Metal-chelate induced nanoparticle aggregation enhanced laser-induced breakdown spectroscopy for ultra-sensitive detection of trace metal ions in liquid samples†
Xiaojiao
Liu
ab,
Qingyu
Lin
c,
Yonghui
Tian
ab,
Wenlong
Liao
c,
Tao
Yang
ab,
Cheng
Qian
ab,
Tianlong
Zhang
 b and
Yixiang
Duan
b and
Yixiang
Duan
 *ab
*ab
aResearch Center of Analytical Instrumentation, Northwest University, 1 Xuefu Ave., Xi'an, Shaanxi 710075, China. E-mail: yduan@nwu.edu.cn
bKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry & Materials Science, Northwest University, Xi'an, Shaanxi 710069, China
cResearch Center of Analytical Instrumentation, Sichuan University, Chengdu, 610064, China
First published on 13th November 2019
Abstract
Rapid detection and efficient removal of heavy metal ions from environmental water are of considerable importance to tackle the water pollution problem. In this work, a simple but efficient strategy named metal-chelate induced nanoparticle aggregation enhanced laser-induced breakdown spectroscopy (MINAELIBS) was proposed for signal enhancement aiming at ultra-sensitive detection of trace levels of metal elements in liquid samples. Unlike the surface contact approach previously reported in the literature, direct mixing of gold nanoparticles (AuNPs) and metal chelates increased the contact area, adhesion force, and uniformity between analytes and AuNPs. As a result, under the optimal conditions, the limits of detection (LODs) of Cd, Cu, Ag, Pb, and Cr were 4.6, 1.5, 3.4, 3.5, and 3.5 ng mL−1, respectively. The LODs of target elements were notably 7–10 times lower than those of chelating reagent enrichment alone and 3–4 orders of magnitude lower compared to traditional LIBS methods, which demonstrates the feasibility of MINAELIBS in analysis of liquid samples at the ppb level. Application of AuNPs for high-performance signal enhancement has undoubtedly pushed the method sensitivity of traditional LIBS analyses to a new level. In addition, the recoveries of all the analytical elements in the environmental water samples were in the range from 88.6% to 109.9%, which further proves the feasibility and potential of MINAELIBS in practical liquid sample analysis.
1. Introduction
With the acceleration of industrial development, water pollution has become a global issue of concern. In particular, metal ions, one of the major pollutants, are mainly from industrial emission and agricultural and domestic activities. With the accumulation of trace heavy metals in biological chains, they will directly have adverse effects on human tissues and organs. Consequently, rapid detection and removal of heavy metals from polluted water have received significant attention from researchers, and multitudinous technologies such as atomic absorption spectrometry (AAS),1 inductively coupled plasma atomic emission spectrometry (ICP-AES),2 and X-ray fluorescence (XRF) spectrometry3 have been developed. However, these traditional techniques involve complex instruments or equipment, which are confined to the laboratory rather than on-site detection.Laser-induced breakdown spectroscopy (LIBS)4,5 is an analytical technique for the rapid, on-site quantitative, and qualitative detection of elemental constituents in samples. At present, LIBS technology has been applied to the analysis of liquid samples.6–10 Nevertheless, it is well known that during the process of laser–matter interaction, liquid sputtering, sloshing and plasma quenching would greatly reduce laser ablation efficiency, signal repeatability, and quantitative accuracy when LIBS is directly used for liquid analysis.11,12 Moreover, sputtering droplets may contaminate the focusing objective and collimating lens. Thus, it is still a challenge for quantitative detection of trace levels of elements in liquid samples. To overcome the above-mentioned inherent defects and improve the analytical capability (sensitivity, accuracy, and repeatability) of LIBS for liquid samples, several measures have been proposed, including employing a double pulse with different geometric positions13–17 and LIBS combined with laser-induced fluorescence (LIBS-LIF).18,19 However, the above techniques require extra system equipment, such as nebulizers, liquid jet injectors, additional power supplies, and magnetic stirrer devices, which increases the complexity and analytical cost.
Matrix transformation is one of the frequently used strategies to conquer the limitation of LIBS in liquid analysis. Adsorbent materials20–25 as a support for liquid samples have been reported in the literature to avoid the inherent drawbacks of LIBS for liquid analysis and to improve the detection limits. Typically, adsorbent materials are immersed in a liquid solution for a while to adsorb the target elements, which is time-consuming and results in uneven distribution of the analytical components on the substrate materials.21,23,24,26 The filter paper involved in such processes is prone to wrinkle during the drying process,20 which is adverse to the analysis of liquid samples. Therefore, although the above-described adsorbent method avoids liquid sputtering, it only achieves a liquid-to-solid phase transition without visible enhancement effects on the signal. Wang et al.27 recently proposed a liquid sample preconditioning strategy based on metal precipitation and membrane separation to improve the sensitivity of LIBS. In their study, the physical enrichment of the target elements was achieved by utilizing a self-made filter to reduce the area of the membrane, and the results showed that the spectral intensity of the elements could be increased about five times. However, the diameter of the commercial membrane is typically too large to reach the same enrichment level as described above when used directly. The smaller the diameter of the membrane is, the weaker its bearing capacity becomes. Therefore, the membrane with a 3 mm diameter cannot meet the detection needs of a large volume of the liquid samples in their study. In addition, such manually cut membranes are vulnerable to contamination, which is negative for accurate detection of trace elements.
With the development of nanotechnology, the optical properties of metal nanoparticles (namely, gold, silver, and copper) have received extensive attention in various fields. Of particular interest are the plasmon resonance phenomena of metal nanoparticles. The surface plasmon resonance (SPR) of metal nanoparticles28 originates from the strong interaction between the light beam and collective conduction free electron oscillations, which produces electromagnetic fields with absorption, scattering, and near-field components. When the dimension of the NPs becomes smaller than the wavelength of the excitation light, the light energy can be controlled within a small spatial area through the local excitation of SPR.29 It is implicit that the local electromagnetic field in these regions will increase dramatically. Local electric field enhancement is one of the most popular optical properties widely applied for liquid analysis, such as the detection of fluoride ions in aquatic samples using surface-enhanced Raman scattering.30
Recently, many researchers have focused their attention on the coupling action between laser electromagnetic fields and the surface plasmon of nanoparticles in LIBS studies. Nanoparticles can act as seed electrons at multiple ignition points upon the occurrence of coupling, and thus plasma is formed with a broad spatial area, which in turn results in more effective ablation and stronger emission signals. Based on the fact mentioned above, the so-called nanoparticle enhanced laser-induced breakdown spectroscopy (NELIBS) has been realized.31 This technology has not only been successfully applied to the analysis of solid samples, such as metal samples31 and transparent samples,32 but also applied to liquid samples. Giacomo and co-workers showed that NELIBS could increase the signal strength of the elements in liquid samples.33 They deposited AuNPs on a glass substrate before the analytical sample and manifested that the signal of the element characteristic line was more than doubled as against the conventional LIBS method. Wen et al. reported a pretreatment method based on the combination of AuNPs and solid–liquid conversion to enhance the LIBS signal for metal ions in the aqueous sample.34 The LODs obtained for Cr, Pb, and Cu were 0.5, 0.5, and 1.1 μg mL−1, respectively. As mentioned above, the pre-processing methods mentioned in both studies contain two steps. During the drying process, both the AuNPs and analytes diffuse from the midpoint to the edge, causing inhomogeneous distribution. Generally, the signal response of an element can be enhanced due to the aggregation of droplets, but the shape and concentration of the sample may be difficult to restrict.35 Another drawback is that the sample processing methods mentioned above result in a minor area of contact between the AuNPs and the analyte particles, and adsorption force is relatively weak.
Seeking improvement of the homogeneity and the adhesion force between the analytes and AuNPs, we mixed the sample solution (Cd, Cu, Ag, Pb, and Cr) in sequence with DDTC and AuNPs, which was named metal-chelate induced nanoparticle aggregation enhanced laser-induced breakdown spectroscopy (MINAELIBS), instead of depositing nanoparticles on the surface of the sample as described in the literature (NELIBS).31,33 First, sodium diethyldithiocarbamate (DDTC) was employed as a chelating agent for the preconcentration of metal ions (MIs), thus forming soluble metal chelates (MIs/DDTC). Second, gold nanoparticles (AuNPs) as a signal-enhancement promoter were directly mixed with the metal chelates, which can lead to a larger contact area between analytes and AuNPs and enhance the adhesion force between them. Third, the final reaction mixture (MIs/DDTC/AuNPs) was analyzed by LIBS. Emission intensities and the signal-to-noise ratios (SNRs) of target elements were significantly enhanced without increasing the complexity of the experimental setups. Lower detection limits and excellent linear ranges were acquired by optimizing various experimental parameters. The natural water sample was investigated as well to verify the feasibility of the proposed method for practical application.
2. Experimental section
2.1. Chemicals and reagents
Stock solutions (1 mg mL−1 of Cr(NO3)2·9H2O, Cd(NO3)2·4H2O, Cu(NO3)2·3H2O, Ag(NO3)2 and Pb(NO3)2) were prepared by dissolving analytical reagents in ultrapure water (18.25 MΩ cm−1) and stored in the dark. The working standard solutions were prepared by a serial dilution process of the stock solution. Nitric acid (HNO3, 65–68%) was purchased from Chengdu Kelong Chemicals Co., Ltd. (Chengdu, China). Aqueous ammonium hydroxide solution (NH3, 25%) was acquired from Guangdong Guanghua Sci-Tech Co., Ltd. (Guangzhou, China). DDTC was purchased from Aladdin Biochemical Sci-Tech Co., Ltd. (Shanghai, China). DDTC (5%, m/v) water solution was prepared before using to avoid slow decomposition. The 0.1 mol L−1 HNO3 water solution and 0.1 mol L−1 NH3 water solution were prepared to adjust pH values. Analytical reagent grade pure chemicals were used throughout the experiments. Ultrapure water was used to prepare all the solutions involved in the experiments.2.2. Synthesis of the AuNPs
AuNPs were prepared by sodium citrate reduction of HAuCl4.36,37 The AuNPs were monodisperse with a diameter of 15.2 ± 0.06 nm, as shown in the ESI (Fig. S1†). All glassware used was thoroughly cleaned in aqua regia (3 parts HCl, 1 part HNO3), cleaned in ultrapure water at least three times, and oven-dried before use. First, 1.0 mL 1% chloroauric acid solution and 46 mL ultrapure water were added into a three-necked flask. After heating to the boiling point, 3 mL 1% sodium citrate solution was added to the boiling solution with good mechanical stirring. Subsequently, the solution turned grey, purple, and finally to a deep wine-red color. After continuous boiling for 30 min, it was set aside to cool to room temperature and the AuNPs were transferred to a brown centrifugal tube. The resultant AuNPs were stored in a refrigerator at 4 °C for further use.2.3. Apparatus
The schematic diagram of the LIBS experimental platform is shown in Fig. 1A. A Q-switched Nd:YAG laser (Nano, Litron, UK) operating at 1064 nm with a pulse width of 6–8 ns and repetition rate of 10 Hz was employed. The laser pulse energy was monitored using a Gentec-EO energy meter. The laser pulse was perpendicularly focused onto the target surface using a high-power micro-spot focusing objective lens (LMH-5X-1064, Thorlabs) with a work distance of 35 mm. Two laser diodes were mounted on a micro-support with adjustable angle to guarantee that the distance from the laser to the sample remained the same during each experiment. A motorized X–Y–Z sample stage was utilized to place the sample to enable a fresh surface for each analysis. The emitted light was processed using an echelle spectrometer (Aryelle 200, Lasertechnik Berlin GmbH, Germany) equipped with an ICCD camera (iStar, Andor, UK). The pulse laser and spectrometer were triggered using a LIBS control box (Lasertechnik Berlin GmbH, Germany).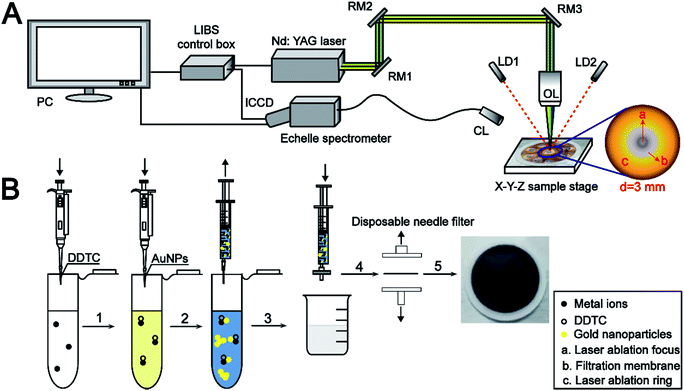 | ||
| Fig. 1 Schematic diagram of the LIBS experimental setup (A) and sample pretreatment (B). RM1, RM2, and RM3: reflection mirror; LD1, LD2: laser-diode; OL: objective lens; CL: collimating lens. | ||
2.4. The sample pretreatment procedure
In this study, the nylon 66 microfiltration membrane (13 mm, 0.22 μm) for rapid separation of the sample was purchased from Jinteng Company (Tianjin, China). A simple sample pretreatment procedure is illustrated in Fig. 1B.5 mL working standard solution was placed in a 10 mL centrifuge tube, and the pH value was adjusted to near 5.0. Then, 300 μL of prepared DDTC (5%, m/v) water solution was added into the above sample solution, and the metal ions rapidly reacted with DDTC to form soluble metal chelates subsequently. At the same time, the sample solution color turns pale yellow. After 1 mL AuNP solution was added to the sample solution, the yellow color suddenly changed to light blue, which indicates that the monodisperse spherical AuNPs were susceptible to the environment causing a higher degree of aggregation. Then, the resultant mixture (MIs/DDTC/AuNPs) consisting of the metal chelates and the AuNPs was rapidly filtered using a 0.22 μm membrane. Ultimately, the membrane was fixed on a sliding glass with double-sided adhesive for LIBS analysis directly.
3. Results and discussion
3.1. Morphological characterization of samples
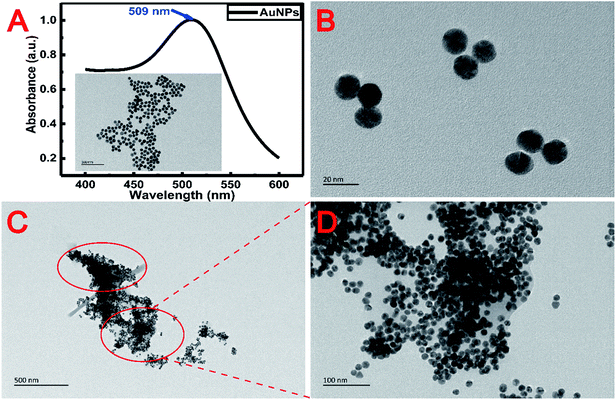
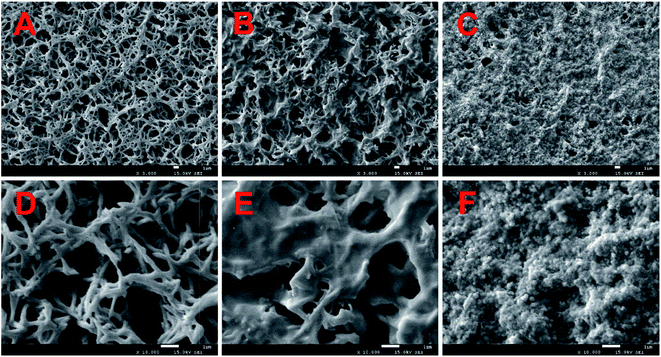
To know the microstructure of the constituent particles of the gold colloidal solution, the AuNPs used in the experiment were characterized by transmission electron microscopy (TEM) (Tecnai G2 F20 S-TWIN, FEI, USA) and UV-vis spectroscopy (Lambda 25, PerkinElmer, USA). Fig. 2A and B show that the AuNPs were spherical and monodisperse with a diameter of about 15 nm. The optical properties of AuNPs were strongly influenced by the surrounding environment in addition to their inherent characteristics, such as size, shape, etc.28 Therefore, as shown in Fig. 2C, a great degree of aggregation occurred when AuNPs were added into the metal chelate solution. This phenomenon can be explained by the fact that the metal chelates readily adsorb hydrogen ions in a slightly acidic reaction system, leading to a positively charged surface that induces the aggregation of negatively charged AuNPs around the metal chelates. Conversely, AuNPs are monodisperse beyond the boundary of the sample solution, as shown in Fig. 2D.The morphology of the sample on the surface of the membrane was characterized by scanning electron microscopy (SEM) (JSM-7500F, JEOL, Japan). The 3000× magnified SEM pictures of the membranes used for the filtration of reagent blank, metal chelates and the resultant mixture (MIs/DDTC/AuNPs) are shown in Fig. 3A–C, respectively. Fig. 3A and B clearly show that the metal chelates were obtained by membrane filtration. Likewise, the gaps between the fibers in Fig. 3C were narrower than those in Fig. 3B, indicating that AuNPs and metal chelates were simultaneously deposited on the membrane due to the electrostatic forces between them. As a consequence, the membrane area covered by the treated sample as shown in Fig. 3C appears to be larger and more uniform than that shown in Fig. 3B. For a closer specification, the corresponding SEM pictures of the membranes at the 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× magnification scale are shown in Fig. 3D–F, respectively. As shown in Fig. 3E, the metal chelates were either adsorbed on or filled between fibers. Oppositely, Fig. 3F clearly indicates that a great number of insoluble particles composed of AuNPs and metal chelates were almost filled in the gap between membrane fibers, which helps to improve the coverage and homogenization of the samples on the membrane surface. Additionally, AuNPs, which acted as seed electrons, can reduce the breakdown threshold of the sample surface, increase the ablation amount of per unit area, and improve the ablation efficiency of the sample.
000× magnification scale are shown in Fig. 3D–F, respectively. As shown in Fig. 3E, the metal chelates were either adsorbed on or filled between fibers. Oppositely, Fig. 3F clearly indicates that a great number of insoluble particles composed of AuNPs and metal chelates were almost filled in the gap between membrane fibers, which helps to improve the coverage and homogenization of the samples on the membrane surface. Additionally, AuNPs, which acted as seed electrons, can reduce the breakdown threshold of the sample surface, increase the ablation amount of per unit area, and improve the ablation efficiency of the sample.
3.2. Optimization of the experimental conditions
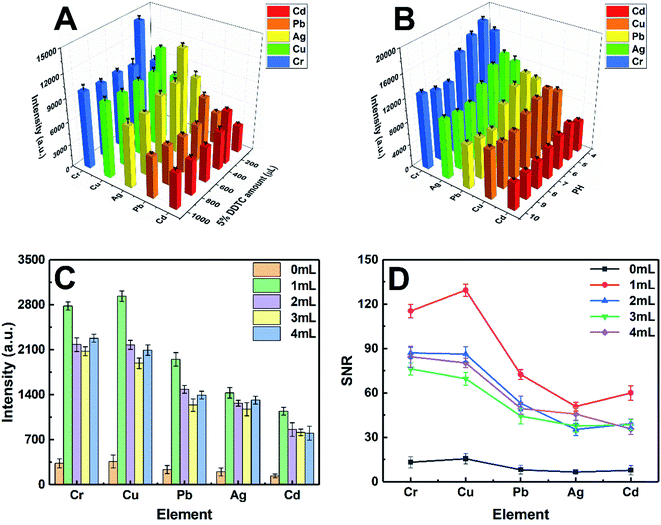
In this study, the experimental conditions at the initial stage have been optimized, including imperative conditions of pretreatment and the system parameters of LIBS. The characteristic spectral lines at 226.502 nm, 327.396 nm, 328.068 nm, 405.781 nm, and 425.433 nm were selected for the quantitative analysis of Cd II, Cu I, Ag I, Pb I, and Cr I, respectively.The pH value of the working standard solution and the amount of DDTC played a vital role in the metal-chelate formation during the enrichment of metal ions and the subsequent LIBS analysis. The effect of the DDTC amount on the enrichment efficiency was investigated in the range of 100–1000 μL, as shown in Fig. 4A. With the volume increase of DDTC from 200 to 300 μL, the signal intensities of the target elements increased gradually. However, when the volume of DDTC was larger than 300 μL, the signal intensities were decreased due to the matrix effect. The results showed that 300 μL of DDTC (5%, m/v) has the best complexing effect on all the target elements. In the chelating reaction system, the preconcentration efficiency of metal ions is closely related to the pH of the system. Thus, a series of experiments were performed by adjusting the pH from 4.0 to 10.0 with diluted HNO3 and NH3 water solution. Fig. 4B indicates that the strongest signal response of target elements was obtained at a pH of 5.0. The signal intensities of the target elements are essentially constant as the pH increased beyond 5.0, which can be rationalized that complete chelation requires appropriate acidity, while high pH may result in precipitation. To enhance the signal response of elements at low concentrations (ppb level), we adopted a strategy of directly mixing the AuNPs with the metal chelates for further enhancement of SNRs without additional equipment and costs. The concentration of target elements was 0.5 μg mL−1. As shown in Fig. 4C and D, the signal intensities and SNRs of the target elements significantly enhanced with AuNPs. The analytical elements have the highest signal intensities and the best SNRs in 1 mL AuNP solution. This phenomenon can be interpreted as that the analytical elements may be wrapped without being fully excited when AuNPs were excessive. Therefore, the optimum amount of the 3.34 nmol L−1 AuNP solution was chosen to be 1 mL. The concentration of AuNP solution was calculated from UV-vis spectra according to the method in the literature.38 The optimization of LIBS system parameters is equally essential. The pulse energy, delay time, and gate width were eventually selected as 36 mJ, 1 μs, and 30 μs, respectively. Under the above optimal conditions, in addition to the ablation of samples at the focal position, three different degrees of laser ablation rings centered on the focal point spot were also observed. Ultimately, the maximum diameter of the ablation ring covered by the plasma can be up to 3 mm (Fig. 1A). Furthermore, throughout the experiment, each parallel sample provided ten sets of data with different fresh points, each set of data being the average of the cumulative results of ten laser ablation events.
3.3. Qualitative and quantitative analyses
According to the sample pretreatment procedure as described in the Experimental section, 5 mL working standard solution containing 0.5 μg mL−1 analytical elements was treated under the optimum conditions. Fig. 5 shows the comparison of the emission spectra of all target elements in metal chelates and metal chelate-induced AuNP aggregation, with the signal response of the latter significantly increased. In addition to the effective enrichment of the target elements with DDTC at the trace level, AuNPs with thermal insulation defects may produce atoms, ions, and electrons when they are excited, thereby increasing the number of particles in the plasma, resulting in more effective ablation. The amount of ablated and excited analytes is directly proportional to the emission spectral intensity in LIBS analysis. Therefore, another reason for signal enhancement is that more samples were ablated in the latter. Except for qualitative analysis, calibration curves in both cases were established using standard solutions in the concentration range from 10 to 1000 ng mL−1 and from 50 to 1000 ng mL−1, respectively, as shown in Fig. 6. The error bar of each data point on the calibration curve represents the standard deviation of ten sets of parallel data. Fig. 6 clearly shows that in the presence of AuNPs, the calibration curves of Cd, Cu, Ag, Pb, and Cr exhibited good linear behavior with high correlation coefficients, and Cu, Ag, Pb, and Cr can be measured at concentrations as low as 10 ppb and Cd at 40 ppb. Linearity was observed over the range of 40–400 ng mL−1, 40–400 ng mL−1, 10–1000 ng mL−1, 40–400 ng mL−1, and 10–800 ng mL−1 for Cd, Cu, Ag, Pb, and Cr, respectively. In addition, the calibration curves of Cd, Cu, and Pb exhibit varying degrees of linear deviation at high concentrations due to self-absorption.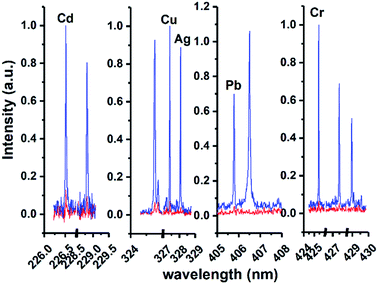 | ||
| Fig. 5 Comparison of emission spectra of all target elements in metal chelates (red lines) and metal chelate induced AuNP aggregation (blue lines). | ||
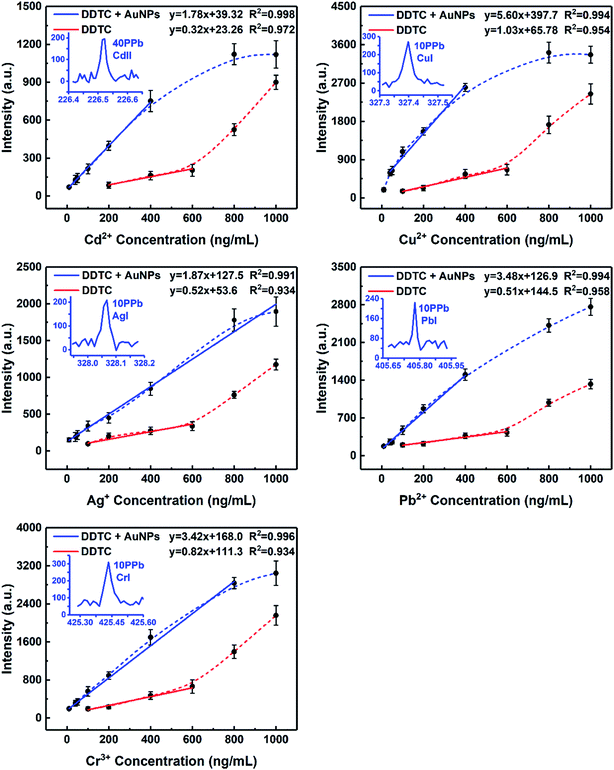 | ||
| Fig. 6 Calibration curves obtained for Cd, Cu, Ag, Pb, and Cr. All target elements were analyzed under optimal experimental conditions. | ||
The LODs of all target elements based on the characteristic lines were approximated according to the IUPAC definition: LOD = 3σB/s, where σB is the standard deviation (SD) of 11 parallel measurements of blank control samples and s is the slope of the calibration curve. The standard deviations, slopes, and LODs calculated for the target elements obtained by MINAELIBS are listed in Table 1. The LODs of Cd, Cu, Ag, Pb, and Cr were 4.6, 1.5, 3.4, 3.5, and 3.5 ng mL−1, respectively. With the same experimental setup (both using ICCD detectors), Table 2 shows the comparison of the LODs (μg mL−1) of target elements in liquid samples obtained with various methods including sample non-pretreatment, DDTC and AuNPs as auxiliary reagents combined with LIBS technology. Obviously, LODs of target elements in the presence of AuNPs were 7–10 times lower than those without AuNPs and decreased by 3–4 orders of magnitude compared to traditional LIBS.6,11,39,40 The results unambiguously demonstrate that metal chelate induced nanoparticle aggregation can enhance LIBS signals and sensitivity making it effective for direct LIBS detection of metal ions in liquid samples.
| Elements | MINAELIBS | ||
|---|---|---|---|
| Standard deviations of blank samples (n = 11) | Slopes of the calibration curve | LODs (ng mL−1) | |
| Cd | 2.66 | 1.78 | 4.5 |
| Cu | 2.83 | 5.60 | 1.5 |
| Ag | 2.11 | 1.87 | 3.4 |
| Pb | 4.09 | 3.48 | 3.5 |
| Cr | 4.04 | 3.42 | 3.5 |
| Element | The LODs of different enrichment methods (μg mL−1) | LODb ratios | ||
|---|---|---|---|---|
| Nonea (literature) | DDTC (this work) | DDTC, AuNPs (this work) | ||
| a Without any pretreatment. b Calculated as the ratio of LOD values obtained with AuNPs and without AuNPs in preprocessing. | ||||
| Cd | 68 (ref. 11) | 0.0446 | 0.0045 | 10 |
| Cu | 7 (ref. 6) | 0.0128 | 0.0015 | 9 |
| Ag | 12 (ref. 39) | 0.0223 | 0.0034 | 7 |
| Pb | 12.5 (ref. 40) | 0.0324 | 0.0035 | 9 |
| Cr | 10 (ref. 6) | 0.0257 | 0.0035 | 7 |
3.4. Method validation
Taking account of the feasibility and practicality of the MINAELIBS method for analysis of environmental water samples, the accuracy of the proposed method was evaluated by analyzing spiked samples and comparing with the results obtained by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (PerkinElmer Optima 8000). The recoveries were calculated by using the ICP-OES results as the reference values.The environmental samples obtained from the natural river (Ming-yuan Lake) were spiked with the analytes with concentrations of 50 ng mL−1, 150 ng mL−1, and 300 ng mL−1, respectively. All parallel samples were analyzed under the optimal conditions, and the results are listed in Table 3. It can be seen that the determined values by MINAELIBS are in satisfactory agreement with the results obtained by ICP-OES. In other words, the results measured by MINAELIBS were close to exact values of the added content of target elements. The recoveries of the spiked samples were calculated according to the definition:
| P = (m2 − m1)/m × 100% |
| Spikeda (ng mL−1) | ICP-AES ± SDb | MINAELIBS | |||
|---|---|---|---|---|---|
| Mean foundc (ng mL−1) | RSD (%) | Recoveryc (%) | |||
| a Real value in environmental water samples. b Mean value ± standard deviation. c Mean value; ND = not determined. | |||||
| Cd | 0 | 2.857 ± 0.25 | ND | — | — |
| 50 | 52.09 ± 0.50 | 48.92 | 10.80 | 92.13 | |
| 150 | 153.5 ± 1.12 | 148.2 | 10.32 | 96.90 | |
| 300 | 304.4 ± 1.53 | 292.8 | 9.42 | 96.65 | |
| Cu | 0 | 10.79 ± 0.26 | ND | — | — |
| 50 | 57.12 ± 1.88 | 62.98 | 8.93 | 104.4 | |
| 150 | 158.0 ± 0.70 | 155.2 | 9.63 | 96.27 | |
| 300 | 311.4 ± 2.41 | 296.6 | 10.30 | 95.23 | |
| Ag | 0 | 5.280 ± 0.07 | ND | — | — |
| 50 | 54.56 ± 1.38 | 60.22 | 10.59 | 109.9 | |
| 150 | 152.0 ± 2.14 | 148.9 | 8.02 | 95.75 | |
| 300 | 304.6 ± 1.85 | 287.2 | 10.36 | 93.97 | |
| Pb | 0 | 2.53 ± 0.13 | ND | — | — |
| 50 | 50.61 ± 0.65 | 49.12 | 7.38 | 93.18 | |
| 150 | 151.2 ± 1.60 | 145.1 | 7.28 | 95.05 | |
| 300 | 304.2 ± 2.31 | 299.5 | 10.18 | 98.99 | |
| Cr | 0 | 13.78 ± 0.22 | ND | — | — |
| 50 | 61.60 ± 1.64 | 58.28 | 10.68 | 89.00 | |
| 150 | 163.3 ± 0.98 | 146.7 | 9.34 | 88.61 | |
| 300 | 311.4 ± 1.31 | 297.3 | 10.91 | 94.51 | |
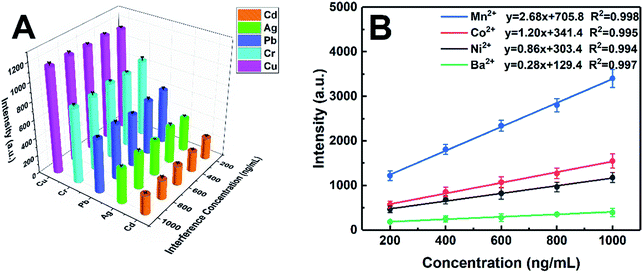
Considering that DDTC is a general chelating agent, the competitive complexing from other heavy metal ions will interfere with the determination of target ions. Therefore, in order to evaluate the selectivity of the proposed method, the possible interference was also investigated. Different concentration levels (200 ng mL−1, 400 ng mL−1, 600 ng mL−1, 800 ng mL−1, and 1000 ng mL−1) of Ni, Co, Mn, and Ba were added as the interferents into the sample solutions containing 100 ng mL−1 of target elements, which means that the concentrations of interferents are up to 10-fold of the targets. All samples were prepared according to the method mentioned in the Experimental section and analyzed under the optimum conditions. As shown in Fig. 7A, the signal intensities of the target elements were basically unchanged within the tested concentration range of the interferents. Moreover, Fig. 7B shows that Mn, Co, Ni, and Ba also exhibited a good linear relationship in the case of coexistence of various ions, with corresponding linear correlation coefficients of Mn, Co, Ni, and Ba being 0.998, 0.995, 0.994, and 0.997, respectively. Thus, the strategy of metal-chelate induced nanoparticle aggregation enhanced signal proposed in this study is applicable for liquid samples with multiple ions coexisting, which reflects the potential of the MINAELIBS technology for analysis of elaborate environmental water.
4. Conclusions
It is well known that liquid splashing and plasma quenching in the process of laser ablation will lead to poor repeatability and low accuracy. Consequently, the direct detection of trace metal ions in liquid samples by LIBS is still a challenge. To overcome this problem, metal-chelate induced nanoparticle aggregation enhanced laser-induced breakdown spectroscopy (MINAELIBS) was proposed to improve the analytical performance of the LIBS method in liquid samples, which not only overcame the defect of liquid splashing, but also increases the adhesion force and uniformity of the sample. Additionally, the coupling action between laser electromagnetic fields and the surface plasmon of AuNPs greatly improved the laser ablation efficiency in the process of laser–matter interaction. Several experimental parameters and conditions affecting the quantitative results were optimized and satisfactory detection limits were obtained, with the LODs of Cd, Cu, Ag, Pb, and Cr being 4.6, 1.5, 3.4, 3.5, and 3.5 ng mL−1, respectively. The LODs of target elements were considerably decreased by 7–10 times compared to those of metal chelate enrichment alone, and they are decreased by 3–4 orders of magnitude compared to traditional LIBS, indicating the effectiveness of the proposed MINAELIBS method to detect trace metal elements in aqueous solution with high sensitivity. The MINAELIBS method has also been successfully applied to the determination of trace metal elements in environmental water samples with excellent repeatability and accuracy. In general, the proposed MINAELIBS method has technical characteristics of simplicity, rapidity and low cost, in line with the development needs of modern analytical methods.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the Startup Funding of Northwest University for setting up the Research Center of Analytical Instrumentation, the Key Research and Development Program of Shaanxi Province of China (2019ZDLSF01-03), and the National Natural Science Foundation of China (61605134).References
- Y. Liu, Y. Guo, S. Meng, F. Feng and X. Chang, Microchim. Acta, 2007, 157, 209–214 CrossRef CAS.
- H. Karami, M. F. Mousavi, Y. Yamini and M. Shamsipur, Anal. Chim. Acta, 2004, 509, 89–94 CrossRef CAS.
- H. Watanabe, S. Berman and D. S. Russell, Talanta, 1972, 19, 1363–1375 CrossRef CAS.
- E. F. Runge, R. W. Minck and F. R. Bryan, Spectrochim. Acta, 1964, 20, 733–736 CrossRef CAS.
- Y. Ding, F. Yan, G. Yang, H. Chen and Z. Song, Anal. Methods, 2018, 10, 1074–1079 RSC.
- P. Fichet, P. Mauchien, J.-F. Wagner and C. Moulin, Anal. Chim. Acta, 2001, 429, 269–278 CrossRef CAS.
- L. St-Onge, E. Kwong, M. Sabsabi and E. B. Vadas, J. Pharm. Biomed. Anal., 2004, 36, 277–284 CrossRef CAS PubMed.
- N. K. Rai and A. K. Rai, J. Hazard. Mater., 2008, 150, 835–838 CrossRef CAS PubMed.
- X. Wang, L. Shi, Q. Lin, X. Zhu and Y. Duan, J. Anal. At. Spectrom., 2014, 29, 1098–1104 RSC.
- Q. Lin, F. Bian, Z. Wei, S. Wang and Y. Duan, J. Anal. At. Spectrom., 2017, 32, 1412–1419 RSC.
- M. S. Cheri and S. H. Tavassoli, Appl. Opt., 2011, 50, 1227–1233 CrossRef PubMed.
- M. Yao, J. Lin, M. Liu and Y. Xu, Appl. Opt., 2012, 51, 1552–1557 CrossRef CAS PubMed.
- S. Nakamura, Y. Ito, K. Sone, H. Hiraga and K.-i. Kaneko, Anal. Chem., 1996, 68, 2981–2986 CrossRef CAS PubMed.
- A. Kumar, F. Y. Yueh and J. P. Singh, Appl. Opt., 2003, 42, 6047–6051 CrossRef PubMed.
- W. Pearman, J. Scaffidi and S. M. Angel, Appl. Opt., 2003, 42, 6085–6093 CrossRef PubMed.
- V. Lazic, F. Colao, R. Fantoni and V. Spizzicchino, Spectrochim. Acta, Part B, 2005, 60, 1002–1013 CrossRef.
- V. I. Babushok, F. C. DeLucia, J. L. Gottfried, C. A. Munson and A. W. Miziolek, Spectrochim. Acta, Part B, 2006, 61, 999–1014 CrossRef.
- S. L. Lui, Y. Godwal, M. T. Taschuk, Y. Y. Tsui and R. Fedosejevs, Anal. Chem., 2008, 80, 1995–2000 CrossRef CAS PubMed.
- H. Loudyi, K. Rifaï, S. Laville, F. Vidal, M. Chaker and M. Sabsabi, J. Anal. At. Spectrom., 2009, 24, 1421–1428 RSC.
- M. A. Gondal and T. Hussain, Talanta, 2007, 71, 73–80 CrossRef CAS PubMed.
- Z. Chen, H. Li, M. Liu and R. Li, Spectrochim. Acta, Part B, 2008, 63, 64–68 CrossRef.
- M. M. Rao, G. P. C. Rao, K. Seshaiah, N. V. Choudary and M. C. Wang, Waste Manag., 2008, 28, 849–858 CrossRef CAS PubMed.
- D. Zhu, J. Chen, J. Lu and X. Ni, Anal. Methods, 2012, 4, 819–823 RSC.
- N. E. Schmidt and S. R. Goode, Appl. Spectrosc., 2002, 56, 370–374 CrossRef CAS.
- K. M. Santos, J. Cortez, I. M. Raimundo, C. Pasquini, E. S. Boa Morte and M. G. A. Korn, Microchem. J., 2013, 110, 435–438 CrossRef CAS.
- C. R. Dockery, J. E. Pender and S. R. Goode, Appl. Spectrosc., 2005, 59, 252–257 CrossRef CAS PubMed.
- X. Wang, Y. Wei, Q. Lin, J. Zhang and Y. Duan, Anal. Chem., 2015, 87, 5577–5583 CrossRef CAS PubMed.
- S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797–4862 CrossRef CAS PubMed.
- K. H. Su, Q. H. Wei, X. Zhang, J. J. Mock, D. R. Smith and S. Schultz, Nano Lett., 2003, 3, 1087–1090 CrossRef CAS.
- X. Li, M. Zhang, Y. Wang, X. Wang, H. Ma, P. Li, W. Song, X. Xia Han and B. Zhao, Talanta, 2018, 178, 9–14 CrossRef CAS PubMed.
- A. De Giacomo, R. Gaudiuso, C. Koral, M. Dell'Aglio and O. De Pascale, Anal. Chem., 2013, 85, 10180–10187 CrossRef CAS PubMed.
- C. Koral, M. Dell'Aglio, R. Gaudiuso, R. Alrifai, M. Torelli and A. De Giacomo, Talanta, 2018, 182, 253–258 CrossRef CAS PubMed.
- A. De Giacomo, C. Koral, G. Valenza, R. Gaudiuso and M. Dell'Aglio, Anal. Chem., 2016, 88, 5251–5257 CrossRef CAS PubMed.
- X. Wen, Q. Lin, G. Niu, Q. Shi and Y. Duan, Appl. Opt., 2016, 55, 6706–6712 CrossRef CAS PubMed.
- C. Zhao and D. Dong, Anal. Chem., 2016, 88, 9869–9870 CrossRef CAS PubMed.
- G. Frens, Nat. Phys. Sci., 1973, 241, 20 CrossRef CAS.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- W. Haiss, N. T. K. Thanh, J. Aveyard and D. G. Fernig, Anal. Chem., 2007, 79, 4215–4221 CrossRef CAS PubMed.
- P. Yaroshchyk, R. J. S. Morrison, D. Body and B. L. Chadwick, Spectrochim. Acta, Part B, 2005, 60, 986–992 CrossRef.
- H. Sobral, R. Sanginés and A. Trujillo-Vázquez, Spectrochim. Acta, Part B, 2012, 78, 62–66 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ja00324j |
| This journal is © The Royal Society of Chemistry 2020 |