Calcium-catalyzed regioselective dehydrative cross-coupling of propargylic alcohols with 1,3-dicarbonyl compounds†
Abstract
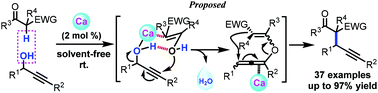
A novel calcium-catalyzed in situ dehydrative cross-coupling reaction of propargylic alcohols with 1,3-dicarbonyl compounds was developed. This catalytic system can suppress the competitive Meyer–Schuster rearrangement, control regioselectivity, and enable the desired process to occur under solvent-free conditions at room temperature with water as the only byproduct. A variety of vicinal tertiary and all-carbon quaternary centers can be obtained in high yields with broad functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...