sci., 2017, 8, 668–673 RSC.
- T. Jiang, Y. Song, D. Du, X. Liu and Y. Lin, ACS Sens., 2016, 1, 717–724 CrossRef CAS.
- Z.-M. Huang, Q.-Y. Cai, D.-C. Ding, J. Ge, Y.-L. Hu, J. Yang, L. Zhang and Z.-H. Li, Sens. Actuators, B, 2017, 242, 355–361 CrossRef CAS.
- J. Pal and T. Pal, RSC Adv., 2016, 6, 83738–83747 RSC.
- L. He, F. Wang, Y. Chen and Y. Liu, Luminescence, 2018, 33, 145–152 CrossRef CAS PubMed.
- H. Yang, Y. Xiong, P. Zhang, L. Su and F. Ye, Anal. Methods, 2015, 7, 4596–4601 RSC.
- X. Yan, Y. Song, X. Wu, C. Zhu, X. Su, D. Du and Y. Lin, Nanoscale, 2017, 9, 2317–2323 RSC.
- H. Ouyang, Q. Lu, W. Wang, Y. Song, X. Tu, C. Zhu, J. N. Smith, D. Du, Z. Fu and Y. Lin, Anal. Chem., 2018, 90, 5147–5152 CrossRef CAS PubMed.
- Y. He, Y. Chai, H. Wang, L. Bai and R. Yuan, RSC Adv., 2014, 4, 56756–56761 RSC.
- H. Begum, M. S. Ahmed and S. Jeon, RSC Adv., 2016, 6, 50572–50580 RSC.
- S. He, B. Zhang, M. Liu and W. Chen, RSC Adv., 2014, 4, 49315–49323 RSC.
- W. Xu, S. Xue, H. Yi, P. Jing, Y. Chai and R. Yuan, Chem. Commun., 2015, 51, 1472–1474 RSC.
- X. Feng, Y. Zhang, J. Song, N. Chen, J. Zhou, Z. Huang, Y. Ma, L. Zhang and L. Wang, Electroanalysis, 2015, 27, 353–359 CrossRef CAS.
- Y. Shu, J. Xu, J. Chen, Q. Xu, X. Xiao, D. Jin, H. Pang and X. Hu, Sens. Actuators, B, 2017, 252, 72–78 CrossRef CAS.
- L. Duan, L. Tong, Y. Xu and L. Sun, Energy Environ. Sci., 2011, 4, 3296–3313 RSC.
- H. Yang, Q. Ming, Z. Tao, M. Ji, L. Shaohua, Z. Xiaodong, Y. Chris and F. Xinliang, Adv. Mater., 2017, 29, 1604480 CrossRef PubMed.
- E. Gil-Santos, C. Baker, A. Lemaitre, C. Gomez, G. Leo and I. Favero, Nat. Commun., 2017, 8, 14267 CrossRef CAS PubMed.
- N. Sakai, Y. Ebina, K. Takada and T. Sasaki, J. Phys. Chem. B, 2005, 109, 9651–9655 CrossRef CAS PubMed.
- Y. Lin, Q. Zhou, D. Tang, R. Niessner and D. Knopp, Anal. Chem., 2017, 89, 5637–5645 CrossRef CAS PubMed.
- J. Li, F. Cheng, H. Huang, L. Li and J.-J. Zhu, Chem. Soc. Rev., 2015, 44, 7855–7880 RSC.
- K. Taekhoon, C. Eun-Jin, C. Youngjoo, K. Minsik, O. Aram, J. Juhong, L. Eun-Sook, B. Hionsuck, H. Seungjoo, S. Jin-Suck, H. Yong-Min and L. Kwangyeol, Angew. Chem., 2011, 123, 10777–10781 CrossRef.
- Y. Chen, D. Ye, M. Wu, H. Chen, L. Zhang, J. Shi and L. Wang, Adv. Mater., 2014, 26, 7019–7026 CrossRef CAS PubMed.
- Z. Zhao, H. Fan, G. Zhou, H. Bai, H. Liang, R. Wang, X. Zhang and W. Tan, J. Am. Chem. Soc., 2014, 136, 11220–11223 CrossRef CAS PubMed.
- P. Zhao, Y. Zhu, X. Yang, J. Shen, X. Jiang, J. Zong and C. Li, Dalton Trans., 2014, 43, 451–457 RSC.
- M. Zhang, L. Xing, H. Ke, Y. J. He, P. F. Cui, Y. Zhu, G. Jiang, J. B. Qiao, N. Lu, H. Chen and H. L. Jiang, ACS Appl. Mater. Interfaces, 2017, 9, 11337–11344 CrossRef CAS PubMed.
- M. Song, T. Liu, C. Shi, X. Zhang and X. Chen, ACS Nano, 2016, 10, 633–647 CrossRef CAS PubMed.
- J. Liu, Q. Chen, W. Zhu, X. Yi, Y. Yang, Z. L. Dong and Z. Liu, Adv. Funct. Mater., 2017, 27, 1605926 CrossRef.
- Q. Chen, L. Feng, J. Liu, W. Zhu, Z. Dong, Y. Wu and Z. Liu, Adv. Mater., 2016, 28, 7129–7136 CrossRef CAS PubMed.
- G. Yang, L. Xu, J. Xu, R. Zhang, G. Song, Y. Chao, L. Feng, F. Han, Z. Dong, B. Li and Z. Liu, Nano Lett., 2018, 18, 2475–2484 CrossRef CAS PubMed.
- H. Fan, G. Yan, Z. Zhao, X. Hu, W. Zhang, H. Liu, X. Fu, T. Fu, X. B. Zhang and W. Tan, Angew. Chem., 2016, 55, 5477–5482 CrossRef CAS PubMed.
- Z. Liu, S. Zhang, H. Lin, M. Zhao, H. Yao, L. Zhang, W. Peng and Y. Chen, Biomaterials, 2018, 155, 54–63 CrossRef CAS PubMed.
- H. Fan, Z. Zhao, G. Yan, X. Zhang, C. Yang, H. Meng, Z. Chen, H. Liu and W. Tan, Angew. Chem., 2015, 54, 4801–4805 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |






