Electrochemical halogenation/semi-pinacol rearrangement of allylic alcohols using inorganic halide salt: an eco-friendly route to the synthesis of β-halocarbonyls†
Abstract
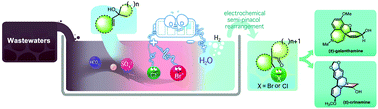
An efficient and eco-friendly electrochemical method involving halogenation/semi-pinacol rearrangement of allylic alcohols using inorganic halide salt as the halogen source to synthesize various β-halocarbonyls bearing an all-carbon α-quaternary center under mild reaction conditions has been developed (X = Br, Cl). Stoichiometric oxidants, metal catalysts, and even external electrolytes were avoided in this method. It was found that the whole reaction system was compatible with various common interfering ions in water and exhibited good selectivity for the bromide ion. Furthermore, the halogen resource can be efficiently extracted from simulated wastewaters to construct key intermediates for the syntheses of natural products (±)-galanthamine and (±)-crinamine. Additionally, this strategy may be promising to treat oil and gas wastewaters and desalt seawater.


 Please wait while we load your content...
Please wait while we load your content...