Developing a sustainable route to environmentally relevant metal–organic frameworks: ultra-rapid synthesis of MFM-300(Al) using microwave heating†
Ieuan
Thomas-Hillman
 a,
Lee A.
Stevens
a,
Marcus
Lange
b,
Jens
Möllmer
b,
William
Lewis
a,
Lee A.
Stevens
a,
Marcus
Lange
b,
Jens
Möllmer
b,
William
Lewis
 cd,
Chris
Dodds
cd,
Chris
Dodds
 a,
Samuel W.
Kingman
a,
Samuel W.
Kingman
 a and
Andrea
Laybourn
a and
Andrea
Laybourn
 *a
*a
aFaculty of Engineering, University of Nottingham, Nottingham, NG7 2RD, UK. E-mail: Andrea.Laybourn@nottingham.ac.uk
bInstitut für Nichtklassische Chemie e.V., Permoserstrasse 15, 04318 Leipzig, Germany
cSchool of Chemistry, University of Nottingham, Nottingham NG7 2RD, UK
dSchool of Chemistry, The University of Sydney, New South Wales 2006, Australia
First published on 1st August 2019
Abstract
NO2, SO2 and CO2 are major air pollutants causing significant environmental and health problems. Metal–organic frameworks (MOFs), in particular [Al2(OH)2(C16O8H6)](H2O)6 (trivial names: NOTT-300/MFM-300(Al)), have shown great promise for capturing these gases. However MOF syntheses often involve toxic solvents and long durations which are inherently energy intensive, an environmental burden, and have serious safety risks. There is a pressing need to develop environmentally-friendly routes to MOFs that require less energy and implement safer solvents particularly when considering scale-up beyond the laboratory for industrial application. We report the rapid synthesis of MFM-300(Al) in aqueous conditions and 10 minutes using microwave heating. This is the fastest reported synthesis of MFM-300(Al) to date with a 99.77% reduction in reaction time compared to the current reported 3-day conventionally heated route. The microwave synthesized sub-micron crystalline material exhibits gas uptake capacities of 8.8 mmol g−1 at 273 K and 1.0 bar for CO2, 8.5 mmol g−1 at 298 K and 0.17 bar for SO2, and 1.9 mmol g−1 at 298 K and 0.01 bar for NO2. These are 26%, 70%, and 90% greater for CO2, SO2, and NO2, respectively, when compared to previously reported MFM-300(Al) materials produced via a 3-day conventionally heated route demonstrating the production of high quality materials in a fraction of the time with enhanced gas properties. Crucially, this offers an opportunity to move from batch to continuous processing owing to reduced reaction times underpinned by targeted heating.
The dioxides of nitrogen, sulfur and carbon (NO2, SO2 and CO2, respectively) are regarded as three of the most problematic air pollutants generated by anthropogenic activity.1–3 NO2 and SO2 are particularly detrimental to the environment3–5 and human health1,6,7 as they contribute to toxic photochemical smog and acid rain.8 Additionally, the escalating level of atmospheric CO2 is a major environmental concern due to its implication in global warming.3,9 The harmful effects of NO2, SO2 and CO2 have provided significant impetus for their removal and the development of a sustainable low-carbon economy.
Metal–organic frameworks (MOFs) are subset of co-ordination polymers that show great potential for gas storage and separation owing to their exceptionally high surface areas and tuneable pore environment and functionalities.8,9 Notably [Al2(OH)2(C16O8H6)](H2O)6 (trivial names: NOTT-300/MFM-300(Al)) has shown exceptional selective reversible adsorption and uptake capacity of CO2, SO2, low-concentration NO2 (5000 to <1 ppm), and ammonia.10–17 A highly sensitive SO2 sensor based on the indium analogue of MFM-300 was also recently reported.18 However, current methods of preparing MFM-300 and indeed many other MOFs, hinders their adoption in industrial gas storage and separation applications.19 MOFs are commonly synthesised via solvothermal batch reactions which involve large volumes of toxic solvent and long reaction times (up to one week).20 The development of routes that reduce the product cost and environmental burden of MOFs is critical for the transfer of these materials from the laboratory to industry and the realisation of the environmental benefits that these materials offer.21 In order to address these synthetic challenges, electrochemical, sonochemical, mechanochemical, spray drying, continuous flow, and microwave methods have been developed.20,22 Of these routes, microwave heating has been shown to offer highly significant benefits such as exceptionally rapid reactions (on the order of seconds),23 control over particle morphology and size, and phase selectivity.21
In this paper we describe an aqueous microwave-assisted method for synthesising MFM-300(Al) on the tens of milligrams scale in 10 minutes. This is the first time a microwave route to MFM-300(Al) has been reported. This route requires significantly less time than the current procedure (3-day solvothermal synthesis at 210 °C)12 and negates the requirement for a corrosive piperazine additive,12 whilst delivering MFM-300(Al) at a higher percentage yield (83 cf. 75%) with a highly uniform particle size and morphology and significantly enhanced uptake capacities for CO2, SO2 and NO2.
A 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
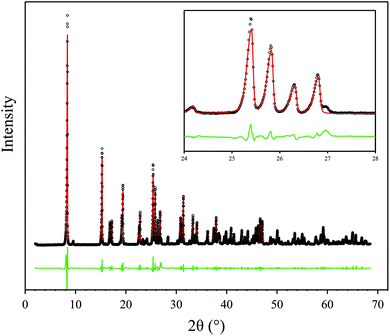
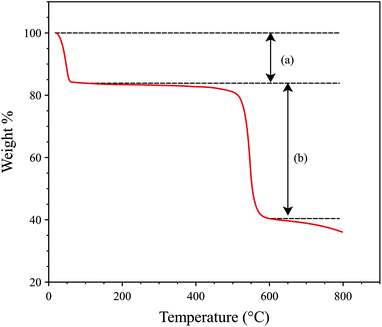
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of aluminium chloride hexahydrate and biphenyl-3,3′,5,5′-tetracarboxylic acid (H4BPTC) in deionized water was heated, with stirring, at 210 °C in a commercial CEM Discover microwave cavity for 10 minutes. Further synthetic details, including method development, are given in the Experimental section. Note: Temperature was determined by an in-built infra-red sensor and therefore represents the average temperature of the reaction mixture. The resulting suspended particles of microwave synthesised MFM-300(Al) (hereby denoted MFM-300(Al)-MW) were found to be 249–790 nm long and between 74–964 nm wide, with a stubby cylindrical to cuboidal morphology (Fig. 1). This differs from the plate-like sub-micron particles previously reported for MFM-300(Al)12 prepared using conventional heating (hereby denoted MFM-300(Al)-CH) and is a likely consequence of the different heating mechanism promoting a different mode of crystal growth.21,23 Reflections in the powder X-ray diffraction (PXRD) pattern of MFM-300(Al)-MW were indexed to the I4122 tetragonal space group, corresponding to the reported crystal structure of MFM-300(Al)-CH (Fig. 2).12 A simulated Le Bail fit24 to the I4122 space group revealed the presence of a low intensity extra reflection at around 27° 2θ (Fig. 2) attributed to residual unreacted linker (H4BPTC). However, there was no detectable evidence of unreacted linker by thermogravimetric analysis, i.e. no loss at the decomposition temperature of H4BPTC (340 °C) was observed (Fig. 3). No peaks corresponding to γ-Al(O)OH25 or other MOF phases beyond MFM-300(Al) were observed. Attempts to synthesise MFM-300(Al) using these conditions with conventional heating for 24 to 72 hours (rather than rapidly using microwaves) gave a mixture of MOF phases and γ-Al(O)OH.25
1 mixture of aluminium chloride hexahydrate and biphenyl-3,3′,5,5′-tetracarboxylic acid (H4BPTC) in deionized water was heated, with stirring, at 210 °C in a commercial CEM Discover microwave cavity for 10 minutes. Further synthetic details, including method development, are given in the Experimental section. Note: Temperature was determined by an in-built infra-red sensor and therefore represents the average temperature of the reaction mixture. The resulting suspended particles of microwave synthesised MFM-300(Al) (hereby denoted MFM-300(Al)-MW) were found to be 249–790 nm long and between 74–964 nm wide, with a stubby cylindrical to cuboidal morphology (Fig. 1). This differs from the plate-like sub-micron particles previously reported for MFM-300(Al)12 prepared using conventional heating (hereby denoted MFM-300(Al)-CH) and is a likely consequence of the different heating mechanism promoting a different mode of crystal growth.21,23 Reflections in the powder X-ray diffraction (PXRD) pattern of MFM-300(Al)-MW were indexed to the I4122 tetragonal space group, corresponding to the reported crystal structure of MFM-300(Al)-CH (Fig. 2).12 A simulated Le Bail fit24 to the I4122 space group revealed the presence of a low intensity extra reflection at around 27° 2θ (Fig. 2) attributed to residual unreacted linker (H4BPTC). However, there was no detectable evidence of unreacted linker by thermogravimetric analysis, i.e. no loss at the decomposition temperature of H4BPTC (340 °C) was observed (Fig. 3). No peaks corresponding to γ-Al(O)OH25 or other MOF phases beyond MFM-300(Al) were observed. Attempts to synthesise MFM-300(Al) using these conditions with conventional heating for 24 to 72 hours (rather than rapidly using microwaves) gave a mixture of MOF phases and γ-Al(O)OH.25
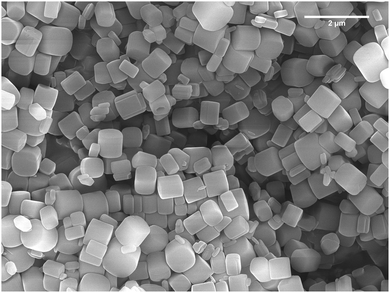 | ||
| Fig. 1 SEM image of MFM-300(Al)-MW synthesised in deionized water in 10 minutes. The material exhibits average particle dimensions of 604 ± 111 × 520 ± 234 nm. | ||
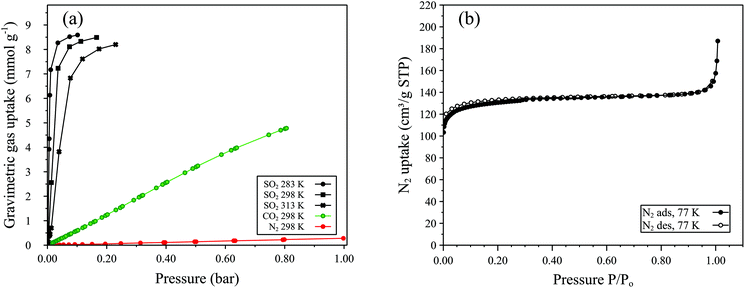
Low pressure CO2 adsorption–desorption isotherms of MFM-300(Al)-MW at ambient temperatures (273–303 K, Fig. 4a) show very high uptake capacities, with a maximum value of 8.8 mmol g−1 at 273 K and 1.0 bar and 4.7 mmol g−1 at 303 K and 1.0 bar. Analysis of the adsorption branch of the CO2 isotherm at 273 K gave a BET surface area of 1272 ± 25 m2 g−1. The non-local density functional theory (NLDFT) model for carbon slit pores26–28 gave a pore size distribution centred at 0.5 nm whereby the total pore volume is reached at 0.6 nm, indicative of a solely microporous material,26 and a cumulative pore volume of 0.58 cm3 g−1 (Fig. 4b). These results differ from the CO2 sorption properties of MFM-300(Al)-CH whereby a maximum uptake of 7.0 mmol g−1 at 273 K and 1.0 bar and cumulative pore volume of 0.38 cm3 g−1 were reported.12 MFM-300(Al)-MW shows negligible N2 sorption at 298 K up to 1 bar (see Fig. 5a), however at 77 K a Type I isotherm, with some Type IV character, is observed (Fig. 5b). From these data, an N2 uptake of 135 cm3 g−1 at ca. 0.5 bar and BET surface area of 515 ± 1 m2 g−1 were calculated (Fig. 5b). These results are significantly different compared to MFM-300(Al)-CH which does not adsorb dinitrogen.10–12 The lack of N2 porosity demonstrated by MFM-300(Al)-CH is not expected for an open porous structure and was ascribed by Yang et al. to restricted diffusion at low temperatures as a result of the narrow pore channels.12 Further work is ongoing to investigate the difference in porosity between microwave and conventionally heated MFM-300(Al) materials.
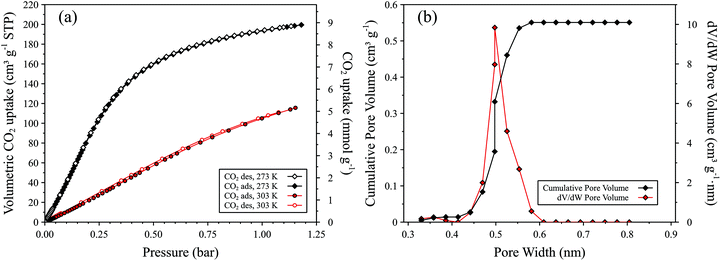 | ||
| Fig. 4 (a) Manometric carbon dioxide gas sorption isotherms (legend inset) and (b) pore size distribution (PSD, red) and cumulative pore volume plot (black) for MFM-300(Al)-MW. Data calculated from the adsorption branch of the CO2 isotherm at 273 K using the NLDFT model for carbon slit pores.26–28 | ||
We also determined the SO2 and NO2 sorption properties of MFM-300(Al)-MW which, like MFM-300(Al)-CH, exhibits exceptional uptake capacities for these environmentally detrimental gases. The high uptake of SO2 and NO2 exhibited by MFM-300(Al) are a result of multiple formal interactions between the bridging μ2-OH functionality of the aluminium secondary building unit with guest molecules, resulting in an efficient packing of SO2 and NO2 within the pore.12,15,16 SO2 adsorption isotherms at 283 K, 298 K and 303 K for MFM-300(Al)-MW collected using 25% SO2 in N2 are shown in Fig. 5a and are summarised in Table 1. As can be seen, MFM-300(Al)-MW exhibits high SO2 uptakes, 8.49 mmol g−1 at 298 K and 0.17 bar partial pressure (P), exceeding the previously reported ∼5 mmol g−1 at the same temperature and pressure,16 and 8.1 mmol g−1 at 273 K and P = 1.0 bar.12 As with the previously reported MFM-300(Al)-CH, the SO2 uptake for MFM-300(Al)-MW (Fig. 5a) show a steep increase at low pressure (<0.01 bar P) followed by a less steep increase up to 0.1 bar P, indicative of a Type I isotherm.12 At a P of 0.10 bar and 298 K, MFM-300(Al)-MW exhibits the second highest reported SO2 uptake capacity compared to other MOFs (Table 1) which is promising for potential applications in SO2 capture from flue gas where the concentration of SO2 is typically <500 ppm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 or for low concentration SO2 gas detection.17 NO2 uptake by MFM-300(Al)-MW was confirmed by measuring single point adsorption at 273 K, 298 K and 313 K using a mixture of 1% NO2 in N2 (see Fig. 6). At a P of 0.01 bar and 298 K, MFM-300(Al)-MW shows an NO2 uptake of 1.89 mmol g−1 which exceeds the reported NO2 uptake of 1.02 mmol g−1 for MFM-300(Al)-CH at the equivalent pressure.16
29 or for low concentration SO2 gas detection.17 NO2 uptake by MFM-300(Al)-MW was confirmed by measuring single point adsorption at 273 K, 298 K and 313 K using a mixture of 1% NO2 in N2 (see Fig. 6). At a P of 0.01 bar and 298 K, MFM-300(Al)-MW shows an NO2 uptake of 1.89 mmol g−1 which exceeds the reported NO2 uptake of 1.02 mmol g−1 for MFM-300(Al)-CH at the equivalent pressure.16
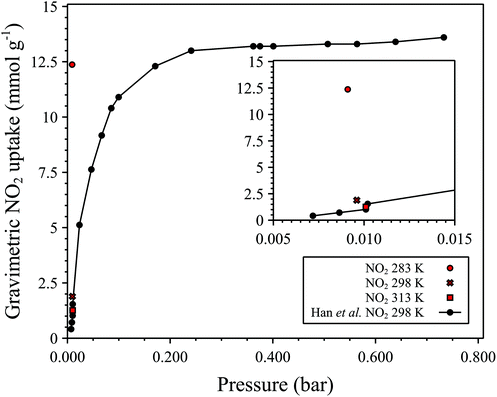 | ||
| Fig. 6 Single point NO2 sorption data collected for MFM-300(Al)-MW at 283 K, 298 K, and 313 K corresponding to partial pressures between 0.009 and 0.01 bar using 1% NO2 in N2 as the sorbate (in red). NO2 isotherm at 298 K reported by Han et al. (ref. 10) for MFM-300(Al)-CH for comparison in black (sorbate is pure NO2). Legend inset. | ||
| Material | SO2 uptake (mmol g−1) | Ref. | ||||
|---|---|---|---|---|---|---|
| Pressure (bar) | Temperature (K) | |||||
| 0.01 | 0.10 | 0.17 | 1.00 | |||
| a Taken from isotherm. b At 0.013 bar. c At 0.074 bar. d At 0.12 bar. | ||||||
| MFM-300(Al)-MW | 7.2 | 8.6 | — | — | 283 | This work |
| 2.6b | 8.1c | 8.5 | — | 298 | This work | |
| 0.5 | 7.6d | 8.0 | — | 313 | This work | |
| MFM-300(Al)-CH | — | — | — | 8.1 | 273 | 12 |
| ∼4.5a | ∼5a | ∼5a | 298 | 16 | ||
| MFM-300(In) | — | — | — | 8.3 | 298 | 34 |
| MOF-177 | 0.3 | 1.0 | 1.5a | 25.7 | 293 | 29 |
| NH2-MIL-125(Ti) | 3.0 | 7.9 | ∼8.6a | 10.8 | 293 | 29 |
| — | 5a | ∼7a | 9.7a | 298 | 35 | |
| MIL-160 | 4.2 | 5.5 | ∼5.8a | 10.8 | 293 | 29 |
| SIFSIX-1-Cu | 3.4 | 8.7 | — | 11.0 | 298 | 36 |
| SIFSIX-2-Cu-i | 4.2 | 6.0 | — | 6.9 | 298 | 36 |
In conclusion, the rapid synthesis of MFM-300(Al) in aqueous conditions using microwave heating has been developed. This route yields MFM-300(Al) on the tens of milligram scale in just 10 minutes with significantly enhanced uptake of polluting dioxides (8.8 cf. 7.0 mmol g−1 CO2 at 273 K and 1 bar,10–12 8.5 cf. ∼5 mmol g−1 SO2 at 298 K and 0.17 bar,12,16 and 1.9 cf. 1.0 mmol g−1 NO2 at 298 K and 0.01 bar.17 Other essential properties of MFM-300(Al) such as surface area (1272 ± 26 m2 g−1) and temperature of decomposition (>500 °C) are maintained. This is the fastest reported synthesis of MFM-300(Al) to date with a 99.77% reduction in reaction time compared to current solvothermal methods (3 days). The efficient and facile procedure in this work represents a key step towards the development of a sustainable route to MFM-300(Al), and possibly other MOFs, by reducing the environmental burden (use of aqueous solvent and very short reaction times). This new method also offers favourable conditions (e.g. short timescales) for development of a continuous and scalable production route using microwave heating. Industry has identified the need for new sustainable routes to MOFs30 as a prerequisite for their use in real-world applications addressing major world-wide concerns in environmental air pollution.
Experimental section
Materials and methods
AlCl3·6H2O (250 mg, 1.04 mmol), biphenyl-3,3′,5,5′-tetracarboxylic acid (H4BPTC, 85 mg, 0.26 mmol) and deionized water (15.0 mL) were added to a 35 mL vial. The vial was sealed and contents stirred to facilitate dissolution of the metal salt. The vial was then placed in to a CEM Discover microwave cavity and heated to 210 °C for 10 minutes (300 W, maximum forward power) under autogenous pressure and with stirring. After the reaction time had elapsed the vial was cooled in the microwave cavity with air and the resultant suspension was centrifuged (4200 revolutions per minute, 20 minutes) and washed with distilled water (ca. 50 mL). The centrifugation and wash step was repeated twice and the supernatant decanted. The white powder product was then dried in an oven at 50 °C for 18 hours. After this time the sample was allowed to rehydrate in the fumehood under ambient atmospheric conditions for 8 hours. Yield (89 mg, 83.5%; for the dehydrated MOF, i.e. [Al2(OH)2(C16O8H6)], calculated based on the linker and TGA analyses). Further details on synthetic development can be found in the ESI.†
Characterisation
Gravimetric SO2 and NO2 adsorption isotherms were recorded using a magnetic suspension balance working in the dynamic flow mode at ambient pressure. Therefore, an inert gas flow of dry nitrogen was used to balance the SO2 concentration between SO2 partial pressure 0.002–0.2. SO2 data were collected at temperatures of 283 K, 298 K, and 313 K and partial pressures up to 0.10, 0.17 and 0.232 bar using 25% SO2 in N2 as the sorbate. Single point NO2 sorption data were collected at 283 K, 298 K, and 313 K corresponding to partial pressures between 0.009 and 0.01 bar using 1% NO2 in N2 as the sorbate. Samples were degassed in a nitrogen flow at 120 °C until no further decrease in weight was observed.
Manometric CO2 and N2 sorption isotherms were recorded using a stainless-steel version of the BELSORP-max sorption analyser at 298 K and up to 0.81 and 1.0 bar, respectively. In a typical measurement between 30 and 60 data points were collected. Samples were degassed at 120 °C for 15 hours under vacuum (10−7 bar) before analysis. Data were plotted using Veusz.32
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
SEM analyses in this work were supported by the Engineering and Physical Sciences Research Council (EPSRC) [under grant EP/L022494/1] and the University of Nottingham. Andrea Laybourn gratefully acknowledges the University of Nottingham for the award of a Nottingham Research Fellowship.References
- M. Kampa and E. Castanas, Human health effects of air pollution, Environ. Pollut., 2008, 151, 362–367 CrossRef CAS PubMed.
- J. N. Galloway, Acid deposition: Perspectives in time and space, Water, Air, Soil Pollut., 1995, 85, 15–24 CrossRef CAS.
- D. W. Keith, Why Capture CO2 from the Atmosphere?, Science, 2009, 325, 1654–1655 CrossRef CAS PubMed.
- P. M. Edwards, S. S. Brown, J. M. Roberts, R. Ahmadov, R. M. Banta, J. A. deGouw, W. P. Dubé, R. A. Field, J. H. Flynn, J. B. Gilman, M. Graus, D. Helmig, A. Koss, A. O. Langford, B. L. Lefer, B. M. Lerner, R. Li, S.-M. Li, S. A. McKeen, S. M. Murphy, D. D. Parrish, C. J. Senff, J. Soltis, J. Stutz, C. Sweeney, C. R. Thompson, M. K. Trainer, C. Tsai, P. R. Veres, R. A. Washenfelder, C. Warneke, R. J. Wild, C. J. Young, B. Yuan and R. Zamora, High winter ozone pollution from carbonyl photolysis in an oil and gas basin, Nature, 2014, 514, 351–354 CrossRef CAS PubMed.
- J. Lelieveld, T. M. Butler, J. N. Crowley, T. J. Dillon, H. Fischer, L. Ganzeveld, H. Harder, M. G. Lawrence, M. Martinez, D. Taraborrelli and J. Williams, Atmospheric oxidation capacity sustained by a tropical forest, Nature, 2008, 452, 737–740 CrossRef CAS PubMed.
- Z. Chen, J.-N. Wang, G.-X. Ma and Y.-S. Zhang, China tackles the health effects of air pollution, Lancet, 2013, 382, 1959–1960 CrossRef.
- V. L. Feigin, G. A. Roth, M. Naghavi, P. Parmar, R. Krishnamurthi, S. Chugh, G. A. Mensah, B. Norrving, I. Shiue, M. Ng, K. Estep, C. J. L. Cercy, M. H. Murray and I. Forouzanfar, Global Burden of Diseases, Risk Factors Study 2013, Stroke Experts Writing Group. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013, Lancet Neurol., 2016, 15, 913–924 CrossRef PubMed.
- E. Barea, C. Montoro and J. A. R. Navarro, Toxic gas removal – metal–organic frameworks for the capture and degradation of toxic gases and vapours, Chem. Soc. Rev., 2014, 43, 5419–5430 RSC.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Carbon Dioxide Capture in Metal–Organic Frameworks, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- X. Han, H. G. W. Godfrey, L. Briggs, A. J. Davies, Y. Cheng, L. L. Daemen, A. M. Sheveleva, F. Tuna, E. J. L. McInnes, J. Sun, C. Drathen, M. W. George, A. J. Ramirez-Cuesta, K. M. Thomas, S. Yang and M. Schröder, Reversible adsorption of nitrogen dioxide within a robust porous metal–organic framework, Nat. Mater., 2018, 17, 691–696 CrossRef CAS PubMed.
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework, Nat. Chem., 2015, 7, 121–129 CrossRef CAS PubMed.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host, Nat. Chem., 2012, 4, 887–894 CrossRef CAS PubMed.
- C. G. Morris, N. M. Jacques, H. G. W. Godfrey, T. Mitra, D. Fritsch, Z. Lu, C. A. Murray, J. Potter, T. M. Cobb, F. Yuan, C. C. Tang, S. Yang and M. Schröder, Stepwise observation and quantification and mixed matrix membrane separation of CO2 within a hydroxy-decorated porous host, Chem. Sci., 2017, 8, 3239–3248 RSC.
- C. P. Krap, R. Newby, A. Dhakshinamoorthy, H. García, I. Cebula, T. L. Easun, M. Savage, J. E. Eyley, S. Gao, A. J. Blake, W. Lewis, P. H. Beton, M. R. Warren, D. R. Allan, M. D. Frogley, C. C. Tang, G. Cinque, S. Yang and M. Schröder, Enhancement of CO2 Adsorption and Catalytic Properties by Fe-Doping of [Ga2(OH)2(L)] (H4L = Biphenyl-3,3′,5,5′-tetracarboxylic Acid), MFM-300(Ga2), Inorg. Chem., 2016, 55, 1076–1088 CrossRef CAS PubMed.
- H. G. W. Godfrey, I. da Silva, L. Briggs, J. H. Carter, C. G. Morris, M. Savage, T. L. Easun, P. Manuel, C. A. Murray, C. C. Tang, M. D. Frogley, G. Cinque, S. Yang and M. Schröder, Ammonia Storage by Reversible Host–Guest Site Exchange in a Robust Metal–Organic Framework, Angew. Chem., Int. Ed., 2018, 57, 14778–14781 CrossRef CAS PubMed.
- X. Han, S. Yang and M. Schröder, Porous metal–organic frameworks as emerging sorbents for clean air, Nat. Rev. Chem., 2019, 3, 108–118 CrossRef CAS.
- T. Jurado-Vázquez, E. Sánchez-González, A. E. Campos-Reales-Pineda, A. Islas-Jácome, E. Lima, E. González-Zamora and I. A. Ibarra, MFM-300: From air pollution remediation to toxic gas detection, Polyhedron, 2019, 157, 495–504 CrossRef.
- V. Chernikova, O. Yassine, O. Shekhah, M. Eddaoudi and K. N. Salama, Highly sensitive and selective SO2 MOF sensor: the integration of MFM-300 MOF as a sensitive layer on a capacitive interdigitated electrode, J. Mater. Chem. A, 2018, 6, 5550–5554 RSC.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastre, Metal-organic frameworks-prospective industrial applications, J. Mater. Chem., 2006, 16, 626–636 RSC.
- N. Stock and S. Biswas, Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- I. Thomas-Hillman, A. Laybourn, C. Dodds and S. W. Kingman, Realising the environmental benefits of metal-organic frameworks: recent advances in microwave synthesis, J. Mater. Chem. A, 2018, 6, 11564–11581 RSC.
- M. Rubio-Martinez, C. Avci-Camur, A. W. Thornton, I. Imaz, D. Maspoch and M. R. Hill, New synthetic routes towards MOF production at scale, Chem. Soc. Rev., 2017, 46, 3453–3480 RSC.
- A. Laybourn, J. Katrib, R. S. Ferrari-John, C. G. Morris, S. Yang, O. Udoudo, T. L. Easun, C. Dodds, N. R. Champness, S. W. Kingman and M. Schroder, Metal-organic frameworks in seconds via selective microwave heating, J. Mater. Chem. A, 2017, 5, 7333–7338 RSC.
- A. Le Bail, H. Duroy and J. L. Fourquet, Ab initio structure determination of LiSbWO6 by X-ray powder diffraction, Mater. Res. Bull., 1988, 23, 447–452 CrossRef CAS.
- X. Bokhimi, J. A. Toledo-Antonio, M. L. Guzmán-Castillo, B. Mar-Mar, F. Hernández-Beltrán and J. Navarrete, Dependence of Boehmite Thermal Evolution on Its Atom Bond Lengths and Crystallite Size, J. Solid State Chem., 2001, 161, 319–326 CrossRef CAS.
- K. S. W. Sing, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984), Pure Appl. Chem., 1985, 57, 603 CAS.
- J. J. Manyà, B. González, M. Azuarab and G. Arner, Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity, Chem. Eng. J., 2018, 345, 631–639 CrossRef.
- A. C. Dassanayake and M. Jaroniec, Activated polypyrrole-derived carbon spheres for superior CO2 uptake at ambient conditions, Colloids Surf., A, 2018, 549, 145–154 CrossRef.
- P. Brandt, A. Nuhnen, M. Lange, J. Möllmer, O. Weingart and C. Janiak, Metal–Organic Frameworks with Potential Application for SO2 Separation and Flue Gas Desulfurization, ACS Appl. Mater. Interfaces, 2019, 11(19), 17350–17358 CrossRef CAS PubMed.
- P. A. Julien, C. Mottillo and T. Friščić, Metal–organic frameworks meet scalable and sustainable synthesis, Green Chem., 2017, 19, 2729–2747 RSC.
- A. A. Coelho, TOPAS and TOPAS-Academic: an optimization program integrating computer algebra and crystallographic objects written in C++, J. Appl. Crystallogr., 2018, 51, 210–218 CrossRef CAS.
- https://veusz.github.io/2019 .
- https://scion-image.software.informer.com/2019 .
- M. Savage, Y. Cheng, T. L. Easun, J. E. Eyley, S. P. Argent, M. R. Warren, W. Lewis, C. Murray, C. C. Tang, M. D. Frogley, G. Cinque, J. Sun, S. Rudić, R. T. Murden, M. J. Benham, A. N. Fitch, A. J. Blake, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Selective Adsorption of Sulfur Dioxide in a Robust Metal–Organic Framework Material, Adv. Mater., 2016, 28, 8705–8711 CrossRef CAS PubMed.
- W. P. Mounfield, C. Han, S. H. Pang, U. Tumuluri, Y. Jiao, S. Bhattacharyya, M. R. Dutzer, S. Nair, Z. Wu, R. P. Lively, D. S. Sholl and K. S. Walton, Synergistic Effects of Water and SO2 on Degradation of MIL-125 in the Presence of Acid Gases, J. Phys. Chem. C, 2016, 120, 27230–27240 CrossRef CAS.
- X. Cui, Q. Yang, L. Yang, R. Krishna, Z. Zhang, Z. Bao, H. Wu, Q. Ren, W. Zhou, B. Chen and H. Xing, Ultrahigh and Selective SO2 Uptake in Inorganic Anion-Pillared Hybrid Porous Materials, Adv. Mater., 2017, 29, 1606929 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Further synthetic details. See DOI: 10.1039/c9gc02375e |
| This journal is © The Royal Society of Chemistry 2019 |