Flow stabilizer on a syringe tip for hand-powered microfluidic sample injection†
Nan
Xiang
 *,
Yu
Han
,
Yuan
Jia
,
Zhiguo
Shi
,
Hong
Yi
and
Zhonghua
Ni
*,
Yu
Han
,
Yuan
Jia
,
Zhiguo
Shi
,
Hong
Yi
and
Zhonghua
Ni
 *
*
School of Mechanical Engineering and Jiangsu Key Laboratory for Design and Manufacture of Micro-Nano Biomedical Instruments, Southeast University, Nanjing, 211189, China. E-mail: nan.xiang@seu.edu.cn; nzh2003@seu.edu.cn
First published on 4th December 2018
Abstract
Precise, portable, low-cost sample injection is strongly demanded for use in point-of-care testing devices in resource-poor settings; however, current microfluidic sample injection techniques are often expensive, bulky and electricity-powered. To address this issue, we propose a novel syringe flow-stabilizer for hand-powered, precise, continuous-flow microfluidic sample injection. Our syringe flow-stabilizer applies the principle of passive flow-resistance compensation to stabilize the unstable sample flow and has the special advantages of easy-to-use, simple structure, low cost and high stability. The flow stabilizing performance of the stabilizer is characterized via a series of experiments and the results show that our stabilizer is capable of outputting a constant flow rate up to several milliliters per minute under a low threshold pressure. Finally, the fabricated syringe flow-stabilizer is integrated with an inertial microfluidic cell concentrator for high-throughput continuous concentration of trace blood cells from large-volume biofluids. The use of our stabilizer makes the concentration performance totally independent of operation. We envision wide applications of our syringe flow-stabilizer as a hand-powered sample injection unit in various point-of-care testing devices in resource-poor settings.
Introduction
The advent of microfluidics has provided important tools for point-of-care testing (POCT) as it offers various advantages such as small footprint, low cost, easy to use, and large-scale integration.1,2 In the past two decades, researchers from different disciplines have made continuous efforts towards all aspects of microfluidics research. Up to now, various microfluidic devices have been successfully invented for on-chip sample pretreatment (e.g., rapid mixing,3 rare cell capturing or sorting,4,5 and so on) and target sample detection (e.g., microflow cytometry6,7). As compared with the sample pretreatment or detection work, relatively less attention has been paid to microfluidic sample injection. At present, syringe pumps are still the most frequently employed equipment for sample injection. Although an accurate driving flow rate can be provided, commercial syringe pumps are expensive, bulky and electricity-powered, which prevent the wide application of these syringe pumps for POCT in resource-poor settings.To address the above issue, various micropumps have been developed for driving the sample flow, which enables the integration of microfluidic sample injection on a chip.8,9 According to the working principle, the reported micropumps can be divided into two categories: the passive and active ones. The passive fluid pumping techniques mainly employed the hydrodynamic phenomena (e.g., capillary force10,11 and surface energy gradient12) to drive the flow. However, these methods have very low pumping throughput and are commonly used for simple quasi-static diagnosis (for example, the capillary force is widely employed for paper microfluidics13). In contrast, the active micropumps utilize the electroosmotic,14 piezoelectric,15 magnetic16 and acoustic streaming17 effects to drive the sample at a controllable flow rate. Although the microactuators (e.g., microelectrodes and interdigital transducers) for inducing these effects can be easily integrated on microfluidic chips, the external units (e.g., digital signal generators and magnets) are still required and difficult to miniaturize. For the commercially-available products from Darwin Microfluidics, the elasticity of the low-cost Tygon tubing is employed to smoothen the small flow oscillations generated by the syringe pump. However, it is impossible to employ these products for stabilizing flows with large fluctuations.
In addition to these methods, the human power may be the ideal and best choice power source for driving the sample flow for point-of-care diagnostics in resource-limited environments. For example, some researchers developed a finger-powered pump in which a polydimethylsiloxane (PDMS) deformable chamber was activated by a human finger to infuse the sample out of the chamber to the target microchannel.18 However, the flow rate generated by finger pressing is highly unstable and the provided pumping throughput is very limited due to the batch working mode. Another interesting example is a hand-powered microfluidic pipette tip which has been successfully employed for the separation of blood plasma or other cell components.19,20 The key technique for enabling hand-powered application is the flow-rate insensitive particle ordering based on hydrophoresis. In later work, a special pipette containing buffer air chambers was developed to maintain the pressure drop during the pushing procedure.21,22 However, the throughput of the microfluidic pipette tip is still not high enough for processing real biological samples with volumes up to several milliliters as hydrophoresis works well at relatively low flow rates. In our previous studies,23,24 we have developed an elastic membrane-based flow regulator for stabilizing gas or liquid flows. However, these flow regulators have a complex structure of five functional layers and require a time-consuming expensive clean-room fabrication process and a precise microscale multi-layer alignment, which prevent the low-cost and disposable applications of these devices in resource-poor settings. In addition, these flow regulators could not work under high hand-powered pressures due to the failure of the flow stabilizing effect and the breakdown of the multilayer PDMS structure.
Herein, we propose a novel syringe flow-stabilizer which can be directly mounted onto the syringe tip to stabilize the sample flow rate generated by hand-powered syringe pushing. We first demonstrate the structure design, the working principle and the prototyping of our syringe flow-stabilizer. Then, the flow stabilizing performance of the stabilizer is explored to verify the conceptual design and understand the flow stabilizing mechanisms. Finally, our syringe flow-stabilizer is integrated with an inertial microfluidic cell concentrator for high-throughput and continuous concentration of trace blood cells from large-volume biofluids under the hand-powered operation. Our syringe flow-stabilizer has a very simple structure and is able to output a stable flow rate up to the level of several milliliters per minute, providing an important sample injection tool for POCT applications in resource-poor settings.
Conceptual design and device fabrication
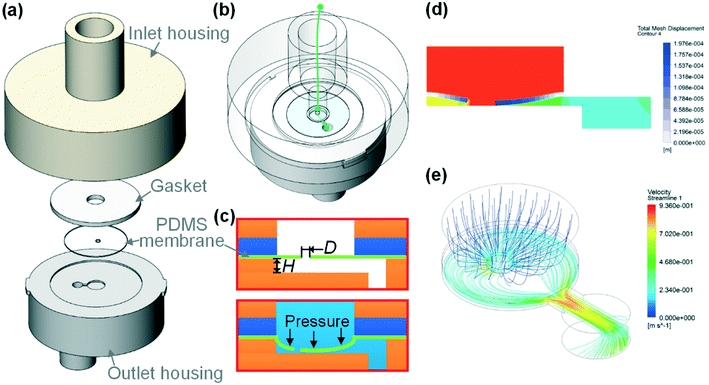
Fig. 1(a) illustrates the structure of our syringe flow-stabilizer which consists of an inlet housing, a silicone gasket, a PDMS elastic membrane and an outlet housing. The above four parts are coaxially stacked in the vertical direction. Then, the inlet housing and the outlet housing are tightly fastened using a bayonet joint. The inlet was designed to be in a female luer lock format, which enables easy connection of the syringe flow-stabilizer with various commercial syringes for hand-powered sample injection. The designed male luer lock outlet on the lower surface of the outlet housing can be connected with downstream microfluidic devices. More detailed CAD drawings of each part are provided in Fig. S1(a and b).† After assembling the four parts, a clear flow path could be formed and the sample fluid injected from the inlet would flow through the hole in the PDMS elastic membrane, then flow along the grooves on the upper surface of the outlet housing, and finally be exported from the outlet (see Fig. 1(b)). As a part of the PDMS elastic membrane is suspended over the centered groove (we name this groove as the deformation cavity), the positive flow pressure would deform the suspended membrane towards the deformation cavity, as illustrated in Fig. 1(c). The deformation degree of the membrane is dependent on the flow pressure applied at the inlet.To demonstrate the working principle of our syringe flow-stabilizer, the nonlinear model of fluid–structure interaction (FSI) was computed. Fig. 1(d and e) and S2(c and d)† illustrate the numerical simulation results of the membrane deformation and the flow velocity in the deformation cavity when a positive flow pressure is applied at the inlet. It is found that the suspended membrane (especially the central region) would deform towards or even be partly in contact with the bottom of the deformation cavity under the positive pressure forces, which results in the increase of the flow resistance (R) of the flow path. Under a constant pressure (P), the output flow rate (Q) can be calculated as Q = P/R. For devices with constant flow resistances, the output flow rate would linearly increase with increasing pressure. In order to output a constant flow rate, an elastic membrane was employed in our syringe flow-stabilizer to dynamically adjust the flow resistance according to the applied pressure (i.e., high flow resistance for high applied inlet pressure and low flow resistance for low inlet pressure). As a pressure increment (ΔP) is generated, the increase in flow resistance (ΔR) caused by the membrane deformation could compensate the pressure increment. Therefore, the output flow rate is possible to maintain a constant value 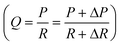 when the pressure (P) applied at the inlet is larger than the threshold pressure. On the basis of this principle, our syringe flow-stabilizer is able to output a constant flow rate (Qc) under the unstable pressures generated by single-handedly pushing the syringe. The only requirement for operators is to push the syringe to generate a driving pressure above the threshold value, which can be easily provided by different male or female operators. Our syringe flow-stabilizer can further be applied for stabilizing the negative-pressure flow when a deformation cavity is designed on the gasket or inlet housing.
when the pressure (P) applied at the inlet is larger than the threshold pressure. On the basis of this principle, our syringe flow-stabilizer is able to output a constant flow rate (Qc) under the unstable pressures generated by single-handedly pushing the syringe. The only requirement for operators is to push the syringe to generate a driving pressure above the threshold value, which can be easily provided by different male or female operators. Our syringe flow-stabilizer can further be applied for stabilizing the negative-pressure flow when a deformation cavity is designed on the gasket or inlet housing.
Fig. S1(c–f)† illustrate the photographs of the fabricated parts and the final assembled prototype of our syringe flow-stabilizer. As a proof-of-concept demonstration, the inlet and outlet housings employed in this work were respectively fabricated in photocurable resins using stereolithography (SLA) three dimensional (3D) printing. As the structures on the housings are relatively large, no advanced clean-room microfabrication is required. In the future, these parts can be massively manufactured in medical plastics using the well-established injection moulding. The actuating component, the elastic membrane, was prepared via spin coating the liquid PDMS over a polyethylene terephthalate (PET) film or directly purchased from the market, and the hole in the central region was cut using a UV laser system. Then, the shaped PDMS membrane was transferred onto the upper surface of the outlet housing and compacted with the silicone gasket and the inlet housing. The silicone gasket directly purchased from the market was employed to enhance the sealing effect. All these parts can be quickly assembled without a precise alignment step. The finished syringe flow-stabilizer has a very small size of Φ20 mm diameter and is 23 mm tall. The special advantages of the simple structure and easy fabrication make our flow stabilizer well suitable for low-cost and disposable applications in resource-poor settings.
Performance characterization
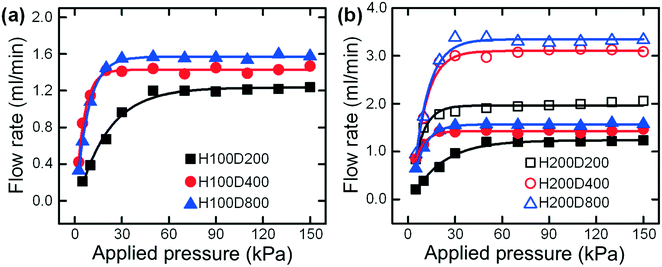
We first set up a gas-driven flow system (see Fig. S3†) to characterize the performance of our syringe flow-stabilizer. Instead of manually pushing the syringe, the compressed air with a controlled stable pressure was employed to pump the sample fluid. The flow rates output by our syringe flow-stabilizer were measured and recorded. In this experiment, the pressures applied to drive the sample flow were determined to be in the range of 2.5–150 kPa according to the empirical pressure values generated by single-handedly pushing the syringe (63–150 kPa measured from different adult operators (N > 5)). On the basis of our previous knowledge,23,24 the hole diameter in the PDMS membrane (D) and the height of the deformation cavity (H) are the two most critical design parameters that would significantly affect the flow stabilizing performance of our syringe flow-stabilizer. Fig. 2(a) illustrates the output flow rates of the syringe flow-stabilizers with different hole diameters (D = 200 μm, 400 μm and 800 μm) when the applied pressure is increased from 2.5 kPa to 150 kPa. It is found that the output flow rates of all these three flow stabilizers would first gradually increase at low applied pressures and then become stable at specific values (i.e., constant flow rate, Qc) when the applied pressures exceed the threshold values. The threshold pressure for device H100D200 (H = 100 μm; D = 200 μm) is estimated to be 50 kPa which is much larger than those of devices with large membrane holes (30 kPa for devices H100D400 and H100D800). The low threshold pressure would benefit the lowering of the power consumption for operating our syringe flow-stabilizer. In addition, a high constant flow rate can be provided by the device with a large membrane hole due to the low initial flow resistance.To explore the effect of the deformation cavity height (H) on the output flow rate, another set of three devices with a cavity height of 200 μm were fabricated. Fig. 2(b) illustrates the output flow rates of these two sets of syringe flow-stabilizers at different applied pressures from 2.5 kPa to 150 kPa. It is found that the devices with a deep deformation cavity of 200 μm show similar flow stabilizing behaviors with increasing pressure, but achieve much higher constant flow rates as compared with the devices with a cavity height of 100 μm. Specifically, the device H200D200 outputs a higher constant flow rate than the device H100D800 which has the highest constant flow rate in the first set of devices with a cavity height of 100 μm. Therefore, it can be concluded that the increase of deformation cavity height has a much significant effect for increasing the output constant flow rate. The quantitative data (i.e., constant flow rates and threshold pressures) for all these two sets of devices are listed in Table 1. As can be seen from this table, the tested syringe flow-stabilizers could output constant flow rates ranging from 1.214 ± 0.022 ml min−1 to 3.325 ± 0.162 ml min−1. At these constant flow rates, the injection of 10 ml liquid samples could be completed within 3–8 min, which is affordable for hand-powered operation. The deviations of the output constant flow rates were found to be smaller than 6% and, a very small flow-rate deviation of 1.81% could be easily achieved for device H100D200. In addition, our flow stabilizer has a better stability for working under the high pressures (e.g., 50–100 kPa) than the previously-reported five-layer PDMS flow regulator24 which may become unstable due to the failure of the flow stabilizing effect at pressures larger than 50 kPa (see Fig. S4†). As the operators may push the syringe at a pressure much larger than the threshold value, the flow stabilizing function at high pressures is very important for hand-powered applications.
| Type | Q c (ml min−1) | Deviation (%) | Threshold pressure (kPa) |
|---|---|---|---|
| H100D200 | 1.214 ± 0.022 | 1.81 | 50 |
| H100D400 | 1.424 ± 0.051 | 3.58 | 30 |
| H100D800 | 1.564 ± 0.035 | 2.24 | 30 |
| H200D200 | 2.023 ± 0.114 | 5.63 | 50 |
| H200D400 | 3.066 ± 0.142 | 4.63 | 30 |
| H200D800 | 3.325 ± 0.162 | 4.87 | 30 |
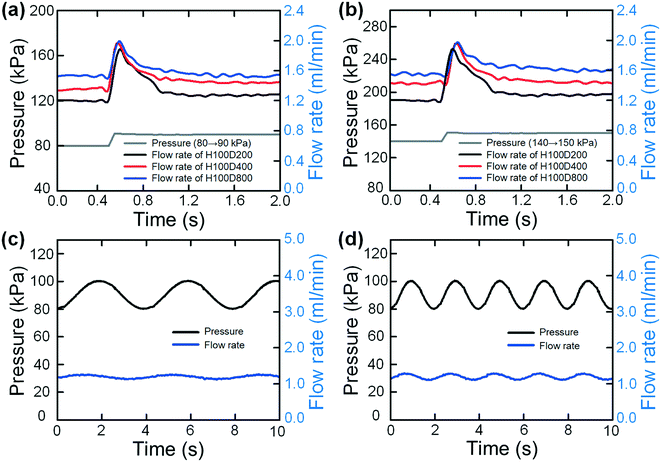
Under the practical conditions of single-handed syringe pushing, a sudden or continuous change of pressure may occur due to the incorrect operations. To explore the flow stabilizing performances under these conditions, we characterized the output flow rates under sudden pressure changes. The pressure-change causes were deliberately selected to be more extreme than the real conditions in order to fully test our flow stabilizer. Fig. 3(a and b) show the output flow rates of the three tested devices (H100D200, H100D400 and H100D800) when the applied pressures are suddenly changed from 80 kPa to 90 kPa and from 140 kPa to 150 kPa, respectively (the applied pressures are all above the threshold values). From these two figures, it is interesting to find that a sudden change of the output flow rates could be clearly observed at the time of pressure increase. After that, the flow rates would quickly recover to the original values due to the flow stabilizing effect of our syringe flow-stabilizer. The whole increasing and re-stabilizing process could be completed within a very short time of less than 0.5 s, which may not affect the performances of most downstream continuous-flow microfluidic applications. For example, the below-mentioned cell concentration application takes about 500 s for processing a 10 ml sample in the hand-powered mode. We have verified that this type of short-time spike in the flow rate would not affect the statistical cell concentration performance (see data below). Instead, without our flow stabilizer, a larger increase in the flow rate will be generated as there is no stabilizing effect to prevent the flow rate from increasing, and finally the flow rate will not recover to the original value.
We also explored the flow stabilizing performance of our syringe flow-stabilizer under dynamically varied pressures. In this experiment, the pressures were set to be in periodic oscillation (sinusoidal wave) forms and the device H100D200 was fabricated and tested. Fig. 3(c and d) show the output flow rates under sinusoidal wave pressures with an amplitude of 20 kPa (changing from 80 kPa to 100 kPa) and two frequencies of 0.25 s−1 and 0.5 s−1. It is interesting to find that our syringe flow-stabilizer could output relatively stable flow rates under the dynamically varied pressures. The flow-rate deviation under the pressure frequency of 0.25 s−1 is 5.84% while the deviation under the pressure frequency of 0.5 s−1 is 7.53%. Even under an extremely large amplitude of 90 kPa (changing from 60 kPa to 150 kPa), the deviation of the output flow rates is about 22% (see Fig. S5†).
Application
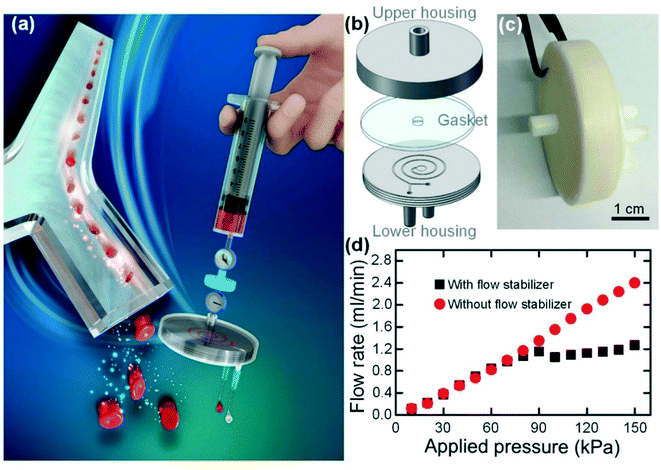
After characterizing the flow stabilizing performances, we further integrated our syringe flow-stabilizer with a “syringe filter” like an inertial microfluidic cell concentrator for high throughput and continuous concentration of blood cells, as illustrated in Fig. 4(a). The concentration of trace blood cells in large-volume biofluid samples (e.g., urine and pleural effusion) would provide an important pretreatment scheme for the early diagnosis of various diseases (e.g., hematuria) in resource-poor settings.25,26 In this integrated device, our syringe flow-stabilizer was employed to stabilize the unstable sample flow generated by manually pushing the syringe. The outlet of the syringe flow-stabilizer was directly inserted into the inlet of the inertial microfluidic cell concentrator using the male/female luer lock connection. Our syringe flow-stabilizer could also be connected with any other devices (e.g., the most popular PDMS microfluidic devices) using commercial tubing and fittings (see Fig. S6(a)† as an example). In this study, we aim to develop a commercial-ready device that can be really used in resource-poor settings, just like the well-known syringe filter for solution filtration in laboratories.When the sample flow is stabilized to be at a specific flow rate, the downstream inertial microfluidic cell concentrator could realize the cell concentration through focusing the cells and removing the blank fluid. As illustrated in Fig. 4(b), the inertial microfluidic cell concentrator consists of three parts, including an upper housing, a circular gasket and a lower housing, and could be quickly assembled via screwing the two housings. As a proof-of-concept demonstration, the upper housing and the lower housing with a semi-open spiral channel were respectively fabricated in photocurable resins using the 3D printing technique. After assembly, the spiral channel was sealed with the silicone gasket and acted as the key structure unit for cell concentration on the basis of inertial focusing. The photographs of the assembled concentrator and the separate housings are illustrated in Fig. 4(c) and S6(b).† The 3D printed channel shows an acceptable cross-section quality, as illustrated in Fig. S6(d).† On the basis of previous experience on inertial microfluidics,27–29 we designed a 3-loop spiral channel with cross-sectional dimensions of 65 μm (HS) × 500 μm (WS). In this channel, the blood cells well satisfy the inertial focusing criterion30 (ap/HS ≥ 0.07, where ap is the particle diameter) and thus could be focused into a cell train. As the particles could be well focused into a narrow particle train near the inner wall, the increase in particle concentration can be easily achieved through removing the cell-free fluid near the outer channel region.
Fig. 4(d) shows the output flow rates of the inertial microfluidic cell concentrator integrated with or without a flow stabilizer (H100D200) at various applied pressures. It is found that the output flow rate of the inertial microfluidic cell concentrator would increase linearly with increasing applied pressure due to the constant flow resistance. In contrast, the integrated device would output a constant flow rate of 1.138 ml min−1 (a flow-rate deviation of 7.9%) when the applied pressure is larger than 80 kPa. In addition, we also examined the flow stabilizing performance of the integrated device under the varied pressures. From the experimental results in Fig. S7,† it is verified that the integration of our syringe flow-stabilizer could effectively reduce the effect of pressure variation on the output flow rate. As the only requirement for inertial focusing is to provide a stable flow rate with a specific value, the integration of our syringe flow-stabilizer makes the performance of the downstream inertial microfluidic cell concentrator totally independent of hand-powered operation.
Before performing the hand-powered cell concentration, we first tested the concentration performances of our 3D printed inertial microfluidic cell concentrator under different flow rates to determine the optimal operating flow rate. The driving flow rate of the diluted blood sample (with a concentration of ∼106 counts per ml) in this experiment was provided by the syringe pump and was controlled to be in the range of 0.6–2.0 ml min−1. To evaluate the concentration performance, an important dimensionless parameter, the cell recovery efficiency (RE = ninner/ntotal), was defined by dividing the cell number (ninner) in the sample collected from the inner outlet by the total cell number (ntotal). Fig. S8† illustrates the REs at different flow rates. It is clearly observed that our 3D printed cell concentrator achieves the best cell recovery efficiency at a flow rate of 1.2 ml min−1. To ensure the optimal concentration performance (i.e., the lowest cell loss), the flow stabilizer H100D200 was selected and employed, which provided a constant flow rate of 1.138 ml min−1 after integrating with the cell concentrator.
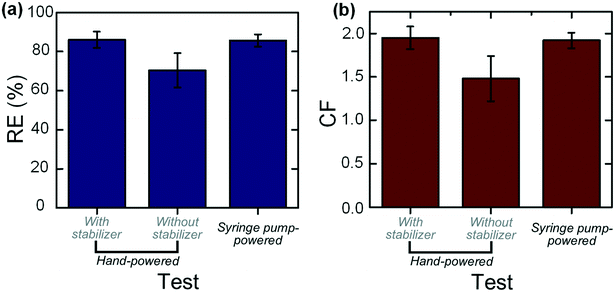
We then applied the integrated device for the hand-powered concentration of trace blood cells from large-volume samples. Blood samples with an initial concentration of 9.48 × 106 counts per ml were prepared and equally filled into several 10 ml syringes. More than three unbiased volunteer operators were asked to push the syringe to drive the blood samples into the cell concentrators integrated with or without the flow stabilizer. These operators did not receive any pre-training or operating instruction. The liquid collected from the inner and outer outlets was respectively sampled and counted. We also defined another dimensionless parameter, the concentration factor (CF = cinner/cinitial, where c is the particle concentration), to quantitatively evaluate the concentration increase of the target samples after processing. The experimental results are shown in Fig. 5. For the tests with the flow stabilizer, a RE of 86.07% (with a deviation of 4.18%) and a CF approaching 2.0 can be achieved, which are identical to those obtained in the syringe pump-powered mode (the driving flow rate was set at 1.2 ml min−1). Further increasing the CF can be realized through a serial concentration step. As a comparison, for tests without the flow stabilizer, the RE varies from 60–83.6% which is heavily different from person to person. The averages of the RE and CF for these tests are respectively 70.38% (with a large deviation of 8.79%) and 1.48 ± 0.26, which are much lower than the values (RE = 86.07% ± 4.18% and CF = 1.95 ± 0.13) from the tests with the stabilizer. For inexperienced operators, it is very difficult to stably push the syringe to output a specific flow rate (the optimal flow rate for cell concentration). For example, various incorrect operations (too fast or too slow injection but with an overall uniform pushing speed, first fast and then slow injection, first slow and then fast injection and so on) would seriously affect the output flow rate and result in the deterioration of concentration performances. However, as proved in the above experimental results, the integration of our flow stabilizer makes the concentration performance totally independent of incorrect operations.
Conclusion
In this work, we developed a novel syringe flow-stabilizer for precise, continuous-flow and low-cost microfluidic sample injection. The syringe flow-stabilizer was designed to be directly mounted onto the syringe tip and has a very simple four-part structure including an inlet housing, a silicone gasket, a PDMS elastic membrane and an outlet housing. The PDMS elastic membrane acts as the microactuator to adjust the flow resistance for compensating the pressure variation. On the basis of this working principle, our syringe flow-stabilizer could stabilize the unstable sample flow generated by hand pushing the syringe. Then, the flow stabilizing performances of our syringe flow-stabilizers were explored via a series of experiments to verify the conceptual design and understand the flow stabilizing mechanisms. The experimental results show that syringe flow-stabilizer is capable of outputting a constant flow rate up to the level of milliliters per minute with a flow-rate deviation smaller than 6% under a low threshold pressure of 30–50 kPa. Even under dynamically varied pressures, our syringe flow-stabilizer could still achieve a good flow stabilizing performance. Finally, we integrated our syringe flow-stabilizer with an inertial microfluidic cell concentrator for high-throughput continuous concentration of trace blood cells from large-volume biofluids under the hand-powered operation. We envision wide applications of our syringe flow-stabilizer as a hand-powered sample injection unit for point-of-care diagnostics devices in resource-poor settings.Experimental section
Experimental setup
The housings in the syringe flow-stabilizer and the inertial microfluidic cell concentrator were fabricated using a laser-based stereolithography (SLA) 3D printing system (3DSL-450S, DigitalManu) in high-resolution photocurable resin (SZUV-W8001). The hole and the circular shape of the silicone gaskets were cut using a UV laser machine (AWAVE 355-10 W-30 K, Advanced Optowave Corporation). To prepare the elastic membrane in the syringe flow-stabilizer, PDMS (Sylgard 184, Dow Corning) was employed due to its excellent elastomeric properties. The PDMS liquid was prepared by mixing the base and the curing agent at a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. After degassing in a vacuum environment, the PDMS liquid was spun onto the PET film to generate a membrane film of 60 μm thick and then cured at 100 °C for 100 min. Of course, the off-the-shelf PDMS membrane could also be purchased from the market. The hole at the center of the membrane film was cut using a UV laser machine, and the whole membrane film was then transferred onto the upper surface of the outlet housing. In the inertial microfluidic cell concentrator, the cross-sectional profile of the spiral channel on the upper surface of the lower housing was replicated using the method described in Fig. S6(c).†
1. After degassing in a vacuum environment, the PDMS liquid was spun onto the PET film to generate a membrane film of 60 μm thick and then cured at 100 °C for 100 min. Of course, the off-the-shelf PDMS membrane could also be purchased from the market. The hole at the center of the membrane film was cut using a UV laser machine, and the whole membrane film was then transferred onto the upper surface of the outlet housing. In the inertial microfluidic cell concentrator, the cross-sectional profile of the spiral channel on the upper surface of the lower housing was replicated using the method described in Fig. S6(c).†
To characterize the flow stabilizing performances of our syringe flow-stabilizers, a gas-driven flow system illustrated in Fig. S3† was set up. The compressed air was regulated via a computer-controlled pressure controller (OB1 Base MkIII, Elveflow) to generate a specific pressure for driving the sample out of the sealed sample reservoir. The sample was then flowed through our syringe flow-stabilizer and the output flow rate was measured using a flow sensor (MFS 5, Elveflow, a measurable flow rate range of 0–±5 ml min−1 and an accuracy up to 10 μL min−1). The values of pressures and flow rates were monitored and recorded via the software Elveflow Smart Interface. When the blood cell concentration application was performed, the driving pressure was provided by single-handedly pushing the 10 ml plastic syringe.
Sample preparation and analysis
Human whole blood was drawn from a healthy consenting volunteer and collected via a 4 ml vacutainer collection tube (BD Biosciences) containing anticoagulant K2EDTA. The study was approved by the institutional committee of the Institutional Ethical Committee (IEC) for Clinical Research of Zhongda Hospital (Southeast University) and informed consent was obtained from the volunteer. All experiments were performed in compliance with the Chinese laws and following the institutional guidelines. To ensure the viability of the blood cells, the blood sample was processed after collection. To mimic the microscopic hematuria sample, the blood sample was then diluted with 0.9% sodium chloride solution (Baxter) to a certain concentration. The concentrations of the initial samples and collected samples were observed under a microscope and then counted using a Countess® II FL Automated Cell Counter (Thermo Fisher Scientific).Numerical simulation
The numerical simulations of the membrane deformation and the flow velocity in the syringe flow-stabilizer were computed using the 3D fluid–structure interaction (FSI) model in ANSYS Workbench. In the FSI model, the PDMS membrane was selected as the linear elastic material. The solid stress–strain model for the membrane was set to be a Young's modulus of 2400 kPa and a Poisson's ratio of 0.49. In the fluid domain, water was assigned as the fluid material. The details on the FSI model and the finite-element method (FEM) mesh are provided in Fig. S2(a and b).†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research work is supported by the National Natural Science Foundation of China (51875103, 51505082, 81727801 and 51775111), the Natural Science Foundation of Jiangsu Province (BK20150606), the Fundamental Research Funds for the Central Universities (2242017K41031), the Six Talent Peaks Project of Jiangsu Province (SWYY-005) and the ZhiShan Young Scholar Fellowship.References
- P. Yager, T. Edwards, E. Fu, K. Helton, K. Nelson, M. R. Tam and B. H. Weigl, Nature, 2006, 442, 412 CrossRef CAS PubMed.
- X. Mao and T. J. Huang, Lab Chip, 2012, 12, 1412–1416 RSC.
- N.-T. Nguyen and Z. Wu, J. Micromech. Microeng., 2005, 15, R1 CrossRef.
- J. M. Jackson, M. A. Witek, J. W. Kamande and S. A. Soper, Chem. Soc. Rev., 2017, 46, 4245–4280 RSC.
- J. Mong and M.-H. Tan, Trends Biotechnol., 2018, 36, 511–522 CrossRef CAS PubMed.
- R.-J. Yang, L.-M. Fu and H.-H. Hou, Sens. Actuators, B, 2018, 266, 26–45 CrossRef CAS.
- W. Tang, D. Tang, Z. Ni, N. Xiang and H. Yi, Anal. Chem., 2017, 89, 3154–3161 CrossRef CAS PubMed.
- D. J. Laser and J. G. Santiago, J. Micromech. Microeng., 2004, 14, R35 CrossRef.
- Y.-N. Wang and L.-M. Fu, Microelectron. Eng., 2018, 195, 121–138 CrossRef CAS.
- Y.-X. Guan, Z.-R. Xu, J. Dai and Z.-L. Fang, Talanta, 2006, 68, 1384–1389 CrossRef CAS.
- R. Safavieh and D. Juncker, Lab Chip, 2013, 13, 4180–4189 RSC.
- G. M. Walker and D. J. Beebe, Lab Chip, 2002, 2, 131–134 RSC.
- D. M. Cate, J. A. Adkins, J. Mettakoonpitak and C. S. Henry, Anal. Chem., 2015, 87, 19–41 CrossRef CAS PubMed.
- K. Bengtsson and N. D. Robinson, Microfluid. Nanofluid., 2017, 21, 178 CrossRef.
- Z. Zhang, J. Kan, G. Cheng, H. Wang and Y. Jiang, Sens. Actuators, A, 2013, 203, 29–36 CrossRef CAS.
- S.-Y. Tang, X. Zhang, S. Sun, D. Yuan, Q. Zhao, S. Yan, L. Deng, G. Yun, J. Zhang, S. Zhang and W. Li, Adv. Funct. Mater., 2018, 28, 1705484 CrossRef.
- X. Y. Du, M. E. Swanwick, Y. Q. Fu, J. K. Luo, A. J. Flewitt, D. S. Lee, S. Maeng and W. I. Milne, J. Micromech. Microeng., 2009, 19, 035016 CrossRef.
- K. Iwai, K. C. Shih, X. Lin, T. A. Brubaker, R. D. Sochol and L. Lin, Lab Chip, 2014, 14, 3790–3799 RSC.
- S. Song, M. S. Kim and S. Choi, Small, 2014, 10, 4123–4129 CAS.
- S. Yan, S. H. Tan, Y. Li, S. Tang, A. J. T. Teo, J. Zhang, Q. Zhao, D. Yuan, R. Sluyter, N. T. Nguyen and W. Li, Microfluid. Nanofluid., 2017, 22, 8 CrossRef.
- B. Kim, S. Oh, D. You and S. Choi, Anal. Chem., 2017, 89, 1439–1444 CrossRef CAS PubMed.
- B. Kim, Y. K. Hahn, D. You, S. Oh and S. Choi, Analyst, 2016, 141, 5753–5758 RSC.
- X. Zhang, Z. Zhu, N. Xiang and Z. Ni, Biomicrofluidics, 2016, 10, 054123 CrossRef.
- X. Zhang, N. Xiang, W. Tang, D. Huang, X. Wang, H. Yi and Z. Ni, Lab Chip, 2015, 15, 3473–3480 RSC.
- R. A. Cohen and R. S. Brown, N. Engl. J. Med., 2003, 348, 2330–2338 CrossRef.
- J. M. Martel, K. C. Smith, M. Dlamini, K. Pletcher, J. Yang, M. Karabacak, D. A. Haber, R. Kapur and M. Toner, Sci. Rep., 2015, 5, 11300 CrossRef CAS.
- N. Xiang, X. Zhang, Q. Dai, J. Cheng, K. Chen and Z. Ni, Lab Chip, 2016, 16, 2626–2635 RSC.
- X. Zhang, Z. Zhu, N. Xiang, F. Long and Z. Ni, Anal. Chem., 2018, 90, 4212–4220 CrossRef CAS.
- N. Xiang, Z. Shi, W. Tang, D. Huang, X. Zhang and Z. Ni, RSC Adv., 2015, 5, 77264–77273 RSC.
- D. Di Carlo, D. Irimia, R. G. Tompkins and M. Toner, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 18892–18897 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8lc01051j |
| This journal is © The Royal Society of Chemistry 2019 |