Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of copper catalysts for click chemistry from distillery wastewater using magnetically recoverable bionanoparticles†
Richard L.
Kimber‡
 *a,
Fabio
Parmeggiani‡
*a,
Fabio
Parmeggiani‡
 b,
Nimisha
Joshi
a,
Alexander M.
Rakowski
c,
Sarah J.
Haigh
b,
Nimisha
Joshi
a,
Alexander M.
Rakowski
c,
Sarah J.
Haigh
 c,
Nicholas J.
Turner
c,
Nicholas J.
Turner
 b and
Jonathan R.
Lloyd
b and
Jonathan R.
Lloyd
 a
a
aSchool of Earth and Environmental Sciences and Williamson Research Centre for Molecular Environmental Science, University of Manchester, Manchester, UK. E-mail: richard.kimber@manchester.ac.uk
bSchool of Chemistry, Manchester Institute of Biotechnology (MIB), University of Manchester, Manchester, UK
cSchool of Materials, University of Manchester, Manchester, UK
First published on 8th July 2019
Abstract
Copper recovered from a whisky distillery waste stream is shown to be an effective catalyst for a range of azide–alkyne “click” reactions. Biogenic nanomagnetite (BNM), produced under mild conditions, was used to rapidly recover and subsequently support the Cu catalyst which could be separated and recycled easily.
The uncatalysed Huisgen 1,3-dipolar cycloaddition of organic azides and alkynes is a slow, high temperature process that affords a mixture of 1,4 and 1,5-disubstitution products.1 In 2002, the groups of Sharpless2 and Meldal3 discovered that copper(I) can greatly accelerate the reaction and markedly improve its regioselectivity. Since then, Cu(I)-catalysed azide–alkyne cycloaddition (CuAAC) has become almost synonymous with “click chemistry”, with applications in drug discovery,4,5 bioconjugation,6,7 materials science,8 and polymer chemistry.9 Click chemistry, a concept introduced by Sharpless in 2001, describes a set of highly efficient reactions which are high yielding, wide in scope, use benign solvents, generates only inoffensive byproducts and are regio/stereospecific.10 Despite the green credentials of click chemistry, the synthesis of CuAAC catalysts often does not follow these same stringent criteria.
There is much interest in the use of supported Cu catalysts due to their increased robustness, ease of recovery and improved reusability, compared to their unsupported counterparts.11,12 To this end, efforts have been made to immobilize CuAAC catalysts on polymers,13 zeolites,14 charcoal,15,16 graphene,17,18 and clay.19 Our previous work demonstrated that under mild aqueous conditions, metal-reducing bacteria can act as both reductant and support for CuAAC catalysts.20 Producing these catalysts with Cu sourced from waste streams would greatly enhance their green credentials, offering numerous advantages over traditional synthesis using Cu salts. Cu recovery from waste provides a low-cost, renewable source of Cu, minimises or actively reduces waste volumes, and recycles and valorises waste metal into highly useful catalysts.
Magnetic iron nanoparticles offer a promising method for simple recovery of metals from waste solutions.21,22 In addition, they have gained increased attention as supports for CuAAC catalysts due to their ease of separation, recovery and reuse.23–25 However, the chemical synthesis of these magnetic nanoparticles often requires high temperature, non-aqueous solvents, stabilizing agents or hazardous chemicals. Microbial metabolism can be harnessed to provide simple, inexpensive, scalable and tunable processes for the synthesis of magnetic nanoparticles under mild conditions.26,27
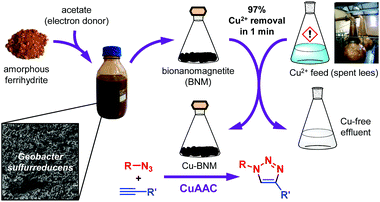
Herein, we present the first synthesis of a CuAAC catalyst using copper recovered from an industrial waste source (Fig. 1). We also demonstrate that the copper can be recovered and subsequently supported using biogenic nanomagnetite (BNM), synthesised under very mild conditions. Due to the magnetic nature of the BNM, the catalyst could easily be separated and reused for up to three cycles. The industrial waste source selected for this study was the spent lees from a whisky distillery located in Scotland. Spent lees are the residues left in the spirit still following the second distillation phase of whisky production, and contain elevated Cu concentrations in addition to a range of fermentation products creating a complex waste source (see ESI†). The lees are normally treated in an effluent plant before being discharged to the environment or applied to land as a soil conditioner. The presence of copper increases the treatment levels required and limits the potential for application to land.
To produce the BNM, cells of the metal-reducing bacterium Geobacter sulfurreducens were added to amorphous ferrihydrite and supplied with acetate as an electron donor. The reaction proceeded at 30 °C and the only solvent used was water. After synthesis, the BNM was easily recovered using a magnet and washed 3 times in water to remove residual biomass. Previous characterization revealed an average particle size of 13.3 ± 0.6 nm.28
Inductively coupled plasma atomic emission spectroscopy (ICP-AES) confirmed the Cu content in the spent less was 49 mg L−1. A BNM concentration of 2.8 mg mL−1 spent lees was chosen for Cu recovery and catalyst synthesis in order to provide sufficient Fe2+ on the BNM surface for potential Cu2+ reduction, and to demonstrate catalytic activity at a relatively low Cu loading. Rapid removal of Cu following BNM addition was confirmed by ICP-AES which revealed >97% of Cu was removed in less than 1 minute. The BNM was then magnetically recovered. Scanning transmission electron microscopy (STEM) and energy-dispersive X-ray spectroscopy (EDX) mapping confirmed the recovered Cu was supported on the BNM (CuleesBNM) with clear evidence of the Cu associated with the Fe signal of the BNM (Fig. 2). Digestion of the recovered CuleesBNM revealed a Cu loading of 2 wt%.
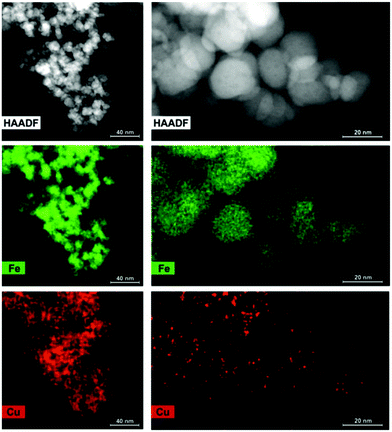 | ||
| Fig. 2 High-angle annular dark field (HAADF) images and corresponding elemental maps of Fe and Cu of the CuleesBNM catalyst. | ||
Characterisation of the CuleesBNM was performed using X-ray photoelectron spectroscopy (XPS). Interestingly, XPS analysis of the spent lees suggested the presence of two Cu species. The Cu 2p3/2 peak at 934.2 eV is characteristic of Cu(II) whereas the peak at 932.8 eV suggests the presence of reduced Cu (Fig. 3a).
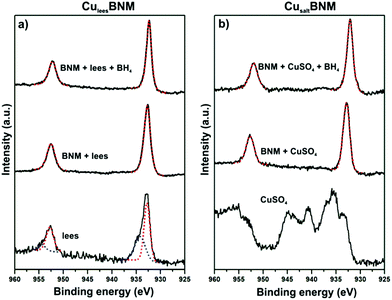 | ||
| Fig. 3 XPS surface analysis of (a) CuleesBNM and (b) CusaltBNM catalysts. Data is displayed as black solid lines whilst fits are displayed as red dotted lines. | ||
However, distinguishing between Cu(I) and Cu(0) is not accurate with this technique.29 After recovery of the Cu by the BNM, only one peak at 932.7 eV was present, suggesting reduction of the Cu(II) had occurred, most likely mediated by the Fe(II) present within and on the surface of the BNM.30,31 A catalyst prepared by the addition of reagent grade CuSO4 (dissolved in water) to BNM (CusaltBNM), giving an identical Cu loading of 2 wt%, was also prepared and characterised (Fig. 3b and ESI†). A similar Cu 2p3/2 peak at 932.8 eV suggested that reduction of Cu(II) by BNM also occurred in the synthesis of the CusaltBNM catalyst (Fig. 3b).
To investigate the potential for using Cu recovered from waste for click reactions, we began by testing the CuleesBNM catalyst in the cycloaddition of benzyl azide 1a and phenylacetylene 2a (Table 1). The activity was compared against the CusaltBNM catalyst. When no additional reducing agent was applied, both the CuleesBNM and CusaltBNM were found to be poorly active (Table 1, entries 3 and 4), likely due to the presence of some residual Cu(II) on the surface of the nanomaterial. Addition of ascorbate to the reaction mixture, leading to reduction of Cu(II) to Cu(I), afforded complete conversion to the corresponding 1,2,3-triazole product 3a (>95%, Table 1, entries 5 and 6). However, ascorbate addition also resulted in dissolution of the CuleesBNM catalyst after prolonged incubation, precluding any potential recyclability, as has been reported previously.24 In order to alleviate this issue, the Cu-BNM catalysts were pre-treated with NaBH4 as the reducing agent. This resulted in a shift in the Cu 2p3/2 peaks of the nanocatalysts of between 0.4 and 0.8 eV (Fig. 3), likely due to reduction of any residual Cu(II). This enabled excellent catalytic yields whilst also preserving the integrity of the catalyst so that it could be easily separated magnetically for potential reuse. The CuleesBNM catalyst prepared from the spent lees waste gave complete conversion after 12 hours and was only slightly less efficient over a shorter incubation time compared to the CusaltBNM catalyst (Table 1, entries 7 and 9). Therefore, all subsequent preparative scale experiments were carried out using the CuleesBNM catalyst.
| Entry | Catalyst | Reducing agent | Time [h] | Yieldb [%] |
|---|---|---|---|---|
a Reaction conditions: 0.25 mmol 1a, 0.25 mmol 2a, 500 μL Cu-BNM suspension, 5 mL H2O/t-BuOH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 25 °C.
b Calculated by 1H NMR (n.d. = product not detected).
c 50 mM sodium ascorbate added to the click reaction mixture.
d Treatment of the Cu-BNM with 10 mM sodium borohydride prior to click reaction. 2), 25 °C.
b Calculated by 1H NMR (n.d. = product not detected).
c 50 mM sodium ascorbate added to the click reaction mixture.
d Treatment of the Cu-BNM with 10 mM sodium borohydride prior to click reaction.
|
||||
| 1 | None | None | 12 | n.d. |
| 2 | BNM | None | 12 | n.d. |
| 3 | CusaltBNM | None | 12 | 45 |
| 4 | CuleesBNM | None | 12 | 43 |
| 5 | CusaltBNM | Ascorbatec | 12 | >95 |
| 6 | CuleesBNM | Ascorbatec | 12 | >95 |
| 7 | CusaltBNM | NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
5 | 56 |
| 8 | CusaltBNM | NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
12 | >95 |
| 9 | CuleesBNM | NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
5 | 47 |
| 10 | CuleesBNM | NaBH4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
12 | >95 |
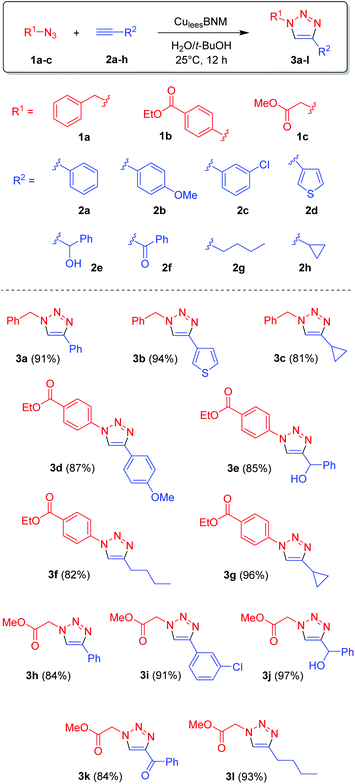
We next investigated the CuAAC of an expanded range of azides (1a–c) and alkynes (1a–h) using the CuleesBNM catalyst, to produce a variety of 1,2,3-triazole products (Scheme 1). The reactions were conducted on a 5 mL scale, with 0.25 mmol of each reagent and 500 μL of the CuleesBNM suspension, at 25 °C for 12 h, and the products were isolated by extraction with ethyl acetate. The catalytic system proved very efficient in all cases, affording almost quantitative conversion and very good isolated yields (81–97%) for 12 different triazoles 3a–l. This also highlights the broad tolerance of this procedure towards different functional groups such as esters, ethers, alcohols, ketones and heterocycles.
Furthermore, the recyclability of the CuleesBNM was assessed by simple magnetic separation and recovery of the catalyst, before being applied to a new reaction cycle. The recyclability test was performed for the synthesis of 3a, by extracting the product and/or leftover starting materials with ethyl acetate, magnetically recovering the particles, rinsing them twice with H2O/EtOH 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and resuspending them in water for a new reaction cycle (Table 2). The recovered catalyst demonstrated good yields for the first three cycles, before a significant decrease in activity at cycle 4. This highlights the advantage of supporting the copper on a magnetic nanomaterial that can be recovered easily. Further optimisation to improve recyclability is being investigated.
1, and resuspending them in water for a new reaction cycle (Table 2). The recovered catalyst demonstrated good yields for the first three cycles, before a significant decrease in activity at cycle 4. This highlights the advantage of supporting the copper on a magnetic nanomaterial that can be recovered easily. Further optimisation to improve recyclability is being investigated.
| Cycle | Yield [%] |
|---|---|
a Reaction conditions for CuAAC: 0.25 mmol 1a, 0.25 mmol 2a, 500 μL CuleesBNM suspension, 5 mL H2O/t-BuOH (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), 25 °C, 12 h. 2), 25 °C, 12 h.
|
|
| 1 | >95 |
| 2 | 90 |
| 3 | 71 |
| 4 | 27 |
| 5 | <5 |
Lastly, in order to prove the scalability of this protocol, a gram-scale synthesis of 3a from 5 mmol of 1a and 2a was performed (employing slightly increased substrate concentrations and decreased catalyst loading, see ESI† for details). Triazole 3a was obtained in 82% isolated yield after extraction and recrystallisation, demonstrating the usefulness of the CuleesBNM as a preparative-scale catalyst for CuAAC reactions.
Conclusions
Herein, we report for the first time CuAAC reactions using copper recovered from an industrial waste stream. In addition, we demonstrate the first use of biogenic magnetic nanoparticles as a simple, green method for recovering and subsequently supporting the CuAAC catalysts. The CuleesBNM catalysts could be reused for up to three cycles following magnetic separation. Conversion of a pre-existing waste into a useful resource for synthetic applications reduces the additional raw resources and associated costs needed for catalyst preparation, demonstrating a previously unexplored opportunity for sustainable CuAAC. The efficient biological synthesis of the magnetic nanoparticles under mild conditions provides additional green benefits by avoiding the need for reducing agents, non-aqueous solvents, high temperatures or hazardous chemicals for producing the catalyst support. Production of BNM from waste iron oxides could further enhance the green credentials of this CuAAC catalyst synthesis method.32 The ability of the BNM to act as both the initial recovery agent and subsequent support for the Cu improves the atom efficiency of the process and reduces the number of steps required for catalyst synthesis. Simple magnetic separation and recovery of the CuleesBNM following the CuAAC adds to the benefits of heterogeneous catalysis. Lastly, the broad applicability of this catalytic system for click reactions has been demonstrated with the synthesis of a panel of 12 different triazoles bearing various functional groups, on preparative scale up to gram-quantities of product. Further work into the physical characterisation of the material and elucidation of the catalytic mechanism is ongoing, in addition to investigating the viability of recovery of Cu and other metals from different waste streams.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank NERC for funding under the Resource Recovery from Waste program (NE/L014203/1) and BBSRC for support (grants BB/L013711/1 and BB/R010412/1). SJH and AMR thank the EPSRC U.K (grants EP/M010619/1, EP/P009050/1, and the NoWNano CDT), and the ERC under the EU's Horizon 2020 program (ERC-2016-STG-EvoluTEM-715502). We gratefully acknowledge Ronald Daalmans and Chivas Brothers for supplying the spent lees. The authors would also like to thank P. Lythgoe, B. Spencer and R. Spiess (University of Manchester) for ICP-AES, XPS and HRMS analyses, respectively.Notes and references
- R. Huisgen, R. Sustmann and G. Wallbillich, Chem. Ber./Recl., 1967, 100, 1786 CrossRef CAS.
- V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596–2599 CrossRef CAS PubMed.
- C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057–3064 CrossRef.
- G. C. Tron, T. Pirali, R. A. Billington, P. L. Canonico, G. Sorba and A. A. Genazzani, Med. Res. Rev., 2008, 28, 278–308 CrossRef CAS PubMed.
- S. G. Agalave, S. R. Maujan and V. S. Pore, Chem. – Asian J., 2011, 6, 2696–2718 CrossRef CAS PubMed.
- H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128–1137 CrossRef CAS PubMed.
- K. Nwe and M. W. Brechbiel, Cancer Biother. Radiopharm., 2009, 24, 289–302 CrossRef CAS PubMed.
- W. Xi, T. F. Scott, C. J. Kloxin and C. N. Bowman, Adv. Funct. Mater., 2014, 24, 2572–2590 CrossRef CAS.
- M. Morten, Macromol. Rapid Commun., 2008, 29, 1016–1051 CrossRef.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS PubMed.
- F. Alonso, Y. Moglie and G. Radivoy, Acc. Chem. Res., 2015, 48, 2516–2528 CrossRef CAS PubMed.
- T. Jin, M. Yan and Y. Yamamoto, ChemCatChem, 2012, 4, 1217–1229 CrossRef CAS.
- C. Girard, E. Önen, M. Aufort, S. Beauvière, E. Samson and J. Herscovici, Org. Lett., 2006, 8, 1689–1692 CrossRef CAS PubMed.
- S. Chassaing, M. Kumarraja, A. Sani Souna Sido, P. Pale and J. Sommer, Org. Lett., 2007, 9, 883–886 CrossRef CAS PubMed.
- B. R. Buckley, R. Butterworth, S. E. Dann, H. Heaney and E. C. Stubbs, ACS Catal., 2015, 5, 793–796 CrossRef CAS.
- B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235–8238 CrossRef CAS PubMed.
- A. Shaygan Nia, S. Rana, D. Dohler, X. Noirfalise, A. Belfiore and W. H. Binder, Chem. Commun., 2014, 50, 15374–15377 RSC.
- V. H. Reddy, Y. V. R. Reddy, B. Sridhar and B. V. S. Reddy, Adv. Synth. Catal., 2016, 358, 1088–1092 CrossRef CAS.
- B. J. Borah, D. Dutta, P. P. Saikia, N. C. Barua and D. K. Dutta, Green Chem., 2011, 13, 3453–3460 RSC.
- R. L. Kimber, E. A. Lewis, F. Parmeggiani, K. Smith, H. Bagshaw, T. Starborg, N. Joshi, A. I. Figueroa, G. v. d. Laan, G. Cibin, D. Gianolio, S. J. Haigh, R. A. D. Pattrick, N. J. Turner and J. R. Lloyd, Small, 2018, 14, 1703145 CrossRef PubMed.
- R. A. Crane and D. J. Sapsford, J. Hazard. Mater., 2018, 347, 252–265 CrossRef CAS PubMed.
- K. Zargoosh, H. Abedini, A. Abdolmaleki and M. R. Molavian, Ind. Eng. Chem. Res., 2013, 52, 14944–14954 CrossRef CAS.
- B. S. P. Anil Kumar, K. Harsha Vardhan Reddy, B. Madhav, K. Ramesh and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 4595–4599 CrossRef CAS.
- R. Hudson, C. J. Li and A. Moores, Green Chem., 2012, 14, 622–624 RSC.
- S. Sabaqian, F. Nemati, M. M. Heravi and H. T. Nahzomi, Appl. Organomet. Chem., 2017, 31, e3660 CrossRef.
- J. R. Lloyd, J. M. Byrne and V. S. Coker, Curr. Opin. Biotechnol., 2011, 22, 509–515 CrossRef CAS PubMed.
- J. M. Byrne, H. Muhamadali, V. S. Coker, J. Cooper and J. R. Lloyd, J. R. Soc., Interface, 2015, 12, 20150240 CrossRef PubMed.
- J. M. Byrne, N. D. Telling, V. S. Coker, R. A. Pattrick, G. van der Laan, E. Arenholz, F. Tuna and J. R. Lloyd, Nanotechnology, 2011, 22, 455709 CrossRef CAS PubMed.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887–898 CrossRef CAS.
- D. B. Johnson, S. Hedrich and E. Pakostova, Front. Microbiol., 2017, 8, 201700211 Search PubMed.
- R. S. Cutting, V. S. Coker, N. D. Telling, R. L. Kimber, C. I. Pearce, B. L. Ellis, R. S. Lawson, G. van der Laan, R. A. D. Pattrick, D. J. Vaughan, E. Arenholz and J. R. Lloyd, Environ. Sci. Technol., 2010, 44, 2577–2584 CrossRef CAS PubMed.
- N. Joshi, J. Filip, V. S. Coker, J. Sadhukhan, I. Safarik, H. Bagshaw and J. R. Lloyd, Front. Environ. Sci., 2018, 6, 201800127 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Supplementary figures and tables, experimental methods, product characterisation data, copies of NMR and HRMS spectra. See DOI: 10.1039/c9gc00270g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |