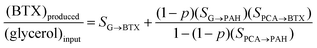Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An improved catalytic pyrolysis concept for renewable aromatics from biomass involving a recycling strategy for co-produced polycyclic aromatic hydrocarbons†
Homer C.
Genuino‡
a,
Inouk
Muizebelt‡
b,
André
Heeres
cd,
Niels J.
Schenk
 b,
Jos G. M.
Winkelman
a and
Hero J.
Heeres
b,
Jos G. M.
Winkelman
a and
Hero J.
Heeres
 *a
*a
aEngineering and Technology Institute Groningen (ENTEG), Faculty of Science and Engineering, University of Groningen, Nijenborgh 4, 9747 AG, Groningen, The Netherlands. E-mail: h.j.heeres@rug.nl
bBioBTX B.V., Zernikeplein 17, 9747 AA, Groningen, The Netherlands
cHanze University of Applied Sciences, Zernikeplein 11, 9747 AS, Groningen, The Netherlands
dSyncom B.V., Kadijk 3, 9747 AT, Groningen, The Netherlands
First published on 25th June 2019
Abstract
Catalytic pyrolysis of crude glycerol over a shaped H-ZSM-5 zeolite catalyst with (partial) recycling of the product oil was studied with the incentive to improve benzene, toluene, and xylene (BTX) yields. Recycling of the polycyclic aromatic hydrocarbon (PAH) fraction, after separation from BTX by distillation and co-feeding with the crude glycerol feed, was shown to have a positive effect on the BTX yield. Further improvements were achieved by hydrogenation of the PAH fraction using a Ru/C catalyst and hydrogen gas prior to co-pyrolysis, and BTX yields up to 16 wt% on feed were obtained. The concept was also shown to be beneficial to other biomass feeds such as e.g., Kraft lignin, cellulose, and Jatropha oil.
Introduction
Aromatic compounds are essential commodity building blocks of the chemical industry. In particular, the low molecular weight mono aromatics benzene, toluene, and xylene (BTX) are important intermediates for the production of various products including liquid fuels, solvents, and polymers.1–3 Typically, BTX is produced via refinery processes of crude oil fractions in steam cracking, steam reforming, and catalytic reforming facilities. In order to achieve a more sustainable route to BTX, a number of studies have been undertaken focusing on the use of renewable resources like biomass.4–6 This development is driven by the wish to green up the petrochemical industry due to pressing concerns about greenhouse gas effects and changes in the supply of bulk aromatics due to the implementation of shale gas-derived streams.7,8A number of routes have been developed for the catalytic conversion of sugar-derived furanics into aromatics via Diels Alder (DA) addition and subsequent dehydration reactions (i.e., a stoichiometric synthesis approach).9–12 Catalytic conversions of bio-based (isobutyl) alcohols,13,14 ethylene,15,16 and pinacol-acrolein17 to aromatics have also been reported. Catalytic pyrolysis, a one-step process with high flexibility in biomass input, offers another promising pathway to aromatics. A number of studies have described the catalytic pyrolysis of a variety of biomass feeds and mixtures thereof using (Brønsted) acidic microporous zeolites as catalysts using in situ and ex situ pyrolysis vapour upgrading concepts.18–21 The H-ZSM-5 zeolite catalyst proved to perform the best due to its high acidity, medium-sized pore structure, high surface area, and outstanding shape-selectivity. In most cases, the liquid organic product phase consists of a mixture of aromatics. The amount of the more valuable BTX in this mixture is highly dependent on the process conditions employed (e.g., WSHV, residence time, temperature) and type of biomass used, but most often it is the minority of the total aromatics formed.22 Aromatic hydrocarbons from H-ZSM-5-catalyzed pyrolysis of lignin, for example, contain considerable amounts of polycyclic aromatic hydrocarbons (PAHs, ≥50%), including (alkylated) naphthalenes, phenanthrenes, anthracenes, and pyrenes.23–25 Such large molecular aromatics are considered to be major coke precursors, responsible for (rapid) catalyst deactivation. As a result, there is a strong demand for catalytic processes converting (mixtures of) biomass in high yields towards BTX, while minimizing PAH yields.
The relatively low selectivity towards mono aromatics and the formation of significant amounts of PAHs and coke are thought to be associated with the relatively high oxygen and low hydrogen content of biomass feedstocks (low H/Ceff ratio26). To promote BTX production, different (co-)pyrolysis strategies have been explored. For instance, zeolite modifications have been tested by introducing transition metals in the framework.27–29 Noble metals were shown to be very effective dopants, but their high cost limit their large-scale utilization. Alternatively, Lu et al. used Mo2N/H-ZSM-5 catalysts, which exhibited catalytic performance for the pyrolysis of pinewood comparable to unmodified H-ZSM-5 in terms of aromatics yield, but afforded lower amounts of PAH.30 For instance, the highest yield of aromatic hydrocarbons was 9 wt%, with 8 wt% of BTX and 1 wt% of PAHs.30 Zhang et al. observed that the combined yield of aromatics and olefins from the H-ZSM-5-catalyzed conversion of different biomass feedstocks increased with higher H/Ceff ratios, accompanied by lower yields of coke.31 Higher H/Ceff ratios of the feeds may also be obtained by co-pyrolysis of biomass with substrates with higher H/Ceff values (e.g., fats, alcohols, plastics), and this strategy was shown to give higher yields of mono aromatics and lower yields of PAH.32–35
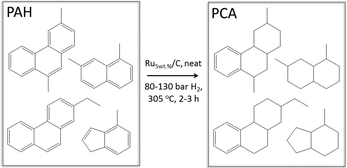
Instead of mitigating PAH formation, we explored herein the use of the co-produced PAHs as a co-feed in catalytic pyrolysis. In addition, the PAH fraction was hydrogenated to a mixture of mainly polycyclic aliphatics (PCA), aiming to increase the H/Ceff ratio, and subsequently utilize this fraction as a co-feed (Fig. 1). The PAHs used in this study were obtained from the ex situ catalytic pyrolysis of crude glycerol (obtained from a biodiesel production process) in a dedicated continuous bench-scale unit. Catalytic hydrogenation of the PAH fraction was performed in a batch-mode reactor using a Ru/C catalyst and hydrogen gas, and the reduced product was catalytically co-pyrolysed with crude glycerol in a small-scale reactor at the gram scale. The synergy between the two feedstocks in terms of liquid and BTX yields was investigated. Finally, the versatility of the concept was tested using other biomass sources such as Kraft lignin, cellulose, and Jatropha oil.
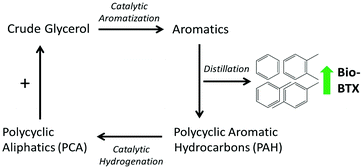 | ||
| Fig. 1 Schematic representation of the concept involving catalytic pyrolysis with efficient recycling of co-produced polycyclic aromatic hydrocarbons (PAHs). | ||
Results and discussion
A representative batch of PAHs, sufficient for all co-feed experiments reported in this work, was obtained from the ex situ catalytic pyrolysis of crude glycerol in a continuous bench-scale unit (200 g h−1). The composition of crude glycerol used is summarized in Table S1 in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 The initial pyrolysis step was conducted at 520 °C, followed by catalytic upgrading of the pyrolysis vapours in a packed bed reactor operated at 536 °C containing a shaped H-ZSM-5/bentonite (60/40) catalyst (200 g, 1–2 mm average particle size). The crude glycerol was converted to an oil phase (19.2 wt% on feed intake, consisting of mainly monocyclic and polycyclic aromatics) and an aqueous phase (25.3 wt%). The BTX was separated from the PAH fraction by means of distillation, giving 40.6 wt% of BTX and 59.4 wt% of PAH. The isolated PAH fraction consists of highly substituted single and multiple aromatic rings (C9–≈C20) with a molecular weight between about 120–500 Da. Two-ring aromatic hydrocarbons (e.g., alkylated naphthalenes) are the major components. Three-ring aromatics (e.g., alkylated phenanthrenes and anthracenes) are also present (Fig. S1–S3†). Under neat conditions, the PAH fraction was hydrogenated over a Ru/C catalyst (Fig. 2). The extent of hydrogenation may be tuned by process conditions. In order to increase the H/Ceff ratio of the PAH fraction, it was subsequently reduced by means of catalytic hydrogenation. Under typical conditions (15–18 g PAH, 0.8–0.9 g Ru5 wt%/C, 305 °C, 80–130 bar H2, 2–3.25 h, neat), the reaction was not complete and a mixture of PAHs, polycyclic aliphatics (PCAs), and mono aromatic polycyclic hydrocarbons (e.g., alkyl-substituted tetralins) was obtained in an isolated yield of 64 wt%. The molecular weight of the reduced product was similar to that of PAH, which ranged from about 100–500 Da. Elemental analysis of the product shows that the level of oxygenates is low as the sum of the C and H percentages is above 98 wt%. Moreover, the 1H NMR spectra of the PAH fraction and the reduced product are considerably different and the amount of aromatic protons present between δ 6.5–8.0 ppm is significantly less in intensity in the reduced product (Fig. S1 in the ESI†). Further information on the molecular composition of the PAH and PCA fraction was obtained from gas chromatography mass spectrometry analysis (GC-MS) and matrix-assisted laser desorption/ionization time-of-flight measurements. Details are given in the ESI (Fig. S2–S3 and Table S2†).
36 The initial pyrolysis step was conducted at 520 °C, followed by catalytic upgrading of the pyrolysis vapours in a packed bed reactor operated at 536 °C containing a shaped H-ZSM-5/bentonite (60/40) catalyst (200 g, 1–2 mm average particle size). The crude glycerol was converted to an oil phase (19.2 wt% on feed intake, consisting of mainly monocyclic and polycyclic aromatics) and an aqueous phase (25.3 wt%). The BTX was separated from the PAH fraction by means of distillation, giving 40.6 wt% of BTX and 59.4 wt% of PAH. The isolated PAH fraction consists of highly substituted single and multiple aromatic rings (C9–≈C20) with a molecular weight between about 120–500 Da. Two-ring aromatic hydrocarbons (e.g., alkylated naphthalenes) are the major components. Three-ring aromatics (e.g., alkylated phenanthrenes and anthracenes) are also present (Fig. S1–S3†). Under neat conditions, the PAH fraction was hydrogenated over a Ru/C catalyst (Fig. 2). The extent of hydrogenation may be tuned by process conditions. In order to increase the H/Ceff ratio of the PAH fraction, it was subsequently reduced by means of catalytic hydrogenation. Under typical conditions (15–18 g PAH, 0.8–0.9 g Ru5 wt%/C, 305 °C, 80–130 bar H2, 2–3.25 h, neat), the reaction was not complete and a mixture of PAHs, polycyclic aliphatics (PCAs), and mono aromatic polycyclic hydrocarbons (e.g., alkyl-substituted tetralins) was obtained in an isolated yield of 64 wt%. The molecular weight of the reduced product was similar to that of PAH, which ranged from about 100–500 Da. Elemental analysis of the product shows that the level of oxygenates is low as the sum of the C and H percentages is above 98 wt%. Moreover, the 1H NMR spectra of the PAH fraction and the reduced product are considerably different and the amount of aromatic protons present between δ 6.5–8.0 ppm is significantly less in intensity in the reduced product (Fig. S1 in the ESI†). Further information on the molecular composition of the PAH and PCA fraction was obtained from gas chromatography mass spectrometry analysis (GC-MS) and matrix-assisted laser desorption/ionization time-of-flight measurements. Details are given in the ESI (Fig. S2–S3 and Table S2†).
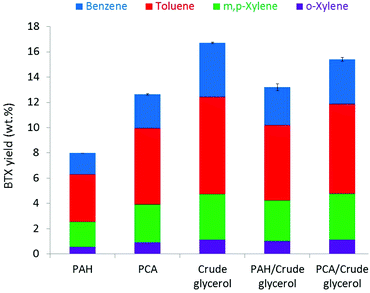
A benchmark ex situ catalytic pyrolysis of crude glycerol was performed in a gram-scale reactor (1 g sample, 3 g H-ZSM-5 catalyst with Si![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al = 23, 550 °C, details are given in the ESI†). Measurements were typically performed in duplicate and the average yields are given. After the reaction, two liquid phases were obtained, an oil phase (32 wt% on feed) and an aqueous phase (30 wt%) (Fig. S4†). In addition, gaseous products (13 wt% yield on feed) and solid products (i.e., ash, carbonaceous materials; 7 wt%) were obtained. The oil phase was analysed in detail and the BTX yield was determined to be 17 wt% on feed, with a selectivity of 28% to benzene, 46% to toluene, and 26% to xylene (Fig. 3). No BTX was found in the aqueous phase, which contained only 0.15 wt% C (TOC analyses). Before the co-pyrolysis experiments of crude glycerol with PAH and PCA, the catalytic pyrolysis of PAH and PCA was first explored to determine their potential to be converted to BTX using the H-ZSM-5 zeolite catalyst. The catalytic pyrolysis of the PAH produced a bio-oil in a 75 wt% yield on feed, together with water (6 wt%), coke on the catalyst (9 wt%), and solid products (8 wt%) (mass balance closure of 98%) (Fig. S5†). A BTX yield of 8 wt% was obtained on feed intake, with toluene being the major product at 4 wt% (Fig. 3). Thus, we conclude that part of the PAHs may be cracked to BTX using the H-ZSM-5 zeolite catalyst. Therefore, recycling of the co-produced PAHs after separation of the BTX is an interesting option to increase the overall BTX yields, as proposed in the concept shown in Fig. 1. Previously, it was reported that dealkylation of (m)ethylated naphthalenes occurs during thermal degradation, which is supported by model studies on 13C-labelled methyl arenes.37 In the presence of a zeolite, the BTX formed by catalytic aromatization of PAH-derived products most likely originates from the alkyl chains of substituted higher aromatics present in the PAH fraction.36
Al = 23, 550 °C, details are given in the ESI†). Measurements were typically performed in duplicate and the average yields are given. After the reaction, two liquid phases were obtained, an oil phase (32 wt% on feed) and an aqueous phase (30 wt%) (Fig. S4†). In addition, gaseous products (13 wt% yield on feed) and solid products (i.e., ash, carbonaceous materials; 7 wt%) were obtained. The oil phase was analysed in detail and the BTX yield was determined to be 17 wt% on feed, with a selectivity of 28% to benzene, 46% to toluene, and 26% to xylene (Fig. 3). No BTX was found in the aqueous phase, which contained only 0.15 wt% C (TOC analyses). Before the co-pyrolysis experiments of crude glycerol with PAH and PCA, the catalytic pyrolysis of PAH and PCA was first explored to determine their potential to be converted to BTX using the H-ZSM-5 zeolite catalyst. The catalytic pyrolysis of the PAH produced a bio-oil in a 75 wt% yield on feed, together with water (6 wt%), coke on the catalyst (9 wt%), and solid products (8 wt%) (mass balance closure of 98%) (Fig. S5†). A BTX yield of 8 wt% was obtained on feed intake, with toluene being the major product at 4 wt% (Fig. 3). Thus, we conclude that part of the PAHs may be cracked to BTX using the H-ZSM-5 zeolite catalyst. Therefore, recycling of the co-produced PAHs after separation of the BTX is an interesting option to increase the overall BTX yields, as proposed in the concept shown in Fig. 1. Previously, it was reported that dealkylation of (m)ethylated naphthalenes occurs during thermal degradation, which is supported by model studies on 13C-labelled methyl arenes.37 In the presence of a zeolite, the BTX formed by catalytic aromatization of PAH-derived products most likely originates from the alkyl chains of substituted higher aromatics present in the PAH fraction.36
The catalytic pyrolysis of the PCA fraction produced a bio-oil in 77 wt% yield on feed, together with water (12 wt%), residual solids (1.3 wt%), and gas (2.3 wt%) (mass balance closure of 93%) (Fig. S5†). The molecular composition of the liquid product was obtained from GC-MS analysis (Table S2†). The BTX yield was 13 wt% on feed, which is considerably higher than that for the PAH experiments (8 wt%) (Fig. 3). This finding is in line with the higher H/Ceff ratio of the PCA fraction (1.71) compared to PAH (1.177). As such, it suggests that the use of a co-feed with a higher aliphatic than aromatic content is advantageous for BTX production. To prove this hypothesis, catalytic co-feeding experiments with crude glycerol and the PCA or PAH fraction were performed using a 1-to-1 wt feed ratio under the same reaction conditions as utilized for the catalytic pyrolysis of the individual feeds. When using PAH as the co-feed, the BTX yield was 13 wt% on feed, which is in between the values obtained for experiments with PAH and crude glycerol individually. Actually the BTX yield for a 1-to-1 mixture of PAH and crude glycerol is about equal to the average value of the yields obtained with these feeds separately (12.5 wt%). Expectedly, co-feeding the PCA with crude glycerol gave a BTX yield of 16 wt% on feed, which is higher than the average yield of the individual components (15 wt%) and may be indicative of a synergistic effect (vide infra). Co-pyrolysis of crude glycerol with PCA also resulted in an increased bio-oil yield as compared to crude glycerol alone (55 wt% vs. 32 wt%), with concomitant decreases in coke deposits on catalyst, residual solids, water, and gaseous products (Fig. S4–S6†). In zeolite-catalysed aromatization of glycerol, the dehydration products acrolein and acetaldehyde are thought to be directly converted to aromatic hydrocarbon via acid-catalysed aldol condensation, after which, they are converted to BTX.38 Our results imply that PCA effectively supplies the available reactive intermediates with the pyrolysis products of crude glycerol for aromatization to BTX.
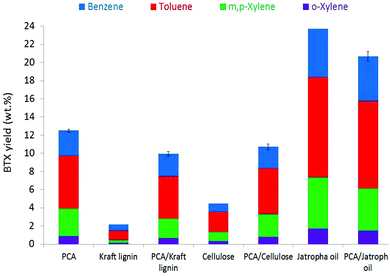
In addition to crude glycerol, Kraft lignin, cellulose, and Jatropha oil were tested as the biomass source under the same catalytic pyrolysis conditions. The BTX yields for the individual components varied between 2 wt% and 24 wt%, with the highest BTX yields obtained from Jatropha oil (Fig. 4). Interestingly, the BTX yields were considerably higher when using the PCA–biomass mixtures as compared to the average BTX yield from the individual feed experiments. For instance, the calculated synergistic effects (defined as the ratio of the actual BTX yields obtained when using a 1-to-1 PCA–biomass wt feed ratio and the average value for a 1-to-1 mixture based on individual feed) were determined to be 131% for PCA–Kraft lignin, 120% for PCA–cellulose, and 113% for PCA–Jatropha oil. These findings suggest that the ease of aliphatic ring-opening in PCA results in smaller fragments for further reactions of the co-pyrolysis vapours in the zeolite catalyst pores, including (de)alkylation, Friedel–Crafts alkylation, DA aromatization, and alike. It seems most likely that in the ex situ pyrolysis with biomass and PCA, the conversion of the latter towards BTX results in the additional formation of hydrogen. The presence of hydrogen in the catalytic pyrolysis results in the stabilization of lignin-derived phenolics and furthermore increases the hydrodeoxygenation of oxygen-containing compounds. This results in an increased formation of small alkanes/alkenes (i.e., C1–C4) that are considered to be the precursors for aromatization to occur and less formation of higher aromatics and gases (see Fig. S6†). This intriguing observation is the focus of subsequent studies and will be reported separately.
To illustrate the potential of the new catalytic pyrolysis concept for BTX production from glycerol, a model study of the process, including recycling, was conducted. Fig. 5 shows a simple diagram of a continuous BTX production process that uses recycling and partial hydrogenation of PAH from the catalytic pyrolysis of glycerol. The catalytic pyrolysis is characterized by the selectivity of the conversion of glycerol to BTX and to PAH (SG→BTX and SG→PAH, respectively), and the selectivity of the conversion of recycled PCA to BTX and PAH (SPCA→BTX and SPCA→PAH, respectively). With a series of separation steps, shown as a single block, separate BTX, gas/water/solids (GWS), and PAH streams are obtained from the pyrolysis output. The PAH stream is partially hydrogenated and returned to the catalytic pyrolysis unit. The relative amount of BTX (wt/wt) obtained from this operation follows from
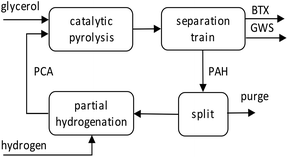 | ||
| Fig. 5 Simplified process flow diagram of continuous BTX production from the catalytic pyrolysis of glycerol with recycling of co-produced PAHs. | ||
Fig. 6 shows the overall selectivity for BTX and the fractional purge versus the recycle ratio calculated using estimated selectivities as SG→BTX = 0.10, SG→PAH = 0.12, SPCA→BTX = 0.40 and SPCA→PAH = 0.30. In this case, with a once through operation, the overall yield of BTX is 10 wt%. With recycling and partial hydrogenation of PAH, the overall yield of BTX is increased to almost 17 wt%. The exact amount of BTX is a function of the bleed, recycle ratio, level of hydrogenation, etc.
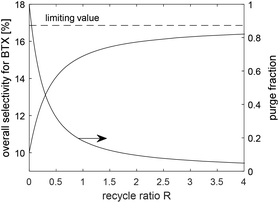 | ||
| Fig. 6 Overall selectivity percentage of BTX and its limiting value vs. the recycle ratio (left axis), and fractional purge vs. the recycle ratio (right axis). | ||
Conclusions
Ex situ catalytic pyrolysis of crude glycerol over a shaped H-ZSM-5 zeolite catalyst with (partial) recycling of the product oil was studied. The new concept involves isolation of the co-produced polycyclic aromatic hydrocarbon (PAH) fraction after separation from the BTX fraction by distillation and subsequent co-feeding with the crude glycerol feed. This was shown to have a positive effect on the BTX yield. The use of a hydrogenated PAH fraction gave higher BTX yields than found for the untreated PAH fraction, indicating that co-feeds with a higher H/Ceff ratio are preferred when aiming for higher BTX yields. Further research will particularly focus on the observed synergistic effects and mechanistic implications of such effects for some of the biomass feeds, which have potential for further optimization of BTX yields, as well as the effects of co-feeds on catalyst stability.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work is part of the research programme, EFRO Valorisatie D: Advanced Integrated Cascading Pyrolysis, which is financed by the “Europees Fonds voor Regionale Ontwikkeling (EFRO)”. Henk van de Bovenkamp (BioBTX B.V.) is acknowledged for comments and discussion.References
- A. M. Niziloek, O. Onel, Y. A. Guzman and C. Floudas, Energy Fuels, 2016, 30, 4970–4998 CrossRef.
- C. Wang, Q. Hao, D. Lu, Q. Jia, G. Li and B. Xu, Chin. J. Catal., 2008, 29, 907–912 CrossRef CAS.
- W. A. Sweeney and P. F. Bryan, BTX Processing, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., 2008, pp. 1–7 Search PubMed.
- S. Gillet, M. Aguedo, L. Petitjean, A. R. C. Morais, A. M. da Costa Lopes, R. M. Lukasik and P. T. Anastas, Green Chem., 2017, 19, 4200–4233 RSC.
- A. Heeres, N. Schenk, I. Muizebelt, R. Blees, B. De Waele, A.-J. Zeeuw, N. Meyer, R. Carr, E. Wilbers and H. J. Heeres, ACS Sustainable Chem. Eng., 2018, 6, 3472–3480 CrossRef CAS PubMed.
- Y.-T. Cheng and G. W. Huber, Green Chem., 2012, 14, 3114–3125 RSC.
- P. J. Dauenhauer and G. W. Huber, Green Chem., 2014, 16, 382–383 RSC.
- P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2013, 52, 11980–11987 CrossRef CAS PubMed.
- S. Thiyagarajan, H. C. Genuino, M. Sliwa, J. C. van der Waal, E. de Jong, J. van Haveren, B. M. Weckhuysen, P. C. A. Bruijnincx and D. A. van Es, ChemSusChem, 2015, 8, 3052–3056 CrossRef CAS PubMed.
- A. Maneffa, P. Priecel and J. A. Lopez-Sanchez, ChemSusChem, 2016, 9, 2736–2748 CrossRef CAS PubMed.
- L. Ni, J. Xin, K. Jiang, L. Chen, D. Yan, X. Lu and S. Zhang, ACS Sustainable Chem. Eng., 2018, 6, 2541–2551 CrossRef CAS.
- A. G. Gayubo, A. T. Aguayo, A. Atutxa, R. Aguado and J. Bilboa, Ind. Eng. Chem. Res., 2004, 43, 2610–2618 CrossRef CAS.
- Z. Li, A. W. Lepore, M. F. Salazar, G. S. Foo, B. H. Davison, Z. Wu and C. K. Narula, Green Chem., 2017, 19, 4344–4352 RSC.
- B. A. Mitchell, Biobased Plastics: Are We There Yet?, September 21, 2015, https://www.acs.org/content/acs/en/pressroom/cutting-edge-chemistry/biobased-plastics-are-we-there-yet.html.
- T. W. Lyons, D. Guironnet, M. Findlater and M. Brookhart, J. Am. Chem. Soc., 2012, 134, 15708–15711 CrossRef CAS PubMed.
- V. Hulea, ACS Catal., 2018, 8, 3263–3279 CrossRef CAS.
- Y. Hu, N. Li, G. Li, A. Wang, Y. Cong, X. Wang and T. Zhang, ChemSusChem, 2017, 10, 2880–2885 CrossRef CAS PubMed.
- A. V. Bridgwater, Biomass Bioenergy, 2012, 38, 68–94 CrossRef CAS.
- C. Liu, H. Wang, A. M. Karim, J. Sun and Y. Wang, Chem. Soc. Rev., 2014, 43, 7594–7623 RSC.
- R. H. Venderbosch, ChemSusChem, 2015, 8, 1306–1316 CrossRef CAS PubMed.
- T. C. Hoff, D. W. Gardner, R. Thilakaratne, K. Wang, T. W. Hansen, R. C. Brown and J.-P. Tessonnier, ChemSusChem, 2016, 9, 1473–1482 CrossRef CAS PubMed.
- A. Meuwese, The sustainability of producing BTX from biomass, MSc. Thesis, University of Groningen, 2013 Search PubMed.
- M. A. Jackson, D. L. Compton and A. A. Boateng, J. Anal. Appl. Pyrolysis, 2009, 85, 226–230 CrossRef CAS.
- H. Zhang, S. Shao, R. Xiao, D. Shen and J. Zeng, Energy Fuels, 2014, 28, 52–57 CrossRef CAS.
- D. J. Mihalcik, A. A. Boateng, C. A. Mullen and N. M. Goldberg, Ind. Eng. Chem. Res., 2011, 50, 13304–13312 CrossRef CAS.
- N. Y. Chen, J. T. F. Degnan and L. R. Koening, Chem. Tech., 1986, 16, 506–511 CAS.
- F. Wang, W. Y. Xiao, L. J. Gao and G. M. Xiao, RSC Adv., 2016, 6, 42984–42993 RSC.
- Y.-W. Suh, H.-S. Jang and K.-B. Bae, Method for Producing Bio-Aromatics from Glycerol, U.S. Pat. Appl. Publ, Industry-University Cooperation Foundation Hanyang University, Seoul, Korea, United States, 2015, p. 11 Search PubMed.
- W. B. Widayatno, G. Guan, J. Rizkiana, J. Yang, X. Hao, A. Tsutsumi and A. Abudula, Appl. Catal., B, 2016, 186, 166–172 CrossRef CAS.
- Q. Lu, H.-Q. Guo, M.-X. Zhou, M.-S. Cui, C.-Q. Dong and Y.-P. Yang, Fuel Process. Technol., 2018, 173, 134–142 CrossRef CAS.
- H. Zhang, Y.-T. Cheng, T. P. Vispute, R. Xiao and G. W. Huber, Energy Environ. Sci., 2011, 4, 2297–2307 RSC.
- S. Wang, J. Chen, Q. Cai, F. Zhang, Y. Wang, B. Ru and Q. Wang, Int. J. Hydrogen Energy, 2016, 41, 16385–16393 CrossRef CAS.
- X. Zhang, H. Lei, S. Chen and J. Wu, Green Chem., 2016, 18, 4145–4169 RSC.
- K. Ding, A. He, D. Zhong, L. Fan, S. Liu, Y. Wang, P. Chen, H. Lei and R. Ruan, Bioresour. Technol., 2018, 268, 1–8 CrossRef CAS PubMed.
- P. Blommel and R. Cortright, US Pat, 9873644B2, 2018 Search PubMed.
- S. He, I. Muizebelt, A. Heeres, N. J. Schenk, R. Blees and H. J. Heeres, Appl. Catal., B, 2018, 235, 45–55 CrossRef CAS.
- A. R. Lea-Langton, G. E. Andrews, K. D. Bartle, J. M. Jones and A. Williams, Fuel, 2015, 158, 719–724 CrossRef CAS.
- T. Q. Hoang, X. Zhu, T. Danuthai, L. L. Lobban, D. E. Resasco and R. G. Mallinson, Energy Fuels, 2010, 24, 3804–3809 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc01485c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |