Transition-metal-free selective pyrimidines and pyridines formation from aromatic ketones, aldehydes and ammonium salts†
Abstract
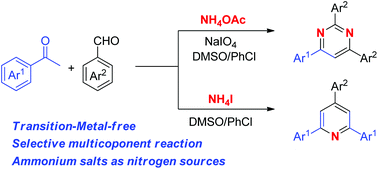
An efficient synthesis of pyrimidines and pyridines has been developed from readily available aromatic ketones, aldehydes and ammonium salts under transition-metal-free conditions. In this strategy, ammonium salts were used as nitrogen sources and only water was generated as a nontoxic byproduct. A catalytic amount of NaIO4 played an important role in the selectivity control, whereas substituted pyridines were dominantly formed in its absence.


 Please wait while we load your content...
Please wait while we load your content...