Improved coalescence stability of monodisperse phospholipid-coated microbubbles formed by flow-focusing at elevated temperatures
Tim
Segers
 *,
Anne
Lassus
,
Philippe
Bussat
,
Emmanuel
Gaud
and
Peter
Frinking
*,
Anne
Lassus
,
Philippe
Bussat
,
Emmanuel
Gaud
and
Peter
Frinking
Bracco Suisse S.A., Route de la Galaise 31, 1228 Geneva, Switzerland. E-mail: t.j.segers@utwente.nl
First published on 28th November 2018
Abstract
Monodisperse phospholipid-coated ultrasound contrast agent (UCA) microbubbles can be directly synthesized in a lab-on-a-chip flow-focusing device. However, high total lipid concentrations are required to minimize on-chip bubble coalescence. Here, we characterize the coalescence probability and the long-term size stability of microbubbles formed using DPPC and DSPC based lipid mixtures as a function of temperature. We show that the coalescence probability can be dramatically reduced by increasing the temperature during bubble formation. Moreover, it is shown that the increased coalescence stability can be explained from an exponential increase of the relative viscosity in the thin liquid film between the colliding bubbles. Furthermore, it was found that the relative viscosity of a DPPC lipid mixture is 7.6 times higher than that of a DSPC mixture and that it can be explained solely from the higher DPPC liposome concentration. Regarding long-term bubble stability, the ratio of the initial on-chip bubble size to the final stable bubble size was always found to be 2.2 for DPPC and DSPC coated bubbles with 10 mol% DPPE-PEG5000, independent of the temperature. Moreover, it was demonstrated that the microbubble suspensions formed at elevated temperatures are highly stable over a time window of 2 to 4 days when collected in a vial. All in all, this work shows that, by increasing the temperature during bubble formation from room temperature to 70 °C, the efficiency of the use of phospholipids in microbubble formation by flow-focusing can be increased by 5 times.
1 Introduction
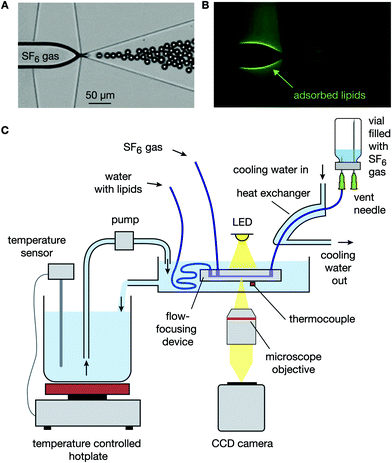
Ultrasound contrast agents (UCA) typically consist of a suspension of phospholipid-coated microbubbles with radii ranging from 0.5 to 5 μm. Upon exposure to ultrasound, the microbubbles oscillate thereby generating harmonic echoes that allow for the visualization and quantification of organ perfusion.1,2 The acoustic response of a microbubble is strongly dependent on the coupling between the ultrasound driving frequency and the microbubble resonance frequency, the latter being inversely proportional to the microbubble size.3 Clinical ultrasound scanners typically operate over a narrow frequency bandwidth, relative to the resonance frequencies of the microbubbles present in a typical UCA. Thus, only a small fraction of the UCA population contributes to the overall echo. Therefore, the sensitivity of contrast enhanced ultrasound imaging, and in particular that of molecular imaging,4 can be increased by 2 to 3 orders of magnitude through the use of monodisperse bubbles that are resonant to the driving ultrasound pulse.5 Moreover, the delivery of drugs and genetic material (e.g., mRNAs or siRNAs) to target cells using functionalized microbubbles and ultrasound6–10 is expected to be more efficient and more precise using a suspension of monodisperse bubbles due to its uniform acoustic response.11Monodisperse bubble suspensions can be obtained through decantation,12 mechanical filtration,13 and centrifugation14 of a polydisperse agent. Polydisperse bubbles can be sorted in microfluidic devices at a higher precision, e.g. microbubbles can be sorted to size in a pinched microchannel15 and they can be sorted to their acoustic response using the primary radiation force.16 Monodisperse microbubbles can also be produced directly using a lab-on-a-chip based microfluidic flow-focusing device. In a flow-focusing device a gas thread is focused between two liquid flows through an orifice where the gas thread destabilizes due to capillary instability and pinches off to release monodisperse bubbles, see Fig. 1A.17–22
The use of flow-focusing methods for the synthesis of stable monodisperse phospholipid-coated microbubbles is non-trivial.23–25 Freshly formed phospholipid-coated microbubbles formed by flow-focusing are inherently unstable, prone to Ostwald ripening, and they always decrease in size during their stabilization.26,27 The diffusive dissolution mechanically compresses the lipid monolayer, thereby increasing its surface pressure and decreasing the Laplace pressure-driven dissolution. Moreover, the mechanical compression results in a barrier for the gas molecules due to the closely packed lipid molecules. During the stabilization process, the total number of lipid molecules in the bubble shell remains unchanged.26 For bubbles formed at room temperature using a mixture of DPPC, DPPA, and DPPE-PEG5000, the ratio of the initial bubble radius Ri to the final stable bubble radius Rf increases with PEG molecular weight Mw, PEG molar fraction ϕPEG, and the propylene glycol mass fraction λPG as follows: RiRf−1 = 1.4 + α(1 + γλPG2)MwϕPEG where α = 1.6 × 10−3 mol g−1 and γ = 30.27 Thus, the bubble size decrease during stabilization originates from both, the primary lipids (factor of 1.4) and the PEGylated lipid. Primary phospholipids are thermodynamically active, i.e. they have a phase transition temperature at which the mobility of the lipids dramatically increases from a solid-like state to a liquid-like behavior.28 Furthermore, the conformation of a PEG chain in an aqueous medium is temperature dependent, i.e. it collapses at increased temperatures.29 However, the stabilization process of microbubbles coated by a mixture of primary and PEGylated phospholipids formed by flow-focusing as a function of the bubble formation temperature has never been studied.
A second complicating factor of flow-focusing methods for the synthesis of monodisperse phospholipid-coated microbubbles is microbubble coalescence in the outlet of the flow-focusing device, particularly at high production rates. Coalescence of the freshly formed microbubbles results in a lower bubble concentration and in a broader microbubble size distribution, both resulting in a lower amount of bubbles resonant to the ultrasound transmit frequency and thus, in a less efficient contrast agent. Moreover, coalescence of monodisperse bubbles with a uniform acoustic response11 results in a polydisperse bubble suspension with a nonuniform acoustic response and thereby in a contrast agent with a decreased potential for molecular- and deep tissue imaging.5 For all these reasons, coalescence should be minimized. At the high lipid concentrations required to minimize bubble coalescence, the efficiency of the phospholipid coating process is low. Typically, less than 0.1% of the total lipid concentration is adsorbed to the microbubble shell, while even at concentrations one order of magnitude lower, the gas–liquid interface is already fully saturated with lipids26,27 (see also Fig. 1B). Recently, it has been shown that the high concentration of free PEGylated liposomes inhibits coalescence through an effective increase of the relative viscosity Φr in the thin film between colliding bubbles.27 In the same paper, a universal power law for Φr has been developed: Φr = 1 + Kc∞2φPEG3Mw4η0−1, with c∞ the total lipid concentration, η0 the bulk viscosity, and K a constant. Moreover, it has been shown that this universal equation for Φr together with the coalescence model for microbubbles in the outlet of a flow-focusing device:27
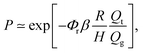 | (1) |
The increase of the relative viscosity Φr in the thin liquid film between colliding bubbles results from the mutual interaction between the PEGylated liposomes and between the liposomes and the PEGylated microbubble surface through surface- and colloidal forces.27,30 Rheology is typically temperature dependent.31 Moreover, surface- and colloidal forces, e.g. steric interactions and the structural force, are reported to increase with an increase in temperature.30,32 Therefore, Φr is expected to be temperature dependent thereby potentially allowing for an increased efficiency of the use of the phospholipids at temperatures exceeding room temperature. Here, microbubble coalescence in the outlet of a lab-on-a-chip flow-focusing device was characterized at temperatures ranging from 15 °C up to 70 °C in order to extend the universal equation for Φr with its temperature dependence. Furthermore, the effect of the bubble formation temperature on microbubble stabilization and long-term size- and concentration stability was measured for DPPC and DSPC based lipid mixtures.
2 Materials and methods
2.1 Phospholipid formulations
Two different phospholipid mixtures were studied. The first one consisted primarily of DPPC that was mixed with DPPA and DPPE-PEG5000 at a molar ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. The total lipid concentration was 2.0 mmol L−1 (2.5 mg mL−1). The second mixture consisted of DSPC mixed with DPPA and DPPE-PEG5000 also at a molar ratio of 8
1, respectively. The total lipid concentration was 2.0 mmol L−1 (2.5 mg mL−1). The second mixture consisted of DSPC mixed with DPPA and DPPE-PEG5000 also at a molar ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, and at a total lipid concentration of 3.9 mmol L−1 (5.0 mg mL−1). The concentration of the DSPC mixture was deliberately chosen twice that of the DPPC mixture since coalescence was more severe for the DSPC based mixture. The lipids were dispersed in phosphate buffered saline (PBS) and they were prepared exactly as described in detail by Segers et al.27 No co-solvents or viscosity increasing chemicals, e.g. propylene glycol and glycerol, were added as they are known to degrade the monodispersity and the stability of the final bubble suspension.27 After sonication of the lipid dispersion, a Zetasizer (Malvern, ZetaSizer, Nano ZSP) was used to measure the liposome size distributions; 10 μL of the concentrated lipid dispersion was diluted in 1 mL of PBS before the measurement.
1, respectively, and at a total lipid concentration of 3.9 mmol L−1 (5.0 mg mL−1). The concentration of the DSPC mixture was deliberately chosen twice that of the DPPC mixture since coalescence was more severe for the DSPC based mixture. The lipids were dispersed in phosphate buffered saline (PBS) and they were prepared exactly as described in detail by Segers et al.27 No co-solvents or viscosity increasing chemicals, e.g. propylene glycol and glycerol, were added as they are known to degrade the monodispersity and the stability of the final bubble suspension.27 After sonication of the lipid dispersion, a Zetasizer (Malvern, ZetaSizer, Nano ZSP) was used to measure the liposome size distributions; 10 μL of the concentrated lipid dispersion was diluted in 1 mL of PBS before the measurement.
2.2 Cooling of the formed microbubbles
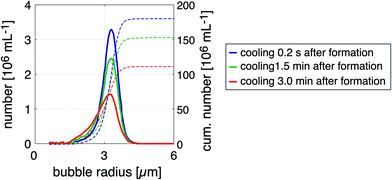
The outlet port of the flow-focusing device was connected to 0.5 mm inner diameter outlet tubing that was passed through 5 mm inner diameter silicone tubing over a length of 10 cm. Water at room temperature was pumped through the 5 mm silicone tubing to cool the freshly formed bubbles. The time between bubble formation and cooling was 200 ms during all experiments except for one additional experiment where the freshly formed bubbles, produced at 50 °C, were cooled down 200 ms, 1.5 min, and 3 min after their formation to investigate the importance of cooling. To this end, the length of the outlet tubing within the thermostatic bath was controlled such that the time between bubble formation and subsequent cooling was varied. The bubbles were collected for 5 min in a vial of which the headspace was filled with SF6 gas, see Fig. 1C. The size distributions of the bubble samples were measured using a coulter counter (Multisizer 3, 30 μm aperture tube, Beckman Coulter, Brea, CA, USA) 1 h after bubble production to allow the bubbles to stabilize to their final size.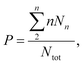 | (2) |
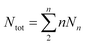 .
.
The gas flow-rate Qg was calculated as follows:
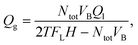 | (3) |
2.3 Long-term stability in the vial: DPPC versus DSPC coated bubbles
In the final experiment, the long-term stability of a microbubble suspension formed using the DPPC based lipid mixture and collected in a vial was characterized and compared to that of a bubble suspension formed using the DSPC based lipid mixture. The bubbles were produced at a temperature of 60 °C and at a liquid flow-rate of 35 μL min−1. The bubbles were collected for 15 min as before, in a vial with a SF6 gas headspace. The size distributions were measured 2 hours, 2 days, 4 days, and 7 days after bubble collection using a coulter counter.3 Results and discussion
3.1 Temperature dependent microbubble coalescence
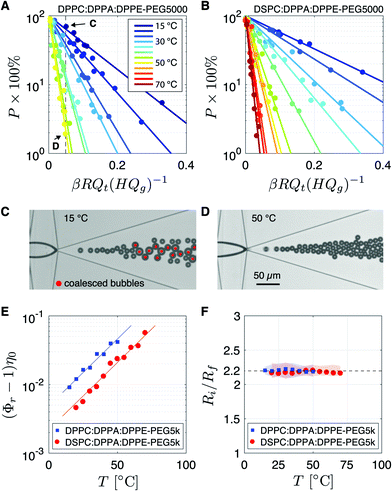
Fig. 2A shows the percentage of coalesced bubbles, formed using the DPPC based lipid mixture, as a function of the dimensionless parameter βRQt(HQg)−1 (see eqn (1)) for temperatures ranging from 15 °C up to 50 °C. For the DSPC based lipid mixture the percentage of coalesced bubbles is plotted in Fig. 2B for temperatures ranging from 20 °C up to 70 °C. Note that for both lipid formulations the coalescence probability of bubbles formed at the same dimensionless value βRQt(HQg)−1 decreases with increasing temperature. To illustrate the dramatic effect of temperature on the bubble coalescence probability, Fig. 2C and D show images of the expanding nozzle of the flow-focusing device for βRQt(HQg)−1 = 0.05 at temperatures of 15 °C and 50 °C, respectively. The percentage of coalesced bubbles at 15 °C (Fig. 2C) was 70%, while at 50 °C (Fig. 2D) it was only 2% (see data points indicated by C and D in Fig. 2A). Thus, the coalescence probability of phospholipid coated monodisperse microbubbles produced by flow-focusing can be dramatically reduced by increasing the temperature during bubble formation.The relative viscosity Φr was obtained by fitting an exponential function (y = exp(−ax) × 100%) to the data points per temperature (see solid lines in Fig. 2A and B). To investigate the contribution of the phospholipids to Φr, (Φr − 1)η0 is plotted in Fig. 2E as a function of temperature. The temperature dependence of η0 was calculated using η0(T) = A × 10B/(T–C), where A = 2.414 × 10−5 Pa s, B = 247.8 K, and C = 140 K.34 The data points collapse on two parallel exponential curves showing that (Φr − 1)η0 ∝ exp(kT), where k = 0.047 K−1. Note that, even though the total lipid concentration of the DSPC mixture was 2 times higher than that of the DPPC mixture, Φr of the DSPC mixture is 2 times lower than that of the DPPC mixture. Thus, since Φr ∝ c∞2,27 at an equal total lipid concentration and for a given temperature, Φr of the DPPC lipid mixture is 7.6 times higher than that of the DSPC lipid mixture.
To investigate the origin of the 7.6 times higher Φr of the DPPC lipid mixture with respect to that of the DSPC mixture, the liposome size distributions are plotted in Fig. 3. Note that the liposome size only slightly increases when the temperature is increased from 25 °C to 60 °C. Also note that the DSPC liposomes are on average 2.8 times larger than the DPPC liposomes whereas the area per lipid molecule is approximately the same for DSPC and DPPC molecules.35,36 The liposome concentration is inversely proportional to the bilayer surface area per liposome. Thus, the liposome concentration of the DPPC lipid mixture is 7.8 times higher than that of the DSPC lipid mixture which is in excellent agreement with the 7.6 higher Φr of the DPPC based lipid mixture suggesting that the higher Φr results purely from the higher liposome concentration and not from the difference in the employed primary phospholipid. Future work may investigate the role of liposome size on coalescence stability in more detail using, e.g., extrusion methods.37 The liposomes formed through sonication of the DPPC and DSPC lipid mixtures are fully covered with surface grafted PEG5000 brushes.38 The PEG brushes shield the DPPC and DSPC molecules from the subphase which explains that Φr is independent of the employed primary phospholipid molecule.
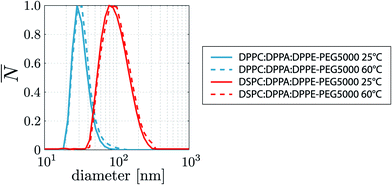 | ||
| Fig. 3 Liposome size distributions of the DPPC and DSPC based lipid formulations studied in this work measured at a temperature of 25 °C and 60 °C. | ||
Using the experimentally obtained scaling of the relative viscosity Φr with temperature, the universal equation for Φr obtained in our previous work27 is extended with the temperature dependence, as follows:
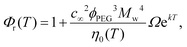 | (4) |
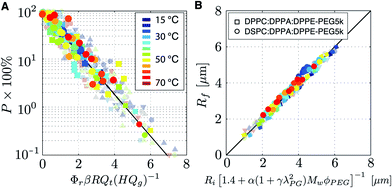 | ||
| Fig. 4 (A) Master plot of the temperature dependent coalescence probability data and (B) of the stabilization data measured for the DPPC:DPPA:DPPE-PEG5000 lipid mixture (squares) and for the DSPC:DPPA:DPPE-PEG5000 lipid mixture (circles). Transparent data points are reproduced from our previous work.27 | ||
Monodisperse bubbles can be produced at high production rates and at room temperature for DPPC based lipid formulations at a total lipid concentration on the order of 10 mg mL−1.26 However, for a DSPC based formulation with the same molar PEG ratio, Φr is 7.6 times lower. Therefore, the total lipid concentration of the DSPC formulation needs to be 2.8 times higher than that of the DPPC formulation in order to produce monodisperse bubbles at room temperature at the same coalescence probability. However, by increasing the temperature from room temperature to 50 °C, eqn (4) shows that the coalescence probability of the DSPC formulation can be lowered such that it is equal to that of the DPPC based formulation used at room temperature. More generally speaking, eqn (4) shows that for any lipid formulation used under certain flow conditions resulting in coalescence probability P, a 5 times lower lipid concentration c∞ results in the very same coalescence probability when the temperature is increased from room temperature to 70 °C. Thus, the use of phospholipids in microbubble formation by flow-focusing becomes 5 times more efficient by increasing the bubble formation temperature from room temperature to 70 °C.
Eqn (4) can also be written in terms of the liposome concentration. However, to allow the equations to be used in practice, where liposome concentration measurements require specialized equipment, it was decided to write eqn (4) in terms of the parameters controlled in the lab, i.e. c∞, ϕPEG, and Mw.
To date, to the best of the author's knowledge, no temperature dependent rheological data is available on an aqueous dispersion of PEGylated liposomes in an extensional flow such as that found in the thin film between colliding bubbles. Nevertheless, it may be speculated that the exponential scaling of Φr with T results from the steric interaction between the liposomes and the monomolecular film around the bubbles. However, in principle the steric interaction force scales linearly with T,28,30 which is very different from the exponential dependence as observed here. Modeling the full temperature dependent liposome–monolayer and liposome–liposome interactions during the drainage of a thin film, by Monte Carlo39 or molecular dynamics40 simulations, may reveal the underlying physics of the observed scaling.
Here, it was demonstrated that the efficiency of the use of surfactants increases with temperature. This may not only be important for the synthesis of bubbles in lab-on-a-chip based devices but also for the stable formation of droplets, e.g. for the formation of phase-change agents.41
3.2 Temperature dependent microbubble stabilization
The bubbles that were formed at the different temperatures and flow conditions were left to stabilize in the flow cell. The ratio of the initial bubble radius Ri to the final stable bubble radius Rf was obtained by fitting a linear function to the data per temperature, see Fig. 2F. The root-mean-square (RMS) of the fitting residuals is represented by the shaded areas. A unique temperature independent relation is found between Ri and Rf where the final bubble size is always found to be 2.2 times smaller than the initial bubble size. The ratio Ri/Rf = 2.2 is exactly as that predicted by the universal equation for the ratio of the initial bubble radius Ri to the final bubble radius that was obtained in our previous work:27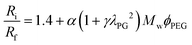 | (5) |
From Langmuir trough surface pressure-area isotherms, it is well known that at a constant surface pressure, the area per lipid molecule increases with temperature.35 The increase in surface area per lipid molecule in the bubble shell of a freshly formed bubble synthesized at a temperature higher than room temperature would lead to an increased ratio of Ri/Rf with respect to that of a bubble formed at room temperature, since Rf of both bubbles is measured at the same temperature, i.e. at room temperature. However, here, we surprisingly found a constant temperature independent ratio of Ri/Rf. This may be explained from the collapse of the PEG chain at elevated temperatures. The state of the PEG chain depends on the affinity of the polymer with the medium. In a poor solvent, a PEG chain collapses and in a good solvent it swells; increasing its excluded volume to above that of a PEG chain conformed as a Gaussian coil in pure water.29,38 An increase in temperature decreases the affinity of the PEG chain with water and thereby its excluded volume. This may cancel out the increase in the area per primary lipid molecule. Nevertheless, to confirm this hypothesis further quantification of the ratio of Ri/Rf is required for bubbles coated with a range of PEG molar ratios.
Even though a constant ratio of Ri/Rf was found here, the conformation, that is, the microstructure of the monomolecular microbubble shell may be different for microbubbles formed at different temperatures.42 The conformation of the microbubble shell may play a role in the acoustic response of the microbubble to an ultrasound driving pulse. Therefore, further research towards acoustic characterization of microbubbles formed at different temperatures would be valuable.
In this study, SF6 gas was used as microbubble filling gas. In our previous studies we used C4F10 and air to fill the microbubbles. For all filling gasses exactly the same ratio between the initial bubble size and the final stable bubble size has been found.26,27 Thus, this confirms once more that the ratio of Ri/Rf of phospholipid-coated microbubbles formed by flow-focusing is independent of the filling gas.26,27 Nevertheless, the lower the aqueous solubility and diffusivity of the microbubble filling gas in water, the longer the time window of the diffusive stabilization process of the freshly formed microbubbles.
A decrease in microbubble stability with temperature has previously been observed for the UCA SonoVue®.43 The mechanisms by which bubble stability decreases at elevated temperatures are, first, the increased diffusivity of the microbubble filling gas. Second, the decreased rigidity and increased mobility of the lipid molecules potentially leading to buckling of the monolayer and to lipid desorption. Third, the increased area per lipid molecule results in an increased gas permeability of the lipid membrane.42,44
The stability of phospholipid molecules in an aqueous dispersion is compromised through, e.g. hydrolization, and the speed of the degradation process increases with temperature.45,46 However, in the preparation process of the lipid mixtures used for microbubble synthesis by flow-focusing it is common to heat the lipid mixture above the lipid phase transition temperature (>55 °C) for the duration of minutes.23,24,26 In the experiments performed in this work, the lipid mixture is located within the thermostatic bath for less than 30 seconds. Therefore, the physico-chemical stability of the phospholipid molecules is most certainly not compromised during microbubble formation at elevated temperatures in a flow-focusing device.
3.3 Microbubble stability in the collection vial as a function of the bubble formation temperature
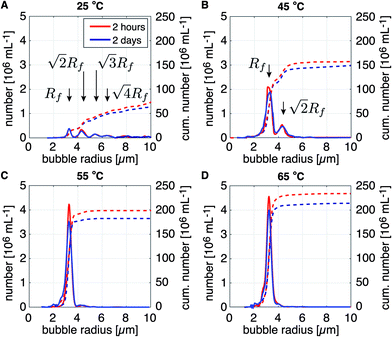
The effect of temperature during bubble formation on the long-term size- and concentration stability of bubble samples collected in vials with a SF6 headspace was investigated. Fig. 6A–D show the size distributions of bubbles formed at temperatures of 25 °C, 45 °C, 55 °C, and 65 °C, respectively. The size distributions were measured 2 hours (solid red curves) and 2 days (solid blue curves) after bubble formation at an initial on-chip bubble radius Ri of 7.0 ± 0.3 μm using the DSPC based lipid mixture. The cumulative bubble concentration per mL is represented by the dotted lines in Fig. 6A–D. Note that the monodispersity of the bubble suspensions increases with the temperature during bubble formation. Also note the distinct maxima in the measured bubble number in Fig. 6A and B. The maximum at the smallest bubble radius (at 3.2 μm) corresponds to the final bubble radius Rf of the initially formed on-chip bubble radius of 7 μm, i.e. the ratio Ri/Rf was 2.2, as before. The second- and higher order maxima in the measured bubble number result from the coalescence of two or more bubbles.Indeed, the position of the higher order maxima in the size distributions can be calculated. Here, it is assumed that the total number of lipid molecules on the surface of a coalesced bubble is the sum of that of the two monodisperse bubbles it originated from. Furthermore, it is known that the surface area of a freshly formed bubble decreases by a factor of (Ri/Rf)2 and that this ratio is independent of Ri.26,27 Thus, the surface area Af,2 of the stable bubble formed due to the coalescence of two monodisperse bubbles is twice the surface area of the stable non-coalesced bubble Af = 4πRf2. The same argument holds for multiple coalesced bubbles such that Af,n = nAf and 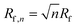 , with n the number of coalesced monodisperse bubbles that form a bigger bubble. The positions of the higher order maxima correspond to the calculated values as indicated in Fig. 6A and B. Thus, indeed, the higher order maxima in the measured bubble number are due to on-chip bubble coalescence.
, with n the number of coalesced monodisperse bubbles that form a bigger bubble. The positions of the higher order maxima correspond to the calculated values as indicated in Fig. 6A and B. Thus, indeed, the higher order maxima in the measured bubble number are due to on-chip bubble coalescence.
Note that apart from an increased monodispersity, the cumulative bubble concentration also increased with temperature, see dotted lines in Fig. 6A–D. In a clinical setting, when the transmit frequency of an ultrasound scanner would be tuned to the resonance frequency of the bubbles with radius Rf, then, for the specific case of the bubble sample produced at a temperature of 25 °C shown in Fig. 6A, only 20 million bubbles per mL would be of a size resonant to the ultrasound driving frequency. However, for the bubble sample produced at 65 °C (Fig. 6D), more than 200 million bubbles per mL will be resonant to the ultrasound driving frequency. Thus, bubble formation using the DSPC based lipid mixture at a temperature of 65 °C resulted in a contrast agent with a 10 times higher sensitivity than that produced at room temperature. Moreover, the echo of the highly monodisperse bubble samples produced at the elevated temperatures have been shown to be more nonlinear than that of a polydisperse agent thereby, e.g. enabling deep tissue imaging without shadowing effects.5
Finally, note that at all temperatures the produced bubble samples were highly stable over time, i.e. the total bubble concentration decreased by only 10–15% over the course of 2 days. No difference in long-term stability can be observed between the bubble samples formed at the different temperatures. Therefore, it can be concluded that bubble formation at elevated temperatures only results in a lower coalescence probability, and, that it does not influence the long-term size- and concentration stability of the bubble suspensions once they have been collected in a vial filled with a SF6 gas headspace.
3.4 Long-term stability in the vial: DPPC versus DSPC coated bubbles
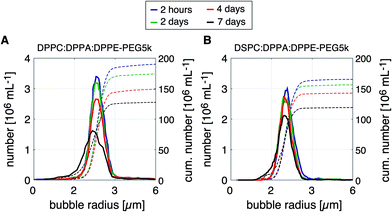
The long-term size- and concentration stability of a microbubble suspension formed using the DPPC based lipid mixture was compared to that of a bubble suspension formed using the DSPC based mixture, see Fig. 7A and B, respectively. For both bubble suspensions, the temperature during bubble formation was 60 °C. The size distributions were measured 2 hours, 2 days, 4 days, and 7 days after bubble collection in vials with a SF6 gas-filled headspace. The cumulative bubble concentration is represented by the dotted lines in Fig. 7A and B.Note that the bubbles formed using the DPPC based lipid mixture were slightly less stable than those formed using the DSPC based mixture. Four days after bubble production, the bubble concentration of the DPPC coated bubbles had decreased by 21% as compared to a decrease of 12% for the DSPC coated bubbles. Seven days after bubble formation, the size distributions of the DPPC and DSPC coated bubbles became wider and shifted towards smaller bubble radii indicating bubble dissolution. Nevertheless, both lipid mixtures resulted in a highly stable bubble suspension for at least two days after their production, both in terms of size- and concentration stability. The time window of 2 to 4 days is sufficiently long for direct in vivo injection, or for further processing.
It has been shown before that microbubble stability against dissolution increases with acyl chain length through an increase of the resistive barrier against gas dissolution, which explains the higher stability of the DSPC coated bubbles.44,47 The stability of the microbubble suspensions produced in this work may be further improved through the use of a microbubble filling gas with a higher molecular weight and a lower aqueous solubility and diffusivity, e.g. C3F8 or C4F10.2,48
Finally, it is of interest to discuss the clinical relevance of the microfluidically synthesized bubble suspensions. The total bubble concentration of the bubble samples (150–250 million bubbles per mL) was similar to the bubble concentration of the clinically available UCA SonoVue® (300 million bubbles per mL). The recommended clinical dose for in vivo injection in human consists of 2.4 mL of SonoVue® and contains approx. 700 million bubbles. The microfluidic production of 2.4 mL of microbubble suspension using the flow-focusing device employed in this work takes at least 1 hour. The production time may be decreased through the use of a different flow-focusing geometry49 and through parallelization.50,51 However, recently, it has been shown that the sensitivity of a monodisperse bubble suspension is 2 to 3 orders of magnitude higher than that of a polydisperse suspension with the same bubble concentration.5 Therefore, the injected dose of a monodisperse bubble suspension driven at resonance can be decreased by 2 to 3 orders of magnitude with respect to that of SonoVue® to achieve the same contrast enhancement. Thus, a clinically relevant dose of monodisperse microbubbles can be produced in less than a minute. Furthermore, the total amount of lipids in such a clinically relevant dose of monodisperse bubbles (0.1–0.3 mg) is approximately equal to that in a recommended SonoVue dose (0.2 mg). All in all, this demonstrates the high clinical potential of microfluidically formed monodisperse microbubbles.
4 Conclusions
The coalescence probability of monodisperse phospholipid-coated microbubbles in the outlet of a flow-focusing device can be dramatically reduced by increasing the temperature during bubble formation. The relative viscosity of the liquid in the thin film between the colliding bubbles increases exponentially with temperature. Furthermore, it was shown that the relative viscosity of a DPPC based lipid mixture is 7.6 times higher than that of a DSPC based lipid mixture at the same total lipid concentration. Therefore, the coalescence probability of bubbles formed using a DSPC based mixture is higher than that of bubbles formed using a DPPC based lipid mixture. The higher relative viscosity of the DPPC based mixture can be explained solely from its 7.6 times higher liposome concentration showing that the relative viscosity is dependent on the liposome concentration and not on the employed primary phospholipid. The results show that the temperature dependent coalescence probability can be predicted by a universal power law of fully independent variables. Furthermore, the ratio of the initial on-chip bubble size to the final stable bubble size was always found to be 2.2 for DPPC:DPPA:DPPE-PEG5000 and for DSPC:DPPA:DPPE-PEG5000 lipid mixtures with 10 mol% PEG, independent of the temperature at which the bubbles were formed. Furthermore, it was shown that the monodispersity of microbubble suspensions formed by flow-focusing at temperatures higher than room temperature is best maintained when the bubbles are rapidly cooled down to room temperature, within seconds after their formation. Moreover, it was demonstrated that microbubble suspensions formed at elevated temperatures are highly stable over a time window of 2 to 4 days with clinically relevant bubble concentrations when collected in a vial with a SF6 headspace. All in all, this work shows that the efficiency of the use of phospholipids in microbubble formation by flow-focusing can be increased by 5 times through an increase of the temperature at which the bubbles are formed from room temperature to 70 °C.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Samir Cherkaoui and Thierry Bettinger for their support to the project. We thank Michel Versluis and Detlef Lohse for stimulating discussions.Notes and references
- J. R. Lindner, Nat. Rev. Drug Discovery, 2004, 3, 527–533 CrossRef CAS.
- T. Segers, N. de Jong, D. Lohse and M. Versluis, Microbubbles for medical applications, RSC Nanosci. Nanotechnol., 2015, 36, 81–101 CAS.
- T. G. Leighton, The Acoustic Bubble, Academic Press, 1994 Search PubMed.
- A. L. Klibanov, Invest. Radiol., 2006, 41, 354–362 CrossRef PubMed.
- T. Segers, P. Kruizinga, M. Kok, G. Lajoinie, N. de Jong and M. Versluis, Ultrasound Med. Biol., 2018, 44(7), 1482–1492 CrossRef PubMed.
- J. M. Tsutsui, F. Xie and R. T. Porter, Cardiovasc. Ultrasound., 2004, 2, 23 CrossRef PubMed.
- S. Hernot and A. L. Klibanov, Adv. Drug Delivery Rev., 2008, 60, 1153–1166 CrossRef CAS PubMed.
- L. E. Deelman, A. E. Declèves, J. J. Rychak and K. Sharma, Adv. Drug Delivery Rev., 2010, 62, 1369–1377 CrossRef CAS PubMed.
- A. R. Carson, C. F. McTiernan, L. Lavery, M. Grata, X. Leng, J. Wang, X. Chen and F. S. Villanueva, Cancer Res., 2012, 72, 6191–6199 CrossRef CAS PubMed.
- H. Dewitte, K. Vanderperren, H. Haers, E. Stock, L. Duchateau, M. Hesta, J. H. Saunders, S. C. De Smedt and I. Lentacker, Theranostics, 2015, 5, 97–109 CrossRef CAS PubMed.
- T. Segers, N. de Jong and M. Versluis, J. Acoust. Soc. Am., 2016, 140, 2506–2517 CrossRef PubMed.
- D. E. Goertz, N. de Jong and A. F. W. van der Steen, Ultrasound Med. Biol., 2007, 33, 1376–1388 CrossRef PubMed.
- M. Emmer, H. J. Vos, D. E. Goertz, A. van Wamel, M. Versluis and N. de Jong, Ultrasound Med. Biol., 2009, 35, 102–111 CrossRef PubMed.
- J. A. Feshitan, C. C. Chen, J. J. Kwan and M. A. Borden, J. Colloid Interface Sci., 2009, 329, 316–324 CrossRef CAS PubMed.
- M. P. Kok, T. Segers and M. Versluis, Lab Chip, 2015, 15, 3716–3722 RSC.
- T. Segers and M. Versluis, Lab Chip, 2014, 14, 1705–1714 RSC.
- S. A. Peyman, R. H. Abou-Saleh, J. R. McLaughlan, N. Ingram, B. R. G. Johnson, K. Critchley, S. Freear, J. A. Evans, A. F. Markham, P. L. Coletta and S. D. Evans, Lab Chip, 2012, 12, 4544–4552 RSC.
- A. M. Gañán-Calvo and J. M. Gordillo, Phys. Rev. Lett., 2001, 87, 274501 CrossRef PubMed.
- S. L. Anna, N. Bontoux and H. A. Stone, Appl. Phys. Lett., 2003, 82, 364–366 CrossRef CAS.
- P. Garstecki, H. A. Stone and G. M. Whitesides, Phys. Rev. Lett., 2005, 94, 164501 CrossRef.
- A. Churchman, V. Mico, J. de Pablo, S. Peyman, S. Freear and S. Evans, Microsyst. Nanoeng., 2018, 4, 17087 CrossRef CAS.
- D. Das, K. Sivasubramanian, C. Yang and M. Pramanik, Sci. Rep., 2018, 8, 6401 CrossRef.
- K. Hettiarachchi, E. Talu, M. L. Longo, P. A. Dayton and A. P. Lee, Lab Chip, 2007, 7, 463–468 RSC.
- E. Talu, R. L. Powell, M. L. Longo and P. A. Dayton, Ultrasound Med. Biol., 2008, 34, 1182–1185 CrossRef PubMed.
- R. Shih and A. P. Lee, Langmuir, 2016, 32, 1939–1946 CrossRef CAS.
- T. Segers, L. de Rond, N. de Jong, M. Borden and M. Versluis, Langmuir, 2016, 32, 3937–3944 CrossRef CAS PubMed.
- T. Segers, D. Lohse, M. Versluis and P. Frinking, Langmuir, 2017, 33, 10329–10339 CrossRef CAS.
- J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, 1992, vol. 2 Search PubMed.
- J. Lee, H. Lee and J. Andrade, Prog. Polym. Sci., 1995, 20, 1043–1079 CrossRef CAS.
- K. Danov, Fluid Mechanics of Surfactant and Polymer Solutions, Springer-Verlag Wien, 2004, ch. 1 Search PubMed.
- H. Barnes, J. Hutton and F. Walters, An introduction to rheology, Elsevier, Amsterdam, 1989, vol. 3 Search PubMed.
- D. Trokhymchuk, A. Henderson, A. Nikolov and D. Wasan, Langmuir, 2001, 17, 4940–4947 CrossRef.
- D. C. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Anal. Chem., 1998, 70, 211–217 CrossRef.
- M. L. F. Nascimentoa and C. Apariciob, Phys. B, 2007, 398, 71–77 CrossRef.
- M. M. Lozano and M. L. Longo, Soft Matter, 2009, 5, 1822–1834 RSC.
- S. Lee, D. H. Kim and D. Needham, Langmuir, 2001, 17, 5544–5550 CrossRef CAS.
- F. Olson, C. Hunt, F. Szoka, W. Vail and D. Papahadjopoulos, Biochim. Biophys. Acta, Biomembr., 1979, 557, 9–23 CrossRef CAS.
- C. Allen, N. Dos Santos, R. Gallagher, G. Chiu, Y. Shu, W. Li, S. Johnstone, A. Janoff, L. Mayer, M. Webb and M. Bally, Biosci. Rep., 2002, 22, 225–250 CrossRef CAS.
- J. Griesbauer, H. Seeger, A. Wixforth and M. F. Schneider, Method for the Monte Carlo Simulation of Lipid Monolayers including Lipid Movement, 2010, arXiv, https://arxiv.org/pdf/1012.4973.pdf Search PubMed.
- E. Boek, J. Padding, W. den Otter and W. Briels, J. Phys. Chem. B, 2005, 109, 19851–19858 CrossRef CAS PubMed.
- D. Bardin, T. Martz, P. Sheeran, R. Shih, P. Dayton and A. Lee, Lab Chip, 2011, 23, 3990–3998 RSC.
- M. Borden, G. Pu, G. Runner and M. Longo, Colloids Surf., B, 2004, 34, 209–223 CrossRef PubMed.
- H. Mulvana, E. Stride, J. Hajnal and R. Eckersley, Ultrasound Med. Biol., 2010, 36, 925–934 CrossRef.
- M. Borden and M. Longo, Langmuir, 2002, 18, 9225–9233 CrossRef CAS.
- H. Frøksjaer, J. Bentz and F. Szoka, in Optimization of Drug Delivery, ed. H. Bundgaard, A. Bagger Hansen and H. Kofod, Munksgaard, Copenhagen, 1982, ch. Stability and storage of liposomes Search PubMed.
- M. Grit, J. de Smidt, A. Struijke and D. Crommelin, Int. J. Pharm., 1989, 50, 1–6 CrossRef CAS.
- M. M. Lozano and M. L. Longo, Langmuir, 2009, 25, 3705–3712 CrossRef CAS PubMed.
- K. Sarkar, A. Katiyar and P. Jain, Ultrasound Med. Biol., 2009, 35, 1385–1396 CrossRef PubMed.
- E. Castro-Hernández, W. van Hoeve, D. Lohse and J. Gordillo, Lab Chip, 2011, 11, 2023–2029 RSC.
- M. Hashimoto, S. S. Shevkoplyas, B. Zasońska, T. Szymborski, P. Garstecki and G. Whitesides, Small, 2008, 4, 1795–1805 CrossRef CAS PubMed.
- E. Amstad, X. Chen, M. Eggersdorfer, N. Cohen, T. Kodger, C. Ren and D. Weitz, Phys. Rev. E, 2017, 95, 043105 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2019 |