The pH effect on the detection of heavy metals in wastewater by laser-induced breakdown spectroscopy coupled with a phase transformation method
Shixiang
Ma
a,
Yun
Tang
a,
Yuyang
Ma
a,
Daming
Dong
 *b,
Lianbo
Guo
*b,
Lianbo
Guo
 *a,
Haihong
Zhu
a,
Jianguo
Liu
a and
Yongfeng
Lu
*a,
Haihong
Zhu
a,
Jianguo
Liu
a and
Yongfeng
Lu
 a
a
aWuhan National Laboratory for Optoelectronics (WNLO), Huazhong University of Science and Technology, Wuhan, Hubei 430074, P. R. China. E-mail: lbguo@hust.edu.cn; Fax: +86-27-87541423; Tel: +86-27-87541423
bNational Engineering Research Center for Information Technology in Agriculture, Beijing Academy of Agriculture and Forestry Sciences, Beijing 10097, China. E-mail: damingdong@hotmail.com
First published on 21st November 2019
Abstract
Heavy metal particles in water are mainly derived from acidic industrial wastewater and seriously endanger the environment and public health. In this work, the pH effect on the detection of toxic metals in wastewater by laser-induced breakdown spectroscopy coupled with a phase transformation method (LIBS-PT) was investigated. Heavy metals of cadmium (Cd) and chromium (Cr) were selected as examples. The results showed that the presence of acids in wastewater inhibited the spectral enhancement of LIBS-PT on a metal substrate. This was mainly due to the presence of the salt floccule formed by the reaction of an acid with a metal substrate on the substrate surface. The floccule content increased as pH decreased, and the corresponding substrate ablation threshold increased. Therefore, more laser energy was used for ablation, resulting in reduced laser energy for ionization and reduced electron density. Eventually, spectral intensity decreased as electron density decreased. However, there was no significant change in plasma temperature. Meanwhile, the determination coefficients (R2) of Cd and Cr were all above 0.99 under the optimal pH 6.5 and on the optimal zinc (Zn) substrate. Limits of detection (LoDs) of 0.0089 mg L−1 and 0.0006 mg L−1 for Cd and Cr were obtained, respectively. The LoDs of Cd and Cr elements met the sewage discharge standard of China. The results indicated that the detection sensitivity of heavy metal elements in acidic wastewater can be significantly improved by optimizing the pH value of the solution using LIBS-PT.
1. Introduction
Acidic and alkaline wastewater are the most common types of industrial pollutants in wastewater, mainly from sources such as steel plants, electroplating and dyeing,1 metallurgy,2 and so on. Heavy metal salts are often found in acidic industrial wastewater, but heavy metal precipitates are formed in alkaline wastewater. Therefore, the detection of heavy metals in acidic industrial wastewater is crucial. Heavy metal elements are highly enriched, toxic, carcinogenic, teratogenic, and difficult to degrade.3 Even very low levels in an aqueous solution can cause serious harm to biological health.4,5 For instance, chromium (Cr) can easily enter human cells, accumulate, and induce gene mutation.6 Cadmium (Cd) can form cadmium-metallothionein (CD-MT) in the human body and cause serious kidney damage.7There are strict standards for the discharge of heavy metals in industrial wastewater. For example, the maximum emission standard of Cd in industrial wastewater is 0.01 mg L−1 in China (No. GB/T 18918-2002). In addition, it is worth emphasizing that the mass fraction of acid in acidic wastewater varies greatly, from less than 1% to higher than 10%. Different levels of acidic contents may affect the detection of metallic elements. Therefore, a fast and sensitive detection method for heavy metals in different types of acidic industrial wastewater is urgently needed.
Laser-induced breakdown spectroscopy (LIBS), a promising elemental analysis technology, is widely applied in many fields.8–12 The advantages of LIBS include rapid in situ analysis, preparation of fewer samples,13,14 and simultaneous multielement detection.15 However, direct analysis of a liquid sample using LIBS is not very satisfactory due to water splashing and quenching of plasma.16–18 An easy sample pretreatment procedure is required for liquid analysis. Various sample disposal measures, such as liquid jets,19 liquid flow,20 aerosol,21 and alcohol-solution mixtures,22 have been reported. However, these methods increase the complexity of equipment required and have poor reproducibility. Because LIBS has advantages when used for solid analysis, the conversion of a liquid phase to a solid phase is currently one of the effective methods for improving detection sensitivity. These include, for example, dispersive liquid–liquid microextraction (DLLME) LIBS using aluminum foil,23 adsorption LIBS using a graphite,24 a paper substrate,25,26 and a wood slice,27 emission enhancement of LIBS based on gold (Au) nanoparticles and a solid-phase substrate,28 enrichment of heavy metals on an electrode by electrodeposition,29 metal precipitation and membrane separation,30 and LIBS coupled with a phase transformation method (LIBS-PT) based on a nonabsorbent solid surface.31–33
LIBS coupled with a phase transformation method is a simple and sensitive method due to easy sample pretreatment and significant spectral enhancement. Aguirre et al.32 analyzed manganese (Mn) using LIBS-PT, and a limit of detection (LoD) of 6 μg g−1 was realized. Yang et al.31 used a magnesium (Mg) alloy for chemical replacement combined with LIBS, and LoDs within the range of 0.016–0.386 μg mL−1 of Cr, lead (Pb), copper (Cu), and Cd elements were achieved. Jijón et al.34 used LIBS-PT to quantitatively analyze lithium (Li) and strontium (Sr) in a solution by using steel as a substrate, and good sensitivity was obtained at about 1 ppm. However, the detection sensitivity of heavy metal elements, such as Cd, still failed to meet the sewage discharge standard of China (0.01 mg L−1 for Cd, No. GB/T 18918-2002) as measured by LIBS or LIBS-PT.27,31,35,36 Meanwhile, the effect of acidic wastewater's properties, such as its pH value, on the detection sensitivity of LIBS-PT was not reported.
In this work, we investigated the effect and mechanism of pH on spectral enhancement for the determination of trace metal elements using the LIBS-PT method by analyzing solutions of different acidities. The Cd and Cr elements were analyzed as examples; and three common substrates (zinc (Zn), Mg alloy, and silicon (Si)), which exhibit different chemical activities, were selected as substrates. Meanwhile, the detection sensitivity of LIBS-PT for detecting trace heavy elements of Cd and Cr in an aqueous solution was studied.
2. Experiment setup and sample preparation
2.1 Experiment setup
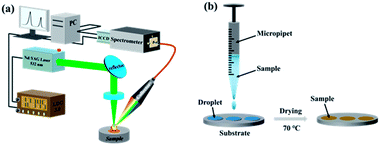
The LIBS experimental setup is shown schematically in Fig. 1(a). A Q-switched Nd:YAG laser (Quantel Ultra 100, wavelength: 532 nm; repetition rate: 20 Hz; pulse width: 7 ns) was used to ablate samples and produce plasma. The pulse energy was 3 mJ, and the focal spot on the sample was about 20 μm. The laser was reflected by a reflector and focused on the surface of a target sample by a 25 mm plano-convex lens. The plasma emission was collected by a light collector and then coupled into a Czerny–Turner spectrometer (Andor Tech., Shamrock 500i, grating: 2400 L mm−1, slit width: 200 μm) equipped with an intensified charge-coupled device (ICCD) camera (Andor Tech., iStar 320T: DH320T-18F-E3-26mm) through a multicore fiber (Avantes). The electron density was measured using a Czerny–Turner spectrometer. But a different echelle spectrometer (Andor Tech., Mechelle 5000, 200–950 nm, λ/Δλ = 5000) was used to evaluate the plasma temperature, because of the narrow observation window of the Czerny–Turner spectrometer, which could only be used in the spectral range of 352–369 nm when detecting Cd I 361.05 nm and 417–433 nm when detecting Cr I 425.43 nm. This meant that the number of spectral lines was not enough to accurately calculate the plasma temperature. A digital delay generator (Wuhan N&D Laser Engineering, LDG3.0) was utilized to trigger the 532 nm laser and the ICCD camera in experiments. The sample was placed on an X–Y–Z motorized translation platform at a speed of 0.5 mm s−1 so that a fresh surface was provided for each laser ablation.To obtain a higher spectral intensity and signal-to-noise ratio (SNR) for Cd and Cr, a delay time of 2 μs and a gate width of 2 μs were optimized. To improve the spectral stability, each spectrum was obtained by scanning the whole sample in a circular ablation area; and seven spectra were taken and averaged for each sample.
2.2 Sample preparation
Unitary aqueous solutions were prepared by dissolving the corresponding amounts of cadmium chloride (CdCl2) or chromic chloride (CrCl3) using deionized water as a solvent, respectively. These analytes were purchased from Sinopharm Chemical Reagent Co., Ltd., and no further treatment was carried out. A series of solutions of different acidities were configured using the hydrochloric acid (HCl) reagent in the pH range from 1 to 6.5. The pH value was measured using a pH meter. Since the concentration of chromium chloride in the solution is at the ppb level, the effect of CrCl3 hydrolysis on pH is almost negligible. The pH value of the 0.8 ppm CrCl3 aqueous solution measured was 6.5, mainly because the pH of the deionized water used was 6.5. In order to avoid the effect of other substances, the pH (6.5) of CrCl3 solution was not adjusted. The concentrations of Cd and Cr were 10 mg L−1 and 1 mg L−1, respectively. In addition, the standard solutions of Cd and Cr with pH values of 2 and 6.5 were formulated, respectively, with concentration ranges of 0.04 to 1 mg L−1 for Cd and 0.02 to 0.8 mg L−1 for Cr. The concentration details are given in Table 1.| Sample no. | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Cd (mg L−1) | 0.04 | 0.06 | 0.1 | 0.3 | 0.5 | 0.8 | 1.0 |
| Cr (mg L−1) | 0.02 | 0.04 | 0.06 | 0.1 | 0.3 | 0.5 | 0.8 |
Three different targets were used as substrates: Zn (99.993–99.995%), Mg alloy (AZ31B, Mg: 95.56 wt%, aluminum (Al): 3.1 wt%, and Zn: 0.82 wt%), and Si wafers (above 99.9999%). The preparation process is shown in Fig. 1(b). Each standard sample was pretreated as follows:
(1) A 5 μL solution was deposited on a substrate using a micropipette for obtaining a CdCl2 solution of 10 mg L−1 Cd and a CrCl3 solution of 1 mg L−1 Cr at pH 1 to 6.5. In addition, a 20 μL solution was deposited on a substrate in an average of four drops using a micropipette for obtaining the standard solution of Cd and Cr with concentrations ranging from 0.02 to 1 mg L−1 with pH values of 2 and 6.5.
(2) The substrate was heated to 70 °C using a heating plate; and after 5 min, a heavy metal layer was prepared in a circular shape with a diameter of 3 mm on the surface of the substrate.
3. Results and discussion
3.1 Comparison of spectral intensity obtained on different substrates and at different pH values
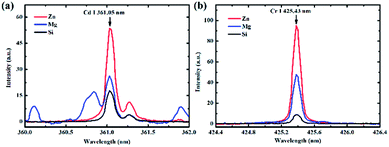
A representative example of the spectra obtained on different substrates at a pH value of 6.5 is presented in Fig. 2. The lines of Cd I 361.05 nm and Cr I 425.43 nm were chosen as analytical spectral lines. Fig. 2 illustrates that the spectral intensities were different on different substrates, and the enhancement effect of the Zn substrate was the best. Therefore, to obtain a better SNR and detection limit, the Zn target was selected as the substrate for quantitative analysis.To investigate the effect of different pH values on the analysis of heavy metal elements in LIBS-PT, the spectra of Cd and Cr on different substrates at different pH values were obtained. The variations of Cd I 361.05 nm at different pH values are shown in Fig. 3(a), and the variations of Cr I 425.43 nm at different pH values are shown in Fig. 3(b). Fig. 3 shows that the spectral intensity of Cd and Cr increased as the pH increased and then remained constant as the pH was increased from 3 to 6.5 on Zn and Mg substrates. However, on the Si substrate, the pH value had no significant effect on the spectral intensity.
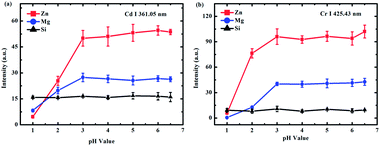 | ||
| Fig. 3 The influence of different pH values on (a) Cd (10 mg L−1 Cd of CdCl2) and (b) Cr (1 mg L−1 Cr of CrCl3) spectral intensity on Zn (red line), Mg (blue line), and Si (black line) substrates. | ||
3.2 Mechanism of the effect of different pH values on LIBS-PT
To further investigate the effect of pH, the products of reactions between solutions and substrates were analyzed. Taking Cd as an example, the chemical equations on Zn and Mg substrates can be expressed as follows:| 2HCl + R = RCl2 + H2, | (1) |
| CdCl2 + R = RCl2 + Cd, | (2) |
| H2O + R = R(OH)2 + H2. | (3) |
To further verify the effect of chloride salt produced by the reactions of eqn (1) and (2) on spectral intensity, scanning electron microscope (SEM) images of the Zn substrate surface after the deposition of CdCl2 solution at different pH values are shown in Fig. 4. The flocculating materials were formed on the surface of the Zn substrate when using pH values of 1 and 2, and more flocculating materials were formed using a pH of 1. No obvious floccus was formed using pH values of 3 to 6.5. The flocculating materials were mainly the product of ZnCl2 of the reaction between HCl and the Zn substrate. Therefore, the different spectral intensities on the Zn substrate under different pH values were caused by the change in the matrix caused by the reaction of the solute and substrate.
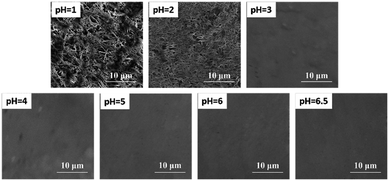 | ||
| Fig. 4 SEM micrographs of a Zn substrate surface after the deposition of 5 μL solution (10 mg L−1 Cd of CdCl2 solution) at different pH values. | ||
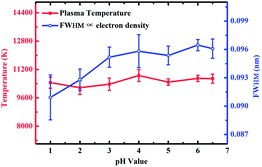
To further confirm the effect of the flocculating materials on the surface of the substrate on the spectral intensity, the plasma temperature and electron density were calculated. The electron density was only related to the full width at half maximum (FWHM) obtained using the same spectral line as that used to calculate the electron density in the Stark broadening method, so the electron density was replaced by the FWHM.37,38 The effect of pH values on plasma temperature and electron density on the Zn substrate is shown in Fig. 5. There was no significant change in plasma temperature when the pH value increases from 1 to 6.5. However, the electron density increased as the pH increased and then remained stable as the pH was increased from 3 to 6.5. Therefore, the difference in the spectral intensity was mainly due to the electron density. The content of ZnCl2 formed on the surface of the substrate increased as the pH decreased. When the laser ablated the sample, as the ZnCl2 content increased, more laser energy was used to ablate ZnCl2, resulting in less ablation of the metal substrate. Zinc chloride had a higher ablation threshold than metal Zn.39,40 Therefore, when the ZnCl2 content increased with decreasing pH, more laser energy was used for ablation, resulting in a decrease in ionization energy,41 which ultimately resulted in a decrease in the spectral intensity.
3.3 Calibration curves and limits of detection
As shown in Fig. 4, flocculating materials were formed under pH values of 1 and 2; however, the metal substrate changed little when the solution pH value was 3 to 6.5. Therefore, to illustrate the effect of pH on the analysis of heavy metal elements in LIBS-PT, representative quantitative analyses of trace Cd and Cr under pH values of 2 and 6.5 on the Zn substrate were conducted. The calibration curves of Cd I 361.05 nm are shown in Fig. 6(a), and the calibration curves of Cr I 425.43 nm are shown in Fig. 6(b). The R2 factor of the calibration curve of Cd I 361.05 nm was 0.999 at a pH of 6.5, better than that at a pH of 2. The same result was obtained for Cr I 425.43 nm.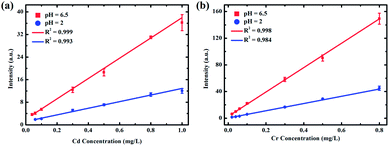 | ||
| Fig. 6 Calibration curves of (a) Cd I 361.05 nm and (b) Cr I 425.43 nm on a Zn substrate at pH values of 2 and 6.5. | ||
The LoDs of Cd at pH values of 6.5 and 2 were 0.0089 and 0.0452 mg L−1, and the LoDs of Cr at pH values of 6.5 and 2 were 0.0006 and 0.0028 mg L−1, respectively. This demonstrates that the LoDs of Cd I 361.05 nm and Cr I 425.43 nm obtained with a pH of 6.5 were lower than those obtained with a pH of 2. More detailed quantitative analysis results are provided in Table 2.
| pH | |||
|---|---|---|---|
| 2 | 6.5 | ||
| Cd | R 2 | 0.993 | 0.999 |
| LoD (mg L−1) | 0.0452 | 0.0089 | |
| RSD | 7.4% | 6.9% | |
| Cr | R 2 | 0.984 | 0.998 |
| LoD (mg L−1) | 0.0028 | 0.0006 | |
| RSD | 6.9% | 5.9% | |
Furthermore, Table 3 shows a comparison of the LoDs of Cd and Cr elements obtained in our work with those obtained in other studies reported in the literature. Obviously, the LoDs obtained in this work were lower than the others. What is more noteworthy is that the LoD of the Cd and Cr elements in this work met the sewage discharge standard of China (0.01 mg L−1 for Cd and 0.1 mg L−1 for Cr, No. GB/T 18918-2002). Therefore, the detection sensitivity of heavy metal elements in an aqueous solution can be improved by adjusting the pH value of the solution in LIBS-PT.
| Element | Method | Substrate | LoDs (mg L−1) | References |
|---|---|---|---|---|
| Cd | Adsorption-LIBS | Word slice | 0.5900 | 27 |
| Absorption paper | 0.4600 | 36 | ||
| CR-SENLIBS | Magnesium alloy | 0.3860 | 31 | |
| Adsorption-LIBS | Ion exchange membranes | 0.2100 | 35 | |
| LIBS-PT | Zinc substrate | 0.0089 | This work | |
| Cr | Adsorption-LIBS | Graphite | 0.5200 | 24 |
| Wood slice | 0.0340 | 27 | ||
| CR-SENLIBS | Magnesium alloy | 0.0160 | 31 | |
| LIBS | Microfiltration membrane | 0.0013 | 30 | |
| LIBS-PT | Zinc substrate | 0.0006 | This work |
4. Conclusions
In summary, we investigated the detection of heavy metal elements in acidic wastewater by using LIBS-PT and the effect of pH on spectral enhancement in LIBS-PT. The results showed that the spectral intensity increased at first, and then changed little on a metal substrate. However, there was no significant change in spectral intensity on a nonmetallic substrate. The main reason is that the presence of flocculating materials formed on the surface of a metal substrate impeded the enhancement of the spectrum when the pH values of the solution to be tested were 1 and 2. In addition, under an optimal pH value of 6.5 and on the optimal Zn substrate, the LoDs were 0.0089 mg L−1 and 0.0006 mg L−1 for Cd and Cr, respectively. The average errors were 6.9% and 5.9% for Cd and Cr, respectively. The LoD of Cd and Cr met the sewage discharge standard of China (0.01 mg L−1 for Cd and 0.1 mg L−1 for Cr, No. GB/T 18918-2002). This work demonstrated that LIBS-PT is a feasible method for detecting heavy metal elements in acidic wastewater by adjusting the acidity of the solution.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (No. 61575073).Notes and references
- R. Kant, Nat. Sci., 2012, 04, 22–26 CAS.
- S. Singh, G. Mahmood and D. Chinche, Int. J. Sci. Eng. Res., 2013, 4, 1840–1854 Search PubMed.
- Z. Li, Z. Ma, T. J. van der Kuijp, Z. Yuan and L. Huang, Sci. Total Environ., 2014, 468–469, 843–853 CrossRef CAS PubMed.
- M. Arora, B. Kiran, S. Rani, A. Rani, B. Kaur and N. Mittal, Food Chem., 2008, 111, 811–815 CrossRef CAS.
- M. S. Islam, M. K. Ahmed, M. Raknuzzaman, M. Habibullah-Al-Mamun and M. K. Islam, Ecol. Indicat., 2015, 48, 282–291 CrossRef CAS.
- A. H. Smith and C. M. Steinmaus, Annu. Rev. Public Health, 2009, 30, 107–122 CrossRef PubMed.
- J. Godt, F. Scheidig, C. Grosse-Siestrup, V. Esche, P. Brandenburg, A. Reich and D. A. Groneberg, J. Occup. Med. Toxicol., 2006, 1, 22 CrossRef PubMed.
- Y. Dixit, M. P. Casado-Gavalda, R. Cama-Moncunill, X. Cama-Moncunill, M. Markiewicz-Keszycka, F. Jacoby, P. J. Cullen and C. Sullivan, J. Food Eng., 2018, 216, 120–124 CrossRef CAS.
- A. De Giacomo, C. Koral, G. Valenza, R. Gaudiuso and M. Dell'Aglio, Anal. Chem., 2016, 88, 5251–5257 CrossRef CAS PubMed.
- Y. Tang, J. Li, Z. Hao, S. Tang, Z. Zhu, L. Guo, X. Li, X. Zeng, J. Duan and Y. Lu, Opt. Express, 2018, 26, 12121–12130 CrossRef CAS PubMed.
- Z. Wang, T.-B. Yuan, Z.-Y. Hou, W.-D. Zhou, J.-D. Lu, H.-B. Ding and X.-Y. Zeng, Front. Phys., 2014, 9, 419–438 CrossRef.
- L.-B. Guo, X.-Y. Li, W. Xiong, X.-Y. Zeng and Y.-F. Lu, Front. Phys., 2016, 11 Search PubMed.
- Z. Zhu, J. Li, Y. Guo, X. Cheng, Y. Tang, L. Guo, X. Li, Y. Lu and X. Zeng, J. Anal. At. Spectrom., 2018, 33, 205–209 RSC.
- R. Yuan, Y. Tang, Z. Zhu, Z. Hao, J. Li, H. Yu, Y. Yu, L. Guo, X. Zeng and Y. Lu, Anal. Chim. Acta, 2019, 1064, 11–16 CrossRef CAS PubMed.
- Z. Hou, Z. Wang, T. Yuan, J. Liu, Z. Li and W. Ni, J. Anal. At. Spectrom., 2016, 31, 722–736 RSC.
- A. Sarkar, D. Alamelu and S. K. Aggarwal, Appl. Opt., 2008, 47, G58–G64 CrossRef CAS PubMed.
- V. Rai, F. Yueh and J. Singh, Laser-Induced Breakdown Spectrosc., 2007, 223–254 CAS.
- N. K. Rai and A. Rai, J. Hazard. Mater., 2008, 150, 835–838 CrossRef CAS PubMed.
- D. Wu, L. Sun, P. Liu, R. Hai and H. Ding, Appl. Spectrosc., 2017, 72, 225–233 CrossRef PubMed.
- X. Cheng, X. Yang, Z. Zhu, L. Guo, X. Li, Y. Lu and X. Zeng, Appl. Opt., 2017, 56, 9144–9149 CrossRef CAS PubMed.
- X. Yang, L. Guo, J. Li, R. Yi, Z. Hao, M. Shen, R. Zhou, K. Li, X. Li, Y. Lu and X. Zeng, Appl. Opt., 2016, 55, 7406–7411 CrossRef CAS PubMed.
- Y. Lan, Y. Lu, X. Dong and R. Zheng, Opt. Express, 2019, 27, 29896–29904 CrossRef PubMed.
- M. A. Aguirre, E. J. Selva, M. Hidalgo and A. Canals, Talanta, 2015, 131, 348–353 CrossRef CAS PubMed.
- Y. Wang, N. Zhao, M. Ma, C. Wang, Y. Yu, D. Meng, J. Liu and W. Liu, Laser Technol., 2013, 37, 808–811 CAS.
- Y. Yu, W. Zhou, H. Qian, X. Su and K. Ren, Plasma Sci. Technol., 2014, 16, 683–687 CrossRef CAS.
- J. Xiu, S. Zhong, H. Hou, Y. Lu and R. Zheng, Appl. Spectrosc., 2014, 68, 1039–1045 CrossRef CAS PubMed.
- Z. Chen, H. Li, M. Liu and R. Li, Spectrochim. Acta, Part B, 2008, 63, 64–68 CrossRef.
- X. Wen, Q. Lin, G. Niu, Q. Shi and Y. Duan, Appl. Opt., 2016, 55, 6706–6712 CrossRef CAS PubMed.
- A. Matsumoto, A. Tamura, R. Koda, K. Fukami, Y. H. Ogata, N. Nishi, B. Thornton and T. Sakka, Anal. Chem., 2015, 87, 1655–1661 CrossRef CAS PubMed.
- X. Wang, Y. Wei, Q. Lin, J. Zhang and Y. Duan, Anal. Chem., 2015, 87, 5577–5583 CrossRef CAS PubMed.
- X. Y. Yang, Z. Q. Hao, C. M. Li, J. M. Li, R. X. Yi, M. Shen, K. H. Li, L. B. Guo, X. Y. Li, Y. F. Lu and X. Y. Zeng, Opt. Express, 2016, 24, 13410–13417 CrossRef CAS PubMed.
- M. A. Aguirre, S. Legnaioli, F. Almodóvar, M. Hidalgo, V. Palleschi and A. Canals, Spectrochim. Acta, Part B, 2013, 79–80, 88–93 CrossRef CAS.
- D. Bae, S.-H. Nam, S.-H. Han, J. Yoo and Y. Lee, Spectrochim. Acta, Part B, 2015, 113, 70–78 CrossRef CAS.
- D. Jijón and C. Costa, J. Phys.: Conf. Ser., 2011, 274, 012077 CrossRef.
- N. E. Schmidt and S. R. Goode, Appl. Spectrosc., 2002, 56, 370–374 CrossRef CAS.
- M. Bukhari, M. A. Awan, I. A. Qazi and M. A. Baig, J. Anal. Methods Chem., 2012, 2012, 823016 Search PubMed.
- A. W. Miziolek, V. Palleschi and I. Schechter, Laser Induced Breakdown Spectroscopy, Cambridge University Press, 2006 Search PubMed.
- S. Ma, Y. Tang, Y. Ma, Y. Chu, F. Chen, Z. Hu, Z. Zhu, L. Guo, X. Zeng and Y. Lu, Opt. Express, 2019, 27, 15091–15099 CrossRef CAS PubMed.
- D. A. C. Aaron, S. Eppler, D. D. Hickmott, M. J. Ferris and A. C. Koskelo, Appl. Spectrosc., 1996, 50, 1175–1181 CrossRef.
- C. Muratore, Plasma Processes Polym., 2010, 4, 847 CrossRef.
- M. A. Ismail, H. Imam, A. Elhassan, W. T. Youniss and M. A. Harith, J. Anal. At. Spectrom., 2004, 19, 489 RSC.
| This journal is © The Royal Society of Chemistry 2020 |